Abstract
Hypercalcemia in sarcoidosis is due to three mechanistic reasons: (1) systemic conversion of 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D by the enzyme 1-α hydroxylase produced by activated monocyte/macrophage system, (2) production of parathormone-related peptide (PTHrP) by the sarcoid granuloma, (3) tissue-level conversion of 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D by 1-αhydroxylase produced by local monocyte/macrophage system in the sarcoid granuloma. We report two cases of one proposed mechanism of hypercalcaemia in sarcoidosis (mechanism 3). Both individuals presented with sarcoidosis and 25-hydroxyvitamin D deficiency and developed symptomatic hypercalcaemia with vitamin D replacement. Given their low serum parathormone and parathormone-related peptide levels, low serum 25-hydroxy vitamin D and normal serum 1,25-dihydroxyvitamin D, the systemic 25-hydroxy vitamin D deficiency may not have reflected an increased activity of vitamin D at the local granulomatous tissue level.
Background
It is known that in certain disease states, serum levels of laboratory parameters may not be accurate measures of tissue levels. Examples include serum potassium levels in diabetic ketoacidosis1 and serum magnesium levels in patients with atrial fibrillation.2 Awareness of these scenarios is important in the management of patients when attempting to treat a measured serum laboratory parameter, especially with unexpected outcomes.
Sarcoidosis is a chronic granulomatous disease that presents with hypercalciuria in 30–50% of patients and hypercalcemia in 10–20% of patients.3–5 Although hypervitaminosis D is what is commonly reported, there have been rare reports where vitamin D deficiency is present.4–6 It is unclear if this represents a true vitamin D deficiency or there is a discrepancy between serum vitamin D levels (being low) and cellular vitamin D levels (being high).
We report two cases of sarcoidosis that presented with hypercalcemia due to one proposed mechanism as indicated by similar laboratory values. One patient had sarcoidosis with vitamin D deficiency, who presented with symptomatic hypercalcemia precipitated by vitamin D supplementation. Similarly, the second patient had sarcoidosis with vitamin D deficiency and presented with severe hypercalcemia secondary to over-the-counter calcium and vitamin D supplementation. The clinical evolution of both patients suggests that their biochemical profile of serum vitamin D deficiency may not have reflected local granulomatous tissue excess of vitamin D.
Case presentation
Case 1
A 54-year-old Caucasian man presented to the emergency room (ER) on 29 October 2010 with symptoms of excessive thirst, nausea, fatigue and increased frequency of urination for 2–3 weeks. He denied having fever, diarrhoea, abdominal pain or dysuria. History was significant for long-standing steroid-dependent pulmonary sarcoidosis (since 2002), hypertension and migraine. He did not have a diagnosis of diabetes mellitus or renal disease. Lung biopsy from 2008 confirmed a diagnosis of sarcoidosis with evidence of non-caseating granulomatous inflammation involving his lung parenchyma (figure 1). He was an ex-smoker (6-pack-years of cigarette smoking), who quit smoking 30 years ago and denied recreational drug use. He was a produce manager at a local store by occupation. Notably, the patient began treatment for vitamin D deficiency diagnosed 6 weeks prior to this ER visit (serum 25-hydroxyvitamin D level: 10 ng/mL (normal range 30–100 ng/mL)), with weekly doses of 50 000 units of oral vitamin D. Since sarcoidosis was quiescent for the last 1 year, over the course of 3 months preceding the ER visit, steroids were tapered from 20 to 9 mg prednisone a day. His other medications included oral valsartan 160 mg daily, aspirin 81 mg daily, zolmitriptan 5 mg daily, omeprazole 20 mg daily and alendronate 70 mg once every week.
Figure 1.
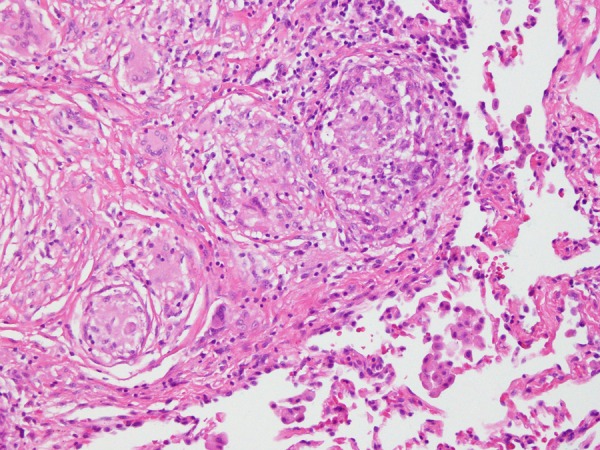
Sarcoid granuloma: lung biopsy showing non-caseating granuloma suggestive of sarcoid granuloma.
