Abstract
One of the lung ultrasound (LUS) signs which can be used to exclude the presence of pneumothorax is the B-line artefact. The presence of a B-line artefact indicates that the two pleural blades are in contact. Most of the research describing LUS for the diagnosis of pneumothorax is limited to pneumothorax without concomitant pleural effusion. This case report presents how a B-line was visualised using LUS in a patient with hydropneumothorax and, in addition, a simple model is used to provide a possible explanation on this phenomenon.
Background
The diagnostic accuracy of lung ultrasound (LUS) for the diagnosis and exclusion of pneumothorax has been documented in several studies.1 2 One of the sonographic signs which can be used to exclude the presence of pneumothorax is the B-line artefact.2 The B-line artefact is seen as a hyperechoic, laser-like, vertical line originating from the pleura and extending to the bottom of the field of view. If lung sliding is present, B-line(s) move in synchrony with breathing.2 The B-line artefact is believed to emerge from the lung tissue located just beneath the visceral pleura. As the ultrasound waves penetrate into the lung tissue with an increased density, it causes resonance which appears as a B-line on the screen.3 4 Since a pneumothorax would prevent the visualisation of the visceral pleura and therefore any B-lines, the presence of a B-line indicates that the two pleural blades are in contact and a pneumothorax can be ruled out.5 Other sonographic signs commonly used to rule out pneumothorax are presence of lung sliding, lung pulse using B-mode and/or sea-shore sign using M-mode.2
In this paper we present a case of hydropneumothorax in which a B-line was visualised using LUS and a simple model to explain how a B-line may appear in hydropneumothorax.
Case presentation
A 19-year-old previously healthy man was admitted to hospital because of dyspnoea, productive cough, fever, right-sided chest pain, and a 10 kg unintended weight loss. No haemoptysis or pedal oedema was noted. The patient had immigrated from Afghanistan to Denmark at the age of 6 years. Seven months prior to admission the patient had been on a 10-day study trip to Uganda. Blood tests showed leukocytosis (16.5×109/L) and elevated C reactive protein (121 mg/L). Other biochemical analyses including electrolytes and tests of liver and renal function were normal. The patient did not use illicit drugs or prescription medicine and had no alcohol abuse.
Investigations
Initial chest X-ray (CXR) revealed a right-sided hydropneumothorax and opacity of the lower lobe (figure 1).
Figure 1.
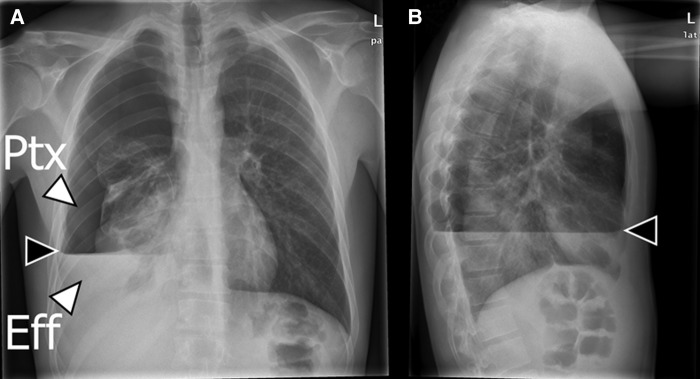
Chest X-ray at admission. (A) Posteroanterior view showing right-sided pneumothorax (Ptx) and effusion (Eff). The interface between the pneumothorax and effusion is marked with a black arrow. (B) Lateral view, the pneumothorax effusion interface is marked with a black arrow.
CT of the chest showed consolidation of the entire right lower lobe, an apico-anterior right-sided pleural effusion and a minor pneumothorax (figure 2).
Figure 2.
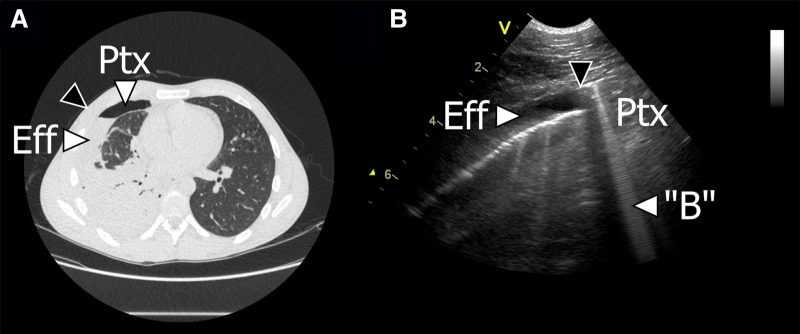
CT and lung ultrasound (LUS) findings. (A) CT of the chest. A small pneumothorax (Ptx) and a pleural effusion (Eff) can be visualised on the right side. The approximate position of the ultrasound transducer, corresponding to image B is marked with a black arrow. (B) LUS findings. A pleural effusion (Eff) is present. Corresponding to the area of the pneumothorax (Ptx), a hyperechoic line representing the parietal pleura can be seen. The pneumothorax effusion interface is marked with a black arrow. A hyperechoic, laser-like, vertical artefact originating from the parietal pleura and extending to the bottom of the field of view is present (B).
LUS of the anterior surface of the right lung revealed that the pleura line was visible, but lung sliding was absent. As the transducer was moved laterally, a minor pleural effusion without septations was seen (figure 2). Owing to motions of breathing, the interface between the pneumothorax and pleural effusion was moving. In B-mode this gave a sense of lung sliding in the area of the pneumothorax, but supplementary M-mode demonstrated the stratosphere sign, which is suggestive of pneumothorax. When further assessing different areas of this interface, a B-line suddenly appeared (figure 2). In the area around the B-line, M-mode still showed stratosphere sign. When scanning the lateral and posterior surface of the right lung, the presence of a total consolidated lower lobe of the right lung was also confirmed.
Diagnostic thoracocenthesis resulted in cloudy, but non-purulent pleural fluid.
Sputum microscopy revealed acid-fast bacilli and a subsequent sputum and pleural fluid culture showed Mycobacterium tuberculosis.
Differential diagnosis
Initially, pneumonia with complicated parapneumonic pleural effusion or bacterial empyema was suspected. Later, when results from sputum microscopy and pleural fluid analysis were reported, the diagnosis was altered to pulmonary tuberculosis with pleural involvement. Owing to the age of the patient, the diagnosis of lung cancer was not suspected, although this would be highly relevant in other patients.
Treatment
When pleural infection was suspected, treatment with piperacillin, tazobactam and ciprofloxacin was initiated, and a chest tube was inserted into the right pleural cavity. As the results from sputum microscopy became available the treatment was immediately changed to antituberculous agents including isoniazid, rifampin, pyrazinamide and ethambutol.
Outcome and follow-up
The patient’s condition gradually improved and 2 weeks after the primary admission he was discharged from hospital.
Discussion
Using LUS, the presence of one or more B-lines may be used to rule out pneumothorax, but as this case illustrates, this may not necessarily be true in hydropneumothorax. LUS showed a vertical, reverberation artefact in the interface between the pleural effusion and the pneumothorax. The artefact had all the hallmarks of a B-line.2 It was a hyperechoic, laser-like, vertical line originating from the pleura and extending to the bottom of the field of view and in addition, it seemed to be moving with lung sliding. If a CXR or CT scanning of the chest had not been performed, the LUS findings might have excluded the presence of a pneumothorax.
We speculated whether the occurrence of bubbles in the interface between pleural effusion and pneumothorax could cause such a ‘pseudo B-line’ as observed in this case. The movement of the artefact, which looked like lung sliding, could then have been the movement of the air/fluid interface in synchrony with the patient’s breathing cycle. To elucidate this potential theory, we simulated the two scenarios described below.
The hydropneumothorax simulator
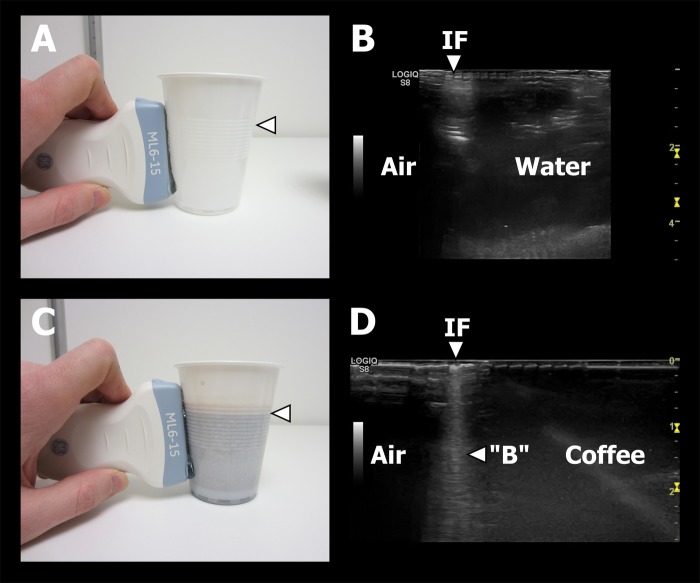
In order to simulate a patient with a hydropneumothorax, a simple hydropneumothorax simulator was devised (figure 3). A standard plastic cup was half-filled with water. The side of the plastic cup on which the transducer was placed was intended to simulate the parietal pleura, the water a pleural effusion, and the air above the water a pneumothorax. The plastic cup was then scanned in the vertical axis. The side of the plastic cup appeared as a bright, hyperechoic, horizontal line in the top of the image. The water appeared as a black, hypoechoic area just below the bright white line representing the wall of the cup. The other side of the cup could be seen below the layer of water. The air also appeared as a black hypoechoic area below the wall of the cup, but the other side of the cup could not be visualised. As expected, no B-line in the interface appeared on the screen.
Figure 3.
Hydropneumothorax simulator. (A) Linear transducer placed on the side of a plastic cup containing water and scanning air (pneumothorax), water (effusion) and the air–water interface (marked with arrow). (B) Picture A's corresponding ultrasound image. The air–water interface (arrow) is seen without the presence of vertical reverberation artefacts. (C) Linear transducer placed on the side of a plastic cup containing coffee when scanning air (pneumothorax), coffee (effusion) and the air–coffee interface (marked with arrow). (D) Picture C's corresponding ultrasound image. The air–coffee interface (arrow) is seen with the presence of a vertical reverberation artefact (‘B’), which has the sonographic hallmarks of a B-line.
The hydropneumothorax with reverberation artefact simulator
In order to simulate a patient with a hydropneumothorax and a vertical reverberation artefact, the hydropneumothorax simulator used above was altered (figure 3). The same type of a standard plastic cup was placed in the coffee machine in the emergency department. It was filled with black coffee, which induced bubbles in the air–coffee interface (figure 4). The transducer-side of the plastic cup once again had to simulate the parietal pleura, the coffee a pleural effusion and the air above the water a pneumothorax. The bubbles placed in the coffee/air interface were to simulate bubbles located on the interface between a pleural effusion and a pneumothorax. The plastic cup was once again scanned in the vertical axis. The ultrasound findings were the same as described above, but in the coffee–air interface a vertical, hyperechoic reverberation artefact, mimicking a B-line, was present. This finding was congruent with the B-lines appearing on LUS in the patient case.
Figure 4.

Cup of coffee from the coffee machine in the emergency department. Several air/coffee bubbles are present in the air–coffee interface (arrow).
Cases with the concurrent presence of B-line(s) and pneumothorax have not previously been published. Most of the research describing LUS for the diagnosis of pneumothorax is limited to pneumothorax without the coexisting presence of a pleural effusion.1 2 Our case demonstrates two potential pitfalls when using LUS for the assessment of pneumothorax in patients with concomitant pleural effusion which, from a clinical setting, are important to be aware of:
Vertical reverberation artefacts mimicking B-lines can be caused by bubbles in the air/pleural effusion interface. Hence sole vertical reverberation artefacts, originating from the parietal pleura and located very close to the surface of a pleural effusion, are not necessarily a ‘true’ B-line.
In hydropneumothorax, the air/pleural effusion interface can move in synchrony with the patient’s breathing cycle. This movement may falsely be interpreted as lung sliding. Supplementary use of either M-mode or Doppler could be helpful to distinguish movement of the interface from lung sliding.
In conclusion, in patients with pleural effusion one should be cautious when using B-lines for ruling out the presence of concomitant pneumothorax. Furthermore, supplementary imaging such as chest CT is advocated when LUS findings are unclear in a patient with possible hydropneumothorax.
Learning points.
Bubbles placed in the air/effusion interface at the parietal pleura may cause a vertical reverberation artefact mimicking a B-line.
In hydropneumothorax, a vertical reverberation artefact similar to a B-line located at the air/effusion interface cannot rule out the presence of pneumothorax.
Movement of the air/pleural effusion interface during the patient's breathing cycle may mimic lung sliding.
Supplementary imaging of the chest CT is advocated when lung ultrasound findings are unclear in a patient with possible hydropneumothorax.
Footnotes
Contributors: All authors contributed to the conception, design, acquisition and interpretation of data; drafting and revising the article for important intellectual content; and final approval of the version to be published.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Alrajhi K, Woo MY, Vaillancourt C. Test characteristics of ultrasonography for the detection of pneumothorax: a systematic review and meta-analysis. Chest 2012;141:703–8 [DOI] [PubMed] [Google Scholar]
- 2.Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 2012;38:577–91 [DOI] [PubMed] [Google Scholar]
- 3.Soldati G, Inchingolo R, Smargiassi A, et al. Ex vivo lung sonography: morphologic-ultrasound relationship. Ultrasound Med Biol 2012;38:1169–79 [DOI] [PubMed] [Google Scholar]
- 4.Via G, Lichtenstein D, Mojoli F, et al. Whole lung lavage: a unique model for ultrasound assessment of lung aeration changes. Intensive Care Med 2010;36:999–1007 [DOI] [PubMed] [Google Scholar]
- 5.Lichtenstein D, Mezière G, Biderman P, et al. The comet-tail artifact: an ultrasound sign ruling out pneumothorax. Intensive Care Med 1999;25:383–8 [DOI] [PubMed] [Google Scholar]