Abstract
Human exposure to polybrominated diphenyl ether (PBDE) can occur via ingestion of indoor dust, inhalation of PBDE-contaminated air and dust-bound PBDEs. However, few studies have examined the pulmonary toxicity of particle-bound PBDEs, mainly due to the lack of an appropriate particle-cell exposure system. In this study we developed an in vitro exposure system capable of generating particle-bound PBDEs mimicking dusts containing PBDE congeners (BDEs 35, 47, 99) and delivering them directly onto lung cells grown at an air-liquid interface (ALI). The silica particles and particle-coated with PBDEs ranged in diameter from 4.3 to 4.5 μm and were delivered to cells with no apparent aggregation. This experimental set up demonstrated high reproducibility and sensitivity for dosing control and distribution of particles. ALI exposure of cells to PBDE-bound particles significantly decreased cell viability and induced reactive oxygen species generation in A549 and NCI-H358 cells. In male Sprague-Dawley rats exposed via intratracheal insufflation (0.6 mg/rat), particle-bound PBDE exposures induced inflammatory responses with increased recruitment of neutrophils to the lungs compared to sham-exposed rats. The present study clearly indicates the potential of our exposure system for studying the toxicity of particle-bound compounds.
Keywords: Particle-bound PBDEs, Lung cells, Air-liquid interface, In vitro toxicity testing, Cytotoxicity
1. Introduction
Polybrominated diphenyl ethers (PBDEs) are commonly used as flame retardants that are added to a wide variety of consumer products, such as upholstered furniture, carpet, building materials, toys and electronic goods (Allen et al. 2008; Birnbaum and Staskal 2004; Harrad et al. 2006). Since 2004, the penta- and octa-brominated diphenyl ether mixtures, two of the three main commercial formulations, have been banned in the European Union (EU) and voluntarily phased out of the USA market because of concerns over the persistence and toxicity of PBDEs. Deca-BDE, the third main technical formulation, is currently being phased out in the EU and its production, importation and use in the USA will cease by the end of 2013 (EPA 2010). Despite efforts to ban commercial PBDE mixtures, PBDEs will remain in the environment and in biological matrices because of their persistence and ability to bioaccumulate. Thus, human exposure to PBDEs will likely continue for decades similar to polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs) even if their production and use are discontinued (Watkins et al. 2011).
PBDEs are persistent, bio-accumulative and have some structural similarities to PCBs and PBBs that can disrupt the immune, reproductive, nervous and endocrine systems in animals (EPA 2010; Gao et al. 2009; He et al. 2009). PBDEs interfere with the endocrine system (thyroid hormone), (Ren et al. 2013) impair neurobehavioral development (Dingemans et al. 2011; He et al. 2009) and induce DNA damage (Gao et al. 2009; He et al. 2008; Lai et al. 2011) in animals and human cells in vitro. Data show that BDE47 and BDE99 disturb the development of primary fetal human neural progenitor cells in vitro via disruption of cellular thyroid hormone signaling (Timm Schreiber 2010). Co-exposure to BDE47 (1-2.5 μM) and BDE99 (5-30 μM), in particular at low doses, induced synergistic oxidative stress-mediated neurotoxicity in human neuroblastoma cells (SK-N-MC cell lines) (Tagliaferri et al. 2010). An in vitro study showed that BDE47 (4 μg/mL) inhibited cell viability, increased lactate dehydrogenase (LDH) leakage, induced reactive oxygen species (ROS), DNA damage and cell apoptosis in human neuroblastoma (SH-SY5Y) cells (He et al. 2008).
