Abstract
A 7-year-old Indian girl presented with symptoms of excessive development of breasts, early menarche, growth of pubic hairs, accelerated growth and abdominal distension. On clinical examination, a large right abdominopelvic mass was palpable. MRI revealed a large, heterogeneous, solid and cystic tumour in the right adnexal region, suggestive of an ovarian neoplasm. The hormonal profile showed markedly elevated oestradiol and low follicle-stimulating hormone levels. Clinical diagnosis of precocious puberty with right ovarian mass was concluded. Right-sided salpingo-oophorectomy was performed. Histopathology showed features consistent with sclerosing stromal tumour of the ovary. Postoperatively, signs and symptoms of precocity gradually regressed and her serum oestradiol level came down to normal. This is the first reported case from India.
Background
Sclerosing stromal tumour (SST) is a rare benign sex cord stromal neoplasm of the ovary which usually occurs in the second and third decades of life.1 Patients with this tumour often present with symptoms of pelvic mass, pain, abdominal distention and menstrual irregularities. Anovulation, virilisation and infertility may be present in these patients because SST is associated occasionally with oestrogen and rarely with androgen secretion.2 Precocious puberty resulting from this tumour is extremely rare.3 4
Case presentation
A 7-year-old Indian girl was presented by her mother to the gynaecology outpatient department with symptoms of excessive development of her breasts over the past 1 year. She had attained menarche 8 months previously. Her mother noticed growth of pubic hairs, accelerated body growth and abdominal distension for the past 4 months. Family history, antenatal, perinatal and developmental history was unremarkable. History of local trauma, sexual abuse, drug intake and any other systemic illness was absent. Her height was 127 cm and weight was 27 kg. She was Tanner stage IV for breast development and Tanner stage III for pubic hair. There were no signs of virilisation such as facial hair, deepening of voice, enlargement of clitoris, adrenocortical and central nervous system dysfunction, hypothyroidism, hyperparathyroidism, Cushing's disease or any endocrinopathy.
On clinical examination, a large right-sided abdominopelvic mass was palpable. The mass was firm in consistency and immobile, measuring about 10×8×4 cm with well-demarcated margins. Pelvic MRI revealed a large, well-defined, heterogeneous solid and cystic mass measuring 10×9×5 cm in the right adnexal region (figure 1A). The right ovary was not separately definable from the mass. Ultrasonography showed an anteverted uterus measuring 5 cm in length with an anteroposterior diameter of 2 cm and endometrial thickness of 4.5 mm. The opposite left ovary appeared normal with a volume of about 3.5 cc.
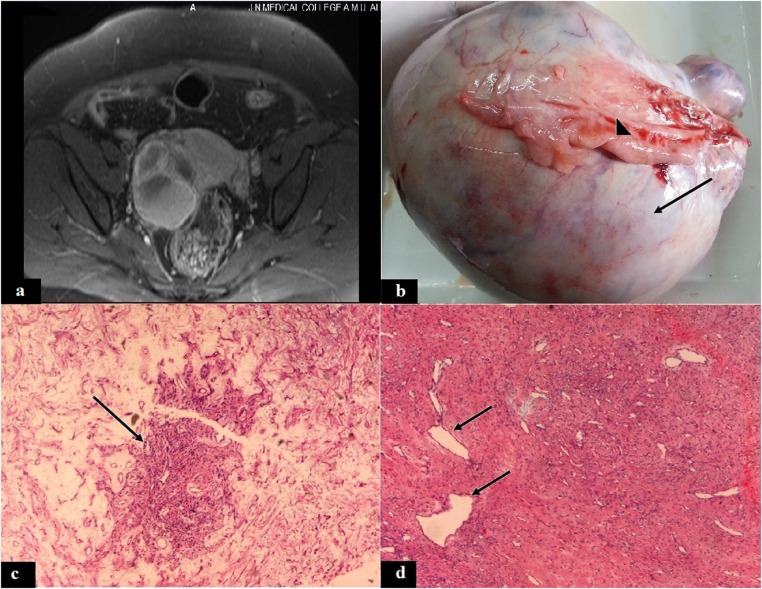
Figure 1.
(A) T2-weighted MRI showing a heterogeneous, solid-cystic tumour in the right adnexal region, (B) ovarian tumour with white surface (arrow) and attached fallopian tube (arrow head), (C) showing a tumour pseudolobule (arrow) with surrounding oedematous stroma (H&E, × 50), (D) pseudolobule with thin-walled hemangiopericytoma-like blood vessels (arrow) and fibrocollagenous stroma (H&E, ×50).
Clinical and MRI findings suggested a diagnosis of ovarian tumour with precocious puberty. Right-sided salpingo-oophorectomy was performed and the specimen was submitted for histopathological diagnosis.
The gross examination of the resected specimen revealed an encapsulated ovarian mass without any capsular breach, measuring 16×12.5×10 cm and weighing 470 g with the fallopian tube measuring 7×3 cm on the tumour surface (figure 1B). The outer surface was white and smooth. The cut surface showed grey–white solid areas with occasionally yellowish foci, rubbery consistency and multiple small cystic spaces without any haemorrhage or necrosis.
H&E-stained sections showed a tumour comprising spindle and round vacuolated clear cells with microcapillary vessels forming pseudolobules separated by hypocellular bands of oedematous fibrocollagenous tissue (figure 1C). Some of the thin-walled vessels showed a hemangiopericytoma-like pattern (figure 1D). Mitosis and necrosis were not appreciable. Tumour was periodic acid Schiff and mucicarmine stains negative, which was indicative of glycol-mucin negativity of the clear cells. The fallopian tube showed normal morphology.
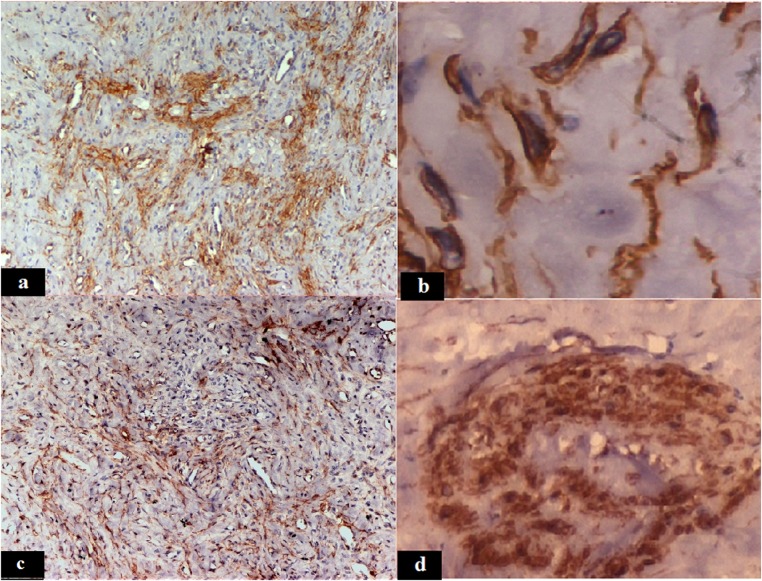
Silver impregnation highlighted the vacuolated cells and blood vessels in the tumour pseudolobules. The Van Gieson stain showed collagen producing spindle cells. The smooth muscle actin immunostain demonstrated the presence of smooth-muscle spindle cells, diffusely distributed in the tumour section with focal accentuation in the vascular areas, suggestive of proliferation of smooth muscle cells in vessel walls (figure 2A, B). Vimentin stain showed a similar pattern of positivity in the tumour section (figure 2C). The cytokeratin marker was negative. A monoclonal anti-α inhibin antibody was used to demonstrate the inhibin expression. The inhibin was positive, expressed as cytoplasmic granular positivity in the vacuolated tumour cells (figure 2D), suggestive of a sex cord stromal origin of the tumour.
Figure 2.
Spindle tumour cells showing smooth muscle actin positivity (A, ×50; B, ×1000) and vimentin positivity (C, ×50), the vacuolated tumour cells showing cytoplasmic positivity for inhibin (D, ×500).
Investigations
Routine haematological investigations and liver and renal function tests were found to be within normal limits. Hormonal profile revealed a markedly elevated serum oestradiol level (440 pg/mL, reference value for prepubertal females <10 pg/mL). Her follicle-stimulating hormone (FSH) level was suppressed (<1 IU/mL). The patient's β-human chorionic gonadotropin (β-hCG; 2.5 mIU/mL), serum carcinoembryonic antigen (1.5 ng/mL), α-fetoprotein (3.5 ng/mL), thyroid stimulating hormone (0.4 mIU/L) and total thyroxine (T4) level (8 μg/dL) were normal for her age.
Differential diagnosis
The differential diagnoses in this case include other oestrogen secreting ovarian neoplasia in children such as the sex cord stromal tumours as juvenile granulosa cell tumour (JGCT), massive ovarian oedema (MOO) and germ cell tumours. Precocious puberty with a fibrocollagenous angiomatous lutein cell ovarian tumour with hyperoestrogenism, in the present case, was diagnostic of ovarian SST, distinguishable from other tumours by its distinctive histological and immunohistochemical characteristics.
Outcome and follow-up
Postoperative recovery of the patient was uneventful. Her serum oestradiol came down to the level of <10 pg/mL within a month. Her menses stopped and signs and symptoms of precocious puberty gradually regressed and disappeared. The patient was under monthly follow-up during the past 6 months and did not show any signs of recurrence but showed normal serum oestradiol and FSH and 24 h urinary oestrogenic hormonal levels. She will be followed up with pelvic ultrasonography, MRI and hormone levels for any recurrence of the tumour every three months for 1 year and then six monthly for the next 5 years. A gonadotropin-releasing hormone (GnRH) stimulation test will be planned in future for distinguishing initiation of normal physiological pubertal hormonal changes from any recurrence of precocious pseudopuberty.
Discussion
Findings in the present case were diagnostic of functional SST of the ovary with precocious puberty. The incidence of this tumour in prepubertal age causing precocious puberty was rarely reported in the earlier literature.3 Presently, SST with hyperoestrogenism occurred with precocious puberty in a 7-year-old Indian girl, not reported earlier by the Indian patients. In only one Chinese girl, SST with signet ring cell tumour characteristics was reported at an early age of 4 years.4
Peripheral or gonadotropin-independent precocious puberty constituted less than 20% of the total precocity cases.5 An elevated level of oestradiol and a suppressed level of FSH, in the present case, suggested that precocity was not central and was GnRH independent, that is, not from hypothalamic-pituitary-ovarian axis dysfunction, but due to the peripheral high-oestrogen production by SST.
Another ovarian tumour known to cause precocious puberty is JGCT which accounts for approximately 10% cases of isosexual precocity in the girls.6 JGCT is a partly solid and cystic tumour similar to SST, causing diagnostic confusion, but histopathologically, it lacks the typical pseudolobular pattern of SST and instead has sheets or nodular aggregates of neoplastic granulosa cells.
MOO causes a marked enlargement of the ovary and may present with signs and symptoms of hormone secretion such as hirsutism and precocious puberty.7 However, the preserved ovarian tissue within the oedematous stroma and absence of heterogeneity distinguish it from the SST.
Germ cell tumours of the ovary may manifest with excessive hCG production with resultant luteinisation of stroma, which may produce steroid hormones resulting in hyperoestrogenism.8 Non-gestational choriocarcinomas are vascular solid tumours, commonly occurring in children and young adults, and may manifest with isosexual precocity (approximately 50% of cases)8; but these tumours present with elevated levels of total and β-hCG which were absent in the present case of SST.
Pure dysgerminomas do not secrete hormones; however, 5% of the tumours may contain syncytiotrophoblasts which produce β-hCG, resulting in oestrogenic manifestations.8 Similarly, mature teratomas occur in young females and constitute about 50% of ovarian tumours in children. Virilisation and hyperoestrogenism due to mature cystic teratomas have been reported, but predominantly in postmenopausal women.9
Unilateral salpingo-oophorectomy for removal of SST ovary, as was performed in the present case, is considered as a fertility-sparing surgery.10 Although the chances of conception are reduced, the patient will be expected to remain fertile because of the normal functions of the opposite ovary and salpinx and will be followed up till normal physiological, hormonal and pubertal changes such as starting of menses will take over.
Learning points.
Sclerosing stromal tumour (SST) is a rare sex cord stromal neoplasm of the ovary with distinct histological and immunohistochemical features.
Rarely, SST may present with precocious puberty.
The diagnosis of SST of the ovary should be concluded by extensive histopathological, hormonal and immunohistochemical examinations to distinguish it from the other ovarian tumours.
Acknowledgments
All the authors wish to thank Dr Anjum Naim for his contribution in the presentation of the figures.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Gwin K, Mariño-Enríquez A, Martel M, et al. Sclerosing stromal tumor: an important differential diagnosis of ovarian neoplasms in childhood and adolescence. Pediatr Dev Pathol 2009;12:366–70 [DOI] [PubMed] [Google Scholar]
- 2.Tanaka YO, Tsunoda H, Kitagawa Y, et al. Functioning ovarian tumors: direct and indirect findings at MR imaging. Radiographics 2004;24:147–66 [DOI] [PubMed] [Google Scholar]
- 3.Chang YW, Hong SS, Jeen YM, et al. Bilateral sclerosing stromal tumor of the ovary in a premenarchal girl. Pediatr Radiol 2009;39:731–4 [DOI] [PubMed] [Google Scholar]
- 4.He Y, Yang KX, Jiang W,et al. Sclerosing stromal tumor of the ovary in a 4-year-old girl with characteristics of an ovarian signet-ring stromal tumor. Pathol Res Pract 2010;206:338–41 [DOI] [PubMed] [Google Scholar]
- 5.Lee CT, Tung YC, Tsai WY. Etiology and clinical features of isosexual precocious puberty in Taiwanese girls: twenty-three years’ experience in National Taiwan University Hospital. J Pediatr Endocrinol Metab 2009;22:947–53 [DOI] [PubMed] [Google Scholar]
- 6.Saeed GA, Farooq N. Precocious pseudopuberty due to juvenile granulosa cell tumor. J Coll Physicians Surg Pak 2003;13:287–8 [PubMed] [Google Scholar]
- 7.Cepni I, Ocal P, Erkan S, et al. Massive edema of the ovary diagnosed with laparoscopic biopsy and frozen section. J Postgrad Med 2005;51:336–7 [PubMed] [Google Scholar]
- 8.Shanbhogue AK, Shanbhogue DK, Prasad SR, et al. Clinical syndromes associated with ovarian neoplasms: a comprehensive review. Radiographics 2010;30:903–19 [DOI] [PubMed] [Google Scholar]
- 9.Hoffman JG, Strickland JL, Yin J. Virilizing ovarian dermoid cyst with leydig cells. J Pediatr Adolesc Gynecol 2009;22:39–40 [DOI] [PubMed] [Google Scholar]
- 10.Tsai HW, Ko CC, Yeh CC, et al. Unilateral salpingo-oophorectomy as fertility-sparing surgery for borderline ovarian tumors. J Chin Med Assoc 2011;74:241–2 [DOI] [PubMed] [Google Scholar]