In the emergency room, clinical examination revealed an alert and oriented patient with dry mucus membranes, blood pressure 134/82 mm Hg, respiratory rate 15/min, heart rate 100/min, temperature 97.6° F and a room air oxygen saturation 98%. Cardiovascular, respiratory, abdominal and neurological examinations were unremarkable.
Case 2
A 60-year-old man presented to the emergency room on 14 July 2013 with increased urination, increased muscle cramping and uncontrolled hypertension. He denied having dysuria, fever, abdominal pain, nausea, vomiting or diarrhoea. The patient's history was significant for pancytopenia, hypertension, splenomegaly, chronic mild hypercalcemia and chronic kidney disease stage IV. Axillary node biopsy from 3 months earlier revealed a diagnosis of sarcoidosis. He reported 1-pack-year cigarette smoking. He was a construction worker. He was taking caltrate D supplements consisting of 600 mg calcium and 800 IU vitamin D twice daily due to vitamin D deficiency diagnosed 3 months earlier (serum 25 hydroxy vitamin D level: 11 ng/mL). Other medications included oral nebivolol 20 mg daily, loratadine 10 mg daily as needed and over-the-counter multivitamins.
In the emergency room, clinical examination revealed an alert and oriented patient with a blood pressure 174/83 mm Hg, respiratory rate 20/min, heart rate 58/min, temperature 98.9° F and an oxygen saturation of 100% on room air. Abdominal examination revealed hepatosplenomegaly. Cardiovascular, respiratory and neurological examinations were unremarkable.
Investigations
Case 1
Laboratory results (table 1) on the day of admission were significant for hypercalcemia (calcium 11.7 mg/dL), normal 25-hydroxyvitamin D levels (44 ng/mL), normal 1, 25-dihydroxyvitamin D (20 pg/mL), prerenal azotaemia (creatinine 1.6 mg/dL), normal intact parathormone (iPTH) levels (32 ng/mL) and normal parathormone-related peptide (PTHrP) levels (<2 pmol/L). Fractional excretion of sodium was 0.75. Urine analysis did not reveal casts, crystals, sediments or protein. Blood counts and liver functions were within normal limits. Ultrasound evaluation of the kidney revealed no evidence for intrinsic parenchymal disease, calcinosis or hydronephrosis.
Table 1.
Admission laboratory parameters
| Laboratory parameters | Case 1 | Case 2 | Normal range |
|---|---|---|---|
| Haemoglobin (g/dL) | 13.4 | 12 | 14–16 |
| Haematocrit (%) | 39.2 | 35.8 | 42–52 |
| Mean corpuscular volume (fL) | 89 | 85.2 | 80–94 |
| White cell count (cell/mm3) | 8910 | 5280 | 4800–10 800 |
| Blood urea nitrogen (mg/dL) | 25 | 55 | 6–20 |
| Serum creatinine (mg/dL) | 1.6 | 4.4 | 0.6–1.2 |
| Serum bicarbonate (mmol/L) | 34 | 24 | 24–28 |
| Anion gap | 7 | 16 | 7–14 |
| Serum sodium (mmol/L) | 139 | 139 | 136–145 |
| Serum potassium (mmol/L) | 4.2 | 4.0 | 3.5–5.1 |
| Serum calcium (mg/dL) | 11.7 | 13.6 | 8.8–10.2 |
| Serum ionised calcium (mg/dL) | 6.2 | 13.5 | 4.5–5.6 |
| Serum phosphorus (mg/dL) | 2.3 | 5.1 | 2.7–4.5 |
| Alkaline phosphatase (U/L) | 64 | 77 | 40–129 |
| Serum magnesium (mg/dL) | 2.1 | 2.0 | 1.7–2.4 |
| Serum albumin (g/dL) | 4.2 | 4.1 | 3.4–4.8 |
| Serum glucose (mg/dL) | 87 | 87 | 70–105 |
| ACE 1 (U/L) | 61 | 155 | 9–67 |
| 25-hydroxyvitamin D levels (ng/mL) | 44 | 11 | 30–80 |
| iPTH | 32 | <3 | 14–72 pg/mL |
iPTH,intact parathormone.
Case 2
Laboratory values (table 1) on the day of admission included elevated serum calcium (13.6 mg/dL), low serum vitamin D (11 ng/mL), normal 1,25-dihydroxyvitamin D (25 pg/mL), low iPTH (<3 pg/mL) and an elevated serum creatinine (4.4 mg/dL). Parathormone-related peptide (PTHrP) levels were normal (<2 pmol/L). Urine analysis was unremarkable. Haematology report showed mild anaemia and thrombocytopenia. Bone marrow aspiration and lymph node biopsy were negative for malignancy; liver functions were within normal limits. Renal ultrasound indicated moderate-to-severe right ureteral hydronephrosis and a right renal calculus. Chest X-ray showed bilateral hilar lymphadenopathy suggestive of sarcoidosis (figure 2).
Figure 2.
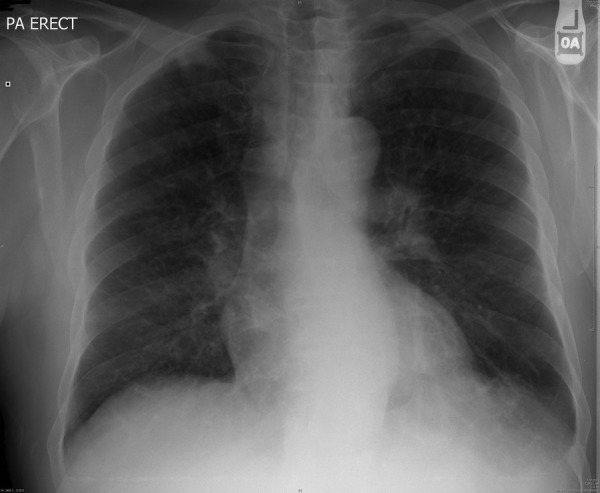
Case 2, X-ray: chest X-ray (posteroanterior) view showing bilateral hilar lymphadenopathy suggestive of sarcoidosis.
Workup for paraproteinemias in both patients was negative and CT imaging of chest, abdomen and pelvis in both patients were negative for malignancy.
Differential diagnosis
Sarcoidosis-associated hypercalcemia.
Hypercalcemia secondary to vitamin D therapy.
Humoral hypercalcemia due to occult malignancy.
Treatment
Case 1
Diagnosis of dehydration induced pre-renal azotemia secondary to hypercalcemia was made. The patient was treated with intravenous fluids; valsartan, magnesium oxide, vitamin D and calcium were withheld. With hydration and conservative measures, within 3 days, symptoms resolved, creatinine decreased to 1.3, calcium to 9.5 mg/dL. The patient was discharged from the hospital. At the time of discharge (2 November 2010), he was continued on prednisone 9 mg daily. However, weekly 50 000 units of vitamin D and valsartan were discontinued.
Case 2
The clinical diagnosis was acute renal failure secondary to obstructive uropathy, hypercalcemia and dehydration superimposed on chronic kidney disease stage IV. Treatment was initiated with intravenous fluids, oral prednisone 60 mg and ureteral stent placement and renal calculus extraction. After 7 days, his creatinine decreased to 3.3 mg/dL and calcium to 10.4 mg/dL and he was discharged from the hospital. Discharge medications included nebivolol and loratidine and oral prednisone 40 mg daily; however, caltrate D was discontinued.
Outcome and follow-up
Case 1
Three weeks from the time of his discharge, in a rheumatology follow-up appointment, the patient was restarted on calcium/vitamin D (600 mg/400 IU one tablet three times a day) by his rheumatologist. At a follow-up pulmonology appointment prednisone was decreased from 9 to 5 mg a day in view of his sarcoidosis being well controlled.
Two months after discharge, at his follow-up primary care visit, though asymptomatic, his calcium had risen to 11.3 mg/dL, creatinine to 1.5 mg/dL with vitamin D levels of 26 ng/mL and iPTH of 5 pg/mL. To treat the recurrence of hypercalcemia and azotemia, vitamin D and magnesium oxide were stopped. Prednisone was continued at 5 mg daily. Resumption of angiotensin receptor blocker (ARB) treatment was deferred.
Further, primary care follow-up after 2 months revealed calcium came down to 9.5 mg/dL, creatinine to 1.3, vitamin D levels of 25 ng/mL and iPTH of 7 pg/mL. Since this time, until August 2013, the patient has had regular follow-up remaining asymptomatic with stable renal function and no clinical evidence of sarcoidosis. He continues to take prednisone 5 mg every day, and has never been restarted on calcium, vitamin D, magnesium oxide or ARB therapy. His calcium has been stable at 9–9.8 mg/dL, creatinine 0.9–1.1 mg/dL, vitamin D 20–38 ng/mL and iPTH 5–13 pg/mL.
Case 2
Within 2 months after the time of his discharge, calcium decreased to 9.2 mg/dL and creatine 1.8 mg/dL and the patient continues to remain clinically stable on oral prednisone 10 mg every other day. In the following 2 months up to November 2013 (4 total months since discharge), calcium levels have been stable from 9.1 to 9.3 mg/dL and creatinine 1.6–1.7 mg/dL and the patient no longer takes any calcium or vitamin D supplements. Renal calculus was found to be of calcium phosphate composition. Repeat renal ultrasound 3 months after discharge indicated resolution of hydronephrosis.
Discussion
The first patient, with a background of pulmonary sarcoidosis, presented with low-to-normal serum vitamin D levels, iPTH levels and PTHrP levels and developed hypercalcemia on vitamin D replacement therapy complicated by acute hypercalcemia-induced pre-renal azotaemia. The second patient had a history of pulmonary sarcoidosis and presented with low vitamin D and iPTH levels. He developed hypercalcemia on caltrate D supplementation complicated by nephrolithiasis and acute kidney injury.
Hypercalcemia in sarcoidosis is known to be due to three mechanistic reasons: (1) systemic conversion of 25- hydroxyvitamin D to 1, 25-dihydroxyvitamin D by the enzyme 1-α hydroxylase produced by activated monocyte/macrophage system that orchestrates sarcoidosis resulting in elevated serum levels of vitamin D and the consequent increase in gastrointestinal absorption of calcium.7 (2) Production of PTHrP by the sarcoid granuloma which causes hypercalcemia via increased renal absorption of calcium and increased bone resorption.5 6 This mechanism is similar to humoral hypercalcemia of malignancy.8 9 In addition to hypercalcemia and normal vitamin D levels, iPTH will be suppressed due to negative feedback from hypercalcemia. (3) Tissue-level conversion of 25-hydroxyvitamin D to 1,25-dihydroxy-vitamin D by 1-α hydroxylase produced by local monocyte/macrophage system in the sarcoid granuloma.7 In this mechanism, in addition to hypercalcemia, normal to suppressed iPTH and PTHrP levels, systemic 25-hydroxy-vitamin D levels may be normal or low as molecular studies have shown that, for unclear reasons, increased vitamin D activity in this mechanism stays at the local tissue levels and does not spill over to measurable systemic levels to cause systemic hypervitaminosis D.7
In both patients, mechanism 1 is unlikely as vitamin D was either low or normal and never presented with hypervitaminosis D. Mechanism 2 can explain some of the presentation as both patients had hypercalcemia and suppressed iPTH levels. However, in mechanism 2, we would expect normal vitamin D and high PTHrP levels. Both patients had normal PTHrP levels.
For both patients, mechanism 3 seems most plausible. Molecular studies have established the possibility that local tissue vitamin D levels can indeed be high when the systemic vitamin D levels are low7 in patients with sarcoidosis. We do not have molecular measures of local tissue vitamin D levels obtained from either patient's sarcoid granulomas to establish this mechanism as the cause of our patients’ hypercalcemia. However, the first patient redeveloped hypercalcemia when his rheumatologist restarted him on calcium with vitamin D, which again resolved after complete cessation of this supplement. Similarly, the second patient developed a hypercalcemia on administration of calcium and vitamin D (caltrate D). The recurrence of hypercalcemia with calcium and vitamin D initiation and resolution with cessation in case 1 and the hypercalcemia seen with caltrate D supplementation in case 2 adds validity to our hypothesis that though serum vitamin D levels were always low in the patients, there seems to be excess vitamin D activity at the cellular level. Also, normal PTHrP levels and normal serum 1,25-dihydroxyvitamin D levels in both patients add validity to this hypothesis.
Unsal et al4 report a patient with hypercalcemia and normal vitamin D levels as a presenting feature of undiagnosed limited renal sarcoidosis. The hypercalcemia persisted even after effectively treating sarcoidosis.4 Although the mechanism behind hypercalcemia was unclear in their case, they hypothesise a mechanism similar to mechanism 3. In contrast, our patients had well controlled, asymptomatic, pulmonary sarcoidosis and mechanism 3 seems most plausible in our patients too.
Hypercalcemia in case 1 coincided with steroid taper. Steroids inhibit gut absorption of calcium.10 So, when steroids were tapered, gut absorption of calcium possibly increased resulting in hypercalcemia. However, since December of 2010 until August of 2013, he has been on the same dose of prednisone (5 mg) that he took during the hypercalcemia episodes but there has been no recurrence of hypercalcemia. Case 2 did not have an increase in calcium levels upon taper of his steroids but his calcium levels also remain stable on prednisone 10 mg every other day.
In conclusion, we report two cases in support of one mechanism of hypercalcemia in sarcoidosis showing the possibility of having high tissue vitamin D activity even when the serum vitamin D levels are low. Therefore, caution needs to be exercised while managing low vitamin D levels in patients with sarcoidosis. However, absence of direct tissue vitamin D and PTHrP levels in our patients needs to be considered while making inferences. Further studies involving larger samples and direct tissue-level measures are needed before conclusions can be drawn with regard to our hypothesis.
Learning points.
In patients with sarcoidosis:
High tissue vitamin D activity in the presence of low serum vitamin D levels is possible.
Caution should be exercised while managing low vitamin D levels.
Steroid withdrawal may be associated with hypercalcemia.
Footnotes
Contributors: JLB, GPSS, HY and LT-H contributed to design of the study, data collection and interpretation, writing of the manuscript and editing it for intellectual contents, and decision to submit the case report for publication.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Aronson PS, Giebisch G. Effects of pH on potassium: new explanations for old observations. J Am Soc Nephrol 2011;22:1981–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dabrowski W, Rzecki Z, Sztanke M, et al. The efficiency of magnesium supplementation in patients undergoing cardiopulmonary bypass: changes in serum magnesium concentrations and atrial fibrillation episodes. Magnes Res 2008;21:205–17 [PubMed] [Google Scholar]
- 3.Zeimer HJ, Greenaway TM, Slavin J, et al. Parathyroid-hormone-related protein in sarcoidosis. Am J Pathol 1998;152:17. [PMC free article] [PubMed] [Google Scholar]
- 4.Unsal A, Basturk T, Koc Y, et al. Renal sarcoidosis with normal serum vitamin D and refractory hypercalcemia. Int Urol Nephrol 2012;25:1779–83. [DOI] [PubMed] [Google Scholar]
- 5.Krikorian A, Shah S, Wasman J. Parathyroid hormone-related protein: an unusual mechanism for hypercalcemia in sarcoidosis. Endocr Pract 2011;17:e84–6 [DOI] [PubMed] [Google Scholar]
- 6.Winnacker JL, Becker KL, Katz S. Endocrine aspects of sarcoidosis. N Engl J Med 1968;278:427. [DOI] [PubMed] [Google Scholar]
- 7.Adams JS, Sharma OP, Gacad MA, et al. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J Clin Invest 1983;72:1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schweitzer DH, Hamdy NA, Frölich M, et al. Malignancy-associated hypercalcaemia: resolution of controversies over vitamin D metabolism by a pathophysiological approach to the syndrome. Clin Endocrinol (Oxf) 1994;41:251. [DOI] [PubMed] [Google Scholar]
- 9.Roskams T, Desmet V. Parathyroid hormone-related peptides: a new class of multifunctional proteins. Am J Pathol 1997;150:779–85 [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman PA, Gesek FA. Calcium transport in renal epithelial cells. Am J Physiol 1993;264Pt 2):F181. [DOI] [PubMed] [Google Scholar]