PBDEs are not permanently bound to the products and can be released from the products into the environment as dust (particle-bound) or as vapor (de Wit 2002). Therefore, PBDEs have been commonly detected in indoor air, house dust and human tissues such as serum and breast milk (Allen et al. 2006; Batterman et al. 2009; Schecter et al. 2003; Vorkamp et al. 2011). Human exposure pathways to PBDEs remain unclear, even though the indoor environment is an important source of exposure to PBDEs used in household products (Allen et al. 2008; Harrad et al. 2006; Vorkamp et al. 2011). The main routes of human exposure to PBDEs appear to occur via food consumption, ingestion of dust and inhalation of PBDE-contaminated air and particle-bound PBDEs, principally in indoor exposure scenarios (Harrad et al. 2006; Huwe et al. 2008; Vorkamp et al. 2011; Wilford et al. 2008). PBDEs were found at high concentrations in house dust (BDE47 and BDE99 were 16.9 and 13.6 ng/g, respectively) and residential indoor air (BDE47 and BDE99 were 134 and 63.7 pg/m3, respectively) (Vorkamp et al. 2011). It has been widely accepted that indoor air and dust concentrations were higher in North America than in continental Europe (Frederiksen et al. 2009). BDE47 and BDE99 were the dominant congeners in indoor air and dust collected from USA urban residences as well as in human tissues (Allen et al. 2006; Batterman et al. 2009; EPA 2010). Interestingly, strongly elevated blood levels of PDBE among aircraft crew and passengers were associated with inhalation exposures (Christiansson et al. 2008). Inhaled PBDEs in dust and corn oil were readily bioavailable and are biologically active in male rats, as indicated by increased transcription of hepatic enzymes. PBDEs and structurally similar semi volatile organic contaminants, such as PCBs and PAHs, are more enriched in the fine indoor particles than coarse particles. Chemicals bound to smaller particles are more bioavailable and have a longer pulmonary residence time (Hwang et al. 2008; Meeker et al. 2009; Paustenbach et al. 1997; Shoeib et al. 2012). These observations support the significance of dust in exposure to particle-bound contaminants.
Few studies have examined pulmonary toxicity of particle-bound PBDEs using in vitro models mainly due to the lack of an appropriate particle-cell exposure system. In some experimental designs, particles are directly added to the cell culture medium for the assessment of particle toxicity. However, these approaches have limitations, including poor reproducibility, changes of particle size due to the aggregation, interactions of particles with components of the medium (e.g., albumin), and dissolution of particles by the medium (Fatisson et al. 2012; Savi et al. 2008). These limitations may account for poor correlation between toxicity of particle-types tested by in vivo insufflation versus in vitro cell culture exposures (Sayes et al. 2007). Differences in cell types, media compositions, exposure concentrations, and particle delivery techniques make comparisons between in vitro toxicity studies difficult.
Inhaled particles first interact with pulmonary surfactants, which are produced by epithelial type II cells and cover the alveolar region to prevent alveolar collapse among other functions. Coating with surfactant may alter surface characteristics and subsequent toxicity of the inhaled particle. The most commonly used in vitro model for assessing pulmonary toxicity of inhaled particles, the liquid cell culture method does not replicate the in vivo conditions of lung cells. In contrast, air-liquid interface (ALI) models more closely reflect in vivo conditions by providing an air-facing surface of epithelial cells with a thin layer of airway surface liquid at the air interface (Jayaraman et al. 2001). Moreover, epithelial cells grown at the ALI have well-differentiated structures and functions compared to cells grown immersed in the medium (Kameyama et al. 2003). These features account for the increase in the use of ALI models for the in vitro toxicity study of particles (Bitterle et al. 2006; Kim et al. 2013; Lenz et al. 2009; Savi et al. 2008; Stringer et al. 1996; Tippe et al. 2002).
The goal of this research was to develop a realistic inhalation exposure model for airborne particle-bound PBDEs. We first coupled an ALI cell exposure system with a particle aerosolizer and exposure chambers allowing the airborne particles to directly interact with the lung cells cultured on commercially available semipermeable membranes. A preliminary study was carried out to validate the reproducibility of the distribution and dose of particles and the impact on biological endpoints, cytotoxicity and ROS using two types of human lung cell lines (A549 and NCI-H358) as a precursor to studies of particle-bound PBDE toxicity. We also evaluated pulmonary responses of the same PBDE-bound particles delivered to rats via intratracheal insufflation to compare the results from in vitro studies to in vivo toxicity.
2. Material and methods
2.1. Materials
Three PBDE congeners—3,3',4-tribromodiphenyl ether (BDE35), 2,2',4,4'-tetrabromodiphenyl ether (BDE47), and 2,2',4,4',5-pentabromodiphenyl ether (BDE99)—were chosen as study compounds on the basis of their prevalence, origin and toxicity (Klo□sener et al. 2008). BDEs 35, 47 and 99 were synthesized by nucleophilic aromatic substitution of the diphenyliodonium salts with bromophenols as described previously (Klo□sener et al. 2008). A spherical silica gel particle (Nucleosil, pore size 100 Å, Macherey-Nagel, Inc., Bethlehem, PA USA) was used as the carrier material.
2.2. Cell culture
Two types of human lung cells (A549 and NCI-H358, American Type Culture Collection, Manassas, VA, USA) were used to evaluate cellular responses of particle-bound PBDEs. A549 cells are transformed human type II alveolar epithelial cells with endocytic properties. Type II cells continuously regenerate the airways epithelium (Stringer et al. 1996). NCI-H358 cells are Clara (club)-type cells that detoxify harmful substances inhaled into the lungs, being one of the few lung cell types in which cytochrome P-450 enzymes are expressed. Cells were cultivated in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS, Atlanta Biologicals, Lawrenceville, GA) and 100 mg/mL penicillin and streptomycin (Invitrogen). The cells were grown in T-cell culture flasks (75 cm2 of growth area) and incubated at 37°C in a humidified atmosphere containing 5% CO2. The culture medium was changed 24 h after subculturing and every 2 days thereafter (up to passage 30). The cells were split 1:10 during each passage.
2.3. Preparation of particle-bound PBDEs
PBDEs are often applied as flame-retarding coatings to a parent backbone material that may range from an inorganic material to an organic polymer (de Wit 2002). PBDEs from these products are then released into the air by volatilization. We therefore generated and characterized coatings of our PBDE congeners on silica particles (Nucleosil) as a model of airborne particle-bound PBDEs to mimic inhalation exposure of mixtures of dust and PBDEs. Spherical silica particles coated with PBDEs were generated as described previously (Klo□sener et al. 2008). Briefly, for the in vitro study, a stock solution of individual PBDE congeners (BDEs 35, 47 or 99) was prepared for each PBDE congener to a concentration of 2 mg/mL diethyl ether. Spherical silica particles (as support materials, 40 mg), stock solution (5 mL) and diethyl ether (5 mL) as a solvent were mixed in a round bottom flask (each PBDE congener represented 20% of the weight of the silica particles). The solvent evaporated under reduced pressure in a rotary evaporator with tumbling at 30 rpm and outside warming to 36°C.
The in vivo study a stock solution was prepared with a total PBDE concentration of 2 mg/mL diethyl ether, containing equal molar amounts of each congener, BDEs 35, 47 and 99. The PBDEs represented 20% of the weight of the silica particles. The silica particles (40 mg), stock solution of PBDE mixture (5 mL) and diethyl ether (5 mL) were mixed together as described above.
2.4. In vitro exposure system for air-delivery of particle-bound PBDEs
An in vitro exposure system was designed for assessing the toxicity of air-delivered particle-bound PBDEs to human lung cells. Figure 1 shows a cross-section representation of our in vitro exposure system. The exposure model consists of two major chambers: (1) a delay chamber to mix particles with air, reduce particle interactions with the chamber (e.g., electrostatics) and thus increase uniform distribution of particles; (2) an exposure chamber holding a semi-permeable cell culture membrane (12 mm of diameter, 1.1 cm2 of growth area, Corning, Inc., NY, USA) to deposit particles to lung cells at an ALI. A dry powder insufflator (model DP-4, Penn-Century, Inc., PA, USA) with an air pump (AP-1, Penn-Century, Inc.) was used to aerosolize particle-bound PBDEs into the delay chamber. Clean air from a laminar flow hood was drawn by an external air pump and passed through the delay chamber where it was combined with the particle aerosol stream and fully mixed, and further into the exposure chamber at 10 mL/min. Cells grown on the transwell membrane were exposed to particle-bound PBDEs via the air at the apical surface in the exposure chamber.
Figure 1.
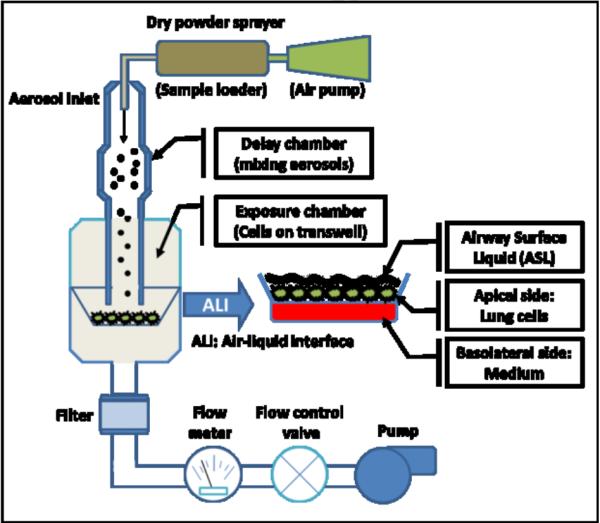
Cross-sectioned representation of the in vitro exposure chamber. The exposure chamber delivers the dry particles to lung cells directly by low air flow (10 mL/min). The in vitro exposure model for particle-bound PBDEs consists of silica particles coated with individual PBDE congeners (BDEs 35, 47, and 99) and a dry powder sprayer connected to a delay chamber and an exposure chamber holding Transwell® inserts.
2.5. Characterization of the in vitro exposure system
To validate and characterize the exposure system, the size, morphology and spatial variability of particles (particle distribution) were determined by scanning electron microscope (SEM, Hitachi S-4800 FE, Tokyo, Japan) and image analysis (ImageJ, NIH, USA). To evaluate the size and morphology of air-delivered particles in the exposure system, silica particles and PBDE-coated silica particles (0.45 ~ 0.65 mg) were aerosolized and deposited onto cell-free transwell membranes treated with culture medium (100 μL) at a flow rate of 10 mL/min for one minute after spraying of the particles into the delay chamber with the dry powder insufflator with air pump. The membranes were then removed from the transwells with a clean scalpel blade. Samples were allowed to air dry, and the membranes of the transwells were mounted on an aluminum SEM stub with double-sided, graphite tape and the edge of the filter was painted with colloidal silver liquid to promote conductivity. The surface of the sample was sputter-coated with gold (K550, Emitech, Ashford, Kent, England) and examined under SEM by observing size and morphology of particles.
To determine the distribution of particles, the membranes were attached to a gridded microscope slide with double-sided tape and photographed at different distances from center of the transwell membrane using a light microscope. The images of particles deposited on the membranes were characterized by ImageJ software (number of particles/2.5 × 10−4 cm2 of image area) to estimate the total number of particles on the transwell (cell growth area, 1.1 cm2). Poisson distribution was used to compute the number of particles deposited within the cell growth area. Particle dosing efficiency was determined by correlation between particle loading mass (0.45 ~ 0.65 mg) in particle aerosolizer and surface cover ratio of particle area versus cell growth area.
2.6. Air-delivery of particle-bound PBDEs to lung cells
For ALI exposure experiments, cells were harvested with 0.25% trypsin-ethylenediamine tetraacetic acid (Trypsin-EDTA, Invitrogen), counted and seeded at a density of 1.5×104 cells/cm2 onto 1.1 cm2 commercially available transwell membranes (0.4 μm, Transwell, Corning). Cells were allowed to attach to the transwell for 12 h. When cells had grown to confluence (> 85% surface covered), they were cultured at the ALI for 12 h prior to exposure to particles. Cells were then washed twice with phosphate buffered saline (PBS) and transferred to the exposure chamber where they were exposed to particle-bound PBDEs at the ALI. Two different mass concentrations of particle-bound PBDEs (28 and 45 μg/cm2) were directly delivered to both A549 and NCI-H358 cells at a flow rate of 10 mL/min for one minute. Airborne exposure of lung cells grown at the ALI was employed to mimic the exposure conditions of epithelial lung cells in vivo. Intracellular ROS and cytotoxicity were determined 4 and 24 h after exposure. The exposure time points for ROS (4 h) and cell viability (24 h) assays were based on previous studies (He et al. 2008; Jin et al. 2010; Tagliaferri et al. 2010) with the assumption that increased oxidative stress may be an early event and cytotoxicity a later event.
2.7. Submerged cell exposure to PBDEs
To facilitate comparison of cellular responses to air-delivered particle-bound PBDEs at the ALI with responses in submerged cultures, PBDE exposures were also performed using a conventional cell culture/PBDE in solution method. For submerged cell exposures, cells (1×105 cells/cm2) were seeded in complete medium into flat-bottom polystyrene 48-well plates (BD Falcon, Franklin Lakes, NJ, USA) and grown to approximately 85-90% of confluency. Cells were allowed to adhere to cell culture plates for 24 h prior to exposure. PBDEs were dissolved in dimethyl sulfoxide (DMSO) and then diluted with PBS with Ca2+ and Mg2+. Before treatment with the compounds, cells were washed with PBS and then exposed to the compounds (5 μM, 10 μM, 20 μM, 40 μM and 80 μM, final concentration) in complete cell culture medium for 24 h at 37 °C in humidified air with 5 % CO2. After exposure, cytotoxicity and intracellular ROS were determined immediately. Control cultures received solvent (DMSO, 0.1% final concentration) alone.
2.8. Cytotoxicity and intracellular ROS measurement
Cell viability, as an indicator of cytotoxicity, was determined by Alamar Blue assay (Serotec, Oxford, UK), a method that is commonly used to assess mitochondrial function. Five mM A amar B ue stock in PBS was di uted to 50 μM with medium. After the incubation period (24 h), cells were washed twice with PBS, fresh cell culture media containing 50 μM A amar Blue was added and cells were incubated for 1 h at 37°C in humidified air with 5% CO2. The fluorescence was monitored at 535 nm excitation and 595 nm emission wavelengths using a TECAN microplate reader (Tecan Group Ltd., Männedorf, Switzerland).
A fluorescent probe, DCFH-DA (2’,7’-dichloro-difluorescin diacetate, Molecular Probes, OR, USA) was used to measure intracellular ROS. DCFH-DA is permeable across cell membranes and is hydro yzed in ce s to 2’,7’-dichlorofluorescin (DCFH). DCFH is then oxidized to 2’,7’-dichloro-fluorescein (DCF) by ROS (Gomes et al. 2005). The fluorescence dye was dissolved in dimethylformamide (DMF) to make a 5 mM stock solution and diluted with Hanks Balanced Salt Solution (HBSS, Invitrogen, Grand Island, NY, USA) to make a 5 μM working solution. Four hours after particle-bound PBDE exposure or exposure to PBDE in solution, cells were washed with HBSS and incubated in HBSS containing 5 μM DCFH-DA for 1 h in the dark at 37° C. DCF fluorescence was measured at 485 nm excitation and 535 nm emission wavelengths using a TECAN microplate reader (Tecan Group Ltd.).
2.9. Animals and intratracheal insufflation
Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA, USA) were exposed to silica (n=2/group) or PBDE-coated silica particles (n=2/group) acutely via intratracheal insufflation at a dose of 0.6 mg/rat. Protocols were approved by the Institutional Animal Care and Use Committee and complied with the NIH Guide for the Care and Use of Laboratory Animals. Animals were acclimatized after arrival for 12 days prior to use. They were housed in an AAALAC-accredited vivarium in polypropylene cages and supplied with feed, a standard rodent chow, water ad libitum, and maintained on a 12-hr light-dark cycle.
Animals were anesthetized by 5% isoflurane inhalation using a precision Fortec vaporizer (Cyprane, Keighley, UK) and prior to intratracheal insufflation with a dry powder insufflator (model DP-4, Penn-Century, Inc.). After exposure, the animals were euthanized by isoflurane inhalation at 2 h, 4 h, 8 h, 12 h, 16 h and 24 h post exposure (silica-exposed animals, shams were euthanized 8 h after exposure) and each lung was lavaged 3 times (total volume, 15 mL) with 0.9% sterile sodium chloride solution (Baxter, Deerfield, IL). Bronchoalveolar lavage (BAL) fluid was collected, processed and the cell pellet was used for enumeration of total and differential cells. Lungs were fixed in 10% formaldehyde-phosphate-buffered saline solution via the canulated trachea. The tissues were subsequently paraffin-embedded, sectioned at 5 μm, and stained with hematoxylin and eosin (H & E) for histopathology evaluation.
2.10. Statistical analysis
All in vitro experiments were conducted in triplicate. Cells exposed to PBDE-free silica particles in ALI exposure conditions or cells exposed to DMSO alone in submerged cell culture conditions were used as controls. The values of cells exposed to particle-bound PBDEs at the ALI or PBDEs in solutions were normalized to control. Statistical analyses were performed using R software (R Development Core Team 2008). Poisson distribution was used to compute the number of particles depositing on transwell membrane, (#/cm2) at 95% confidence intervals using the in vitro exposure system. A p-value less than 0.05 was considered statistically significant. All data are expressed as means ± standard error (SE) unless otherwise noted.
3. Results
3.1. Characterization of the in vitro exposure system
The size silica particles used as support materials and particles coated with PBDEs ranged in diameter from 4.4 to 4.6 μm (Figure 2A). Particles presented as discrete spheres with no apparent particle aggregation after deposition on the transwell membrane in the exposure system (Figures 2B and C). As shown in Figure 3, particle deposition was highest at a distance 2 mm from center and decreased along one side of the membranes. The SEM images also show that the particles were quite evenly deposited over the membrane at 2 mm (Figure 2B) and 2.8 mm (Figure 2C) from center.
Figure 2.
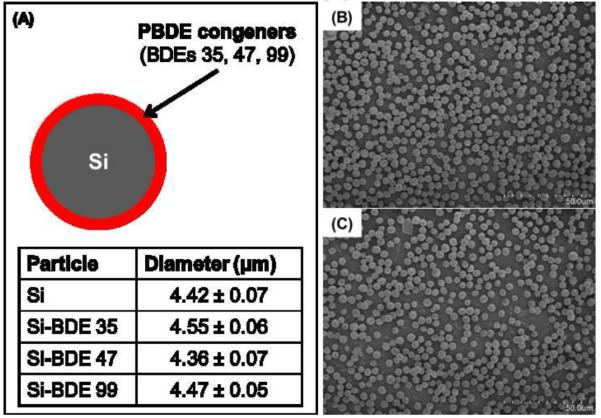
Particle-bound PBDEs were synthesized by coating silica (Si) particles with individual PBDE congeners (BDEs 35, 47 and 99). Particle size (μm) of silica and silica coated with individual PBDE congeners was analyzed by measuring the diameter of 100 random particles by scanning electron microscopy (SEM) and ImageJ software (NIH, USA) after air-delivery of particles onto the transwell in the exposure system (A). Data are expressed as means ± standard error (SE). The deposited particles on the membrane at 2 mm (B) and 2.8 mm (C) from center are shown.
Figure 3.
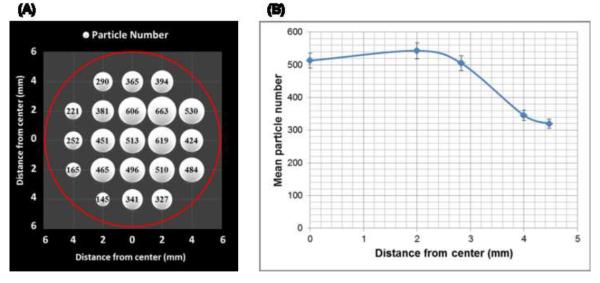
The spatial uniformity of particle deposition in the system was evaluated by counting particles with a light microscope equipped with Image software (Image J) in different distances from center of transwell membrane. (A) The number of deposited particles on the transwell showing positions of delivered particles. The circle indicates the entire transwell (diameter, 12 mm). (B) The number of particles deposited on the transwell at five distances (0, 2, 2.8, 4, 4.5 mm) along the membrane radius. Data are expressed as means ± standard error (SE).
The Poisson distribution was used to compute the total number of particles deposited on the transwell membranes to evaluate the particle dosing efficiency in our exposure chamber. As shown in Table 1 and Figure 4, particle number deposited strongly correlated with particle mass loading (R2=0.97). The surface covered by particles ranged from 27 to 42% of the growth area, depending on the dose of particles (Figure 4).
Table 1.
The 95% confidence intervals for tine number of particles on transwell (#/cm2) are computed based on Poisson distribution assumption.
| Particle loading mass (mg) |
95% confidence intervals |
||
|---|---|---|---|
| aLower Bound (LB) | bUpper Bound (UB) Interval | Length (UB-LB) | |
| 0.45 | 1,731,184 | 1,746,725 | 15,541 |
| 0.50 | 1,913,572 | 1,929,911 | 16,338 |
| 0.55 | 2,120,580 | 2,137,777 | 17,196 |
| 0.60 | 2,581,970 | 2,600,940 | 18,971 |
| 0.65 | 2,737,362 | 2,756,893 | 19,531 |
The lower bounds of the 95% confidence interval for mean
The upper bounds of the 95% confidence interval for mean
Figure 4.
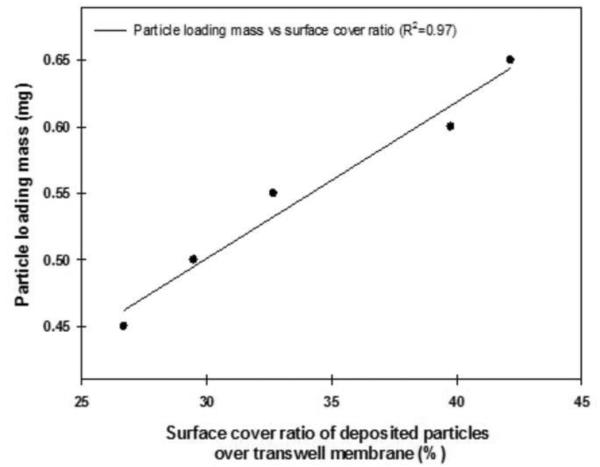
Particle dosing efficiency determined by the correlation between particle loading mass and surface cover ratio.
3.2. Cellular responses to air-delivery of particle-bound PBDEs
As shown in Figure 5A, 24 h after ALI exposure to PBDE-coated particles, statistically significant decreases in the viability of A549 cells were observed in response to doses of 28 and 45 μg BDE35-coated particles (p<0.001 and p<0.05, respectively) compared to control cells that were exposed to silica particles alone. A significantly decreased viability of A549 cells at a dose of 28 μg BDE47-coated particles (p<0.01) was found, whereas BDE99-bound particles did not significantly decrease cell viability. No significant reduction in the viability of NCI-H358 cells in all treatment groups.
Figure 5.
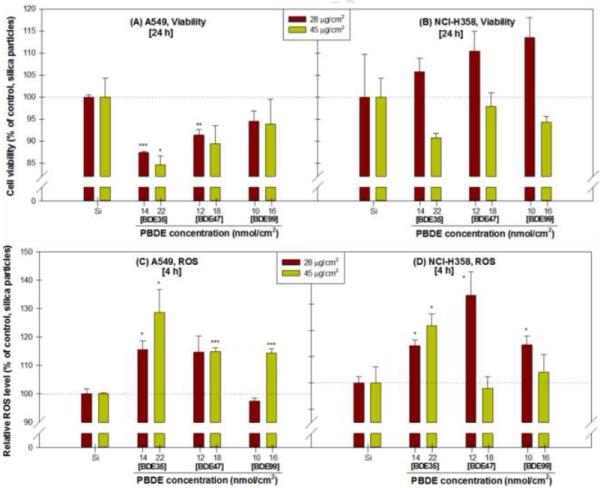
Effects of particle-bound PBDEs (BDEs 35, 47 and 99) treatment on the viability at 24 h (A and B) and reactive oxygen species at 4 h (ROS, C and D) in A549 and NCI-H358 cells at an air-liquid interface, expressed as % of control. Two mass concentrations (28 and 45 μg/cm2) particle-bound PBDEs were directly delivered to lung cells with air. Data are expressed as means ± standard error (SE). * p < 0.05, ** p < 0.01, *** p < 0.001, compared with the control group.
As shown in Figure 5C and D, 4 h after ALI exposure, a significant increase in ROS in both cell lines was detected in response to the doses of 28 and 45 μg BDE35-coated particles (p<0.05) compared to control (silica particles alone). BDE47-bound particles significantly increased the intracellular ROS in A549 (at 45 μg, p<0.001) and NCI-H358 cells (at 28 μg, p<0.05). ALI exposure of cells to BDE99-bound particles significantly increased ROS in A549 (at 45 μg, p<0.001) and NCI-H358 cells (at 28 μg, p<0.05).
3.3. Cellular responses to PBDEs in solution exposure system
As shown in Figure 6, the viability of A549 cells treated with BDE47 was significantly lower only at a concentration of 40 μM (p<0.01) and cell viability following exposure to BDE99 decreased at concentrations of 20 (p<0.01), 40 (p<0.05) and 80 μM (p<0.05). The levels of intracellular ROS in A549 cells (Figure 6 C) were examined at 4 h after PBDE treatment in solution. A significant increase in ROS was detected in response to BDE35 at 5 (1.3-fold), 10 (1.3-fold) and 20 μM (1.2-fold) compared to control (DMSO alone). Exposure of cells to BDE47 resulted in significant increases in ROS at 5 (1.4-fold) and 10 μM (1.5-fold) compared to control. BDE99 produced a significant increase in ROS from 1.4-, 1.5- and 1.5-fold at concentrations of 5, 10 and 20 μM, respectively. In NCI-H358 cells exposed to 5 μM BDE47 and all concentrations of BDE99, the intercellular ROS level was significantly increased compared to the control (p<0.05, Figure 6D).
Figure 6.
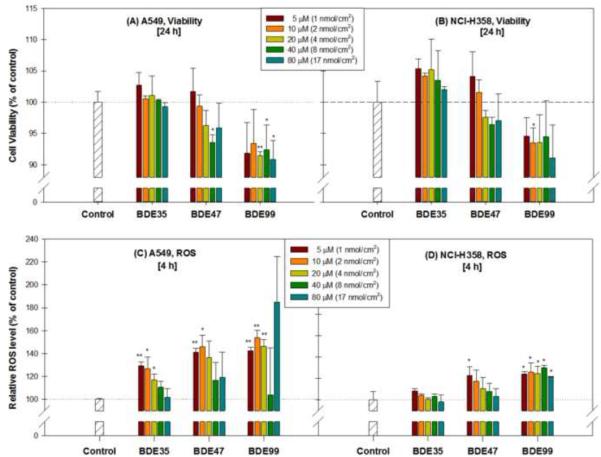
Effects of PBDEs (BDEs 35, 47 and 99) dissolved in culture medium on viability at 24 h (A and B) and on intracellular reactive oxygen species formation at 4 h (C and D) in A549 and NCI-H358 cells, expressed as % of control. Data are expressed as means ± standard error (SE). * p < 0.05, ** p < 0.01, compared with the control group.
3.4. Intratracheal insufflation
To evaluate pulmonary responses to PBDE-coated particles, we examined BAL fluids and lung histopathology in rats exposed acutely to silica particles or PBDE-coated particles at different post-exposure time (Figure 7). The number of macrophages in BAL fluids from animals exposed to PBDE-coated particles significantly increased over time, reaching a peak at 8 h post-exposure, p < 0.05, and then slowly decreasing thereafter (Figure 7A). A significant increase in the number of neutrophils over time was observed also reaching a maximum at 8 h post exposure, p < 0.05, and then slowly declining thereafter. PBDE-coated particles caused a significant inflammation in the lungs represented by a high recruitment of neutrophils (70% + 4), that were slowly decreasing to 41% (+ 12) at 24 h after exposure (Figure 7A). The total number of cells in the BAL fluid at the 24 h time point was approaching the number of cells in shams at the 8 h time point.
Figure 7.
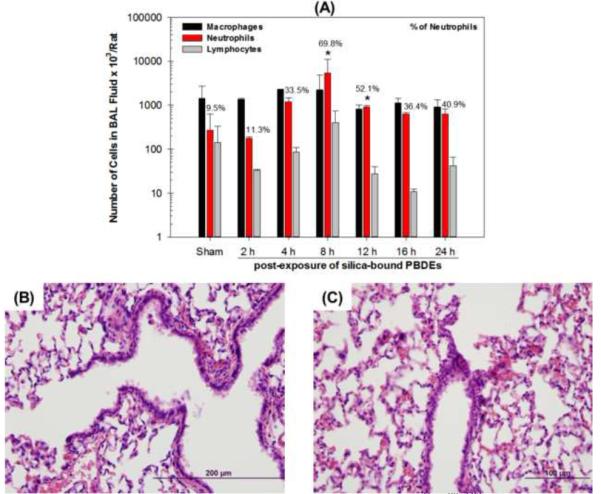
Number of cells and percentage of neutrophils in BAL fluid (A) and micrographs of lung sections stained with H&E from rats exposed acutely via intratracheal insufflation of silica (B) and particle-coated with mixtures of PBDE congeners (C, 0.6 mg/rat). Data are expressed as means ± standard deviation (SD). * p < 0.05 significantly different from sham-exposed animals.
Histopathologic evaluations of lung tissues showed neutrophilic peribronchiolar infiltration (at 8 to 16 h post-exposure) that became less severe with a mixed inflammatory cell population by 24 h post-exposure (Figure 7B and C). Within the airways and the terminal bronchiolar-alveolar duct junctions, there were focal accumulations of macrophages, often containing phagocytosed particles, and small number of neutrophils was most prominent at 8 to 16 h post-exposure to PBDE-coated particles (data not shown). Phagocytosed particles in PDBE-exposed rats appeared as round, pinkish, slightly refractile spheroids within macrophages or neutrophils which had severely peripheralized nuclei, but particles were not observed in the cells of silica only treated rats.
4. Discussion
We generated and characterized coatings of PBDE congeners on silica particles as a model of airborne particle-bound PBDEs to mimic inhalation exposure of mixtures of dust and PBDEs. The in vitro exposure model employing an ALI was built to assess potential pulmonary toxicity of these particle-bound PBDEs. Our results demonstrate that the exposure system fulfills several important requirements. First, the system is able to be used to reproducibly generate and deliver particles with no apparent aggregation and cell damage by airflow. The size of the spherical silica and PBDE coated particles is in the range of respirable particles (smaller than 3 μm in adults and 5 μm in children) (Brown et al. 2013), including dusts that contain PBDEs. Second, particles were evenly deposited over the membrane except that relatively fewer particles were deposited at the outer edge of the membrane (at 4.5 mm from the center). This decrease was likely due to the outer diameter (4 mm) of the aerosol delivery tube being smaller than that of the transwell membrane and the higher velocity of the outer particles (Desantes et al. 2006; Tippe et al. 2002). An uneven distribution of particles could cause artifactual, localized, cellular responses in overexposed areas on the transwell due to excessive localized particle loading. Our results show that the risk of such uneven responses was relatively small in our exposure set-up. Third, the exposure system is able to be used to provide an ALI for lung cells to mimic airborne exposure and avoid interactions between particles and culture medium. Our results demonstrate that the new in vitro exposure system allows for dosing and surface cover ratio control of particles.
We recognize that choosing the appropriate endpoints and cell types based on the biological effects of a test substance and biology of the target organ is a key to the establishment of in vitro toxicity systems. Non-small lung carcinoma cell lines (A549 and NCI-H358) are widely used to study the toxicity of airborne particles in lung. While primary human lung epithelial cells may be a better choice for the toxicity assessment of inhalable particles, primary cells are more difficult to obtain and have limited growth potential. A main goal of this work was not to elicit mechanisms in responses to particle-bound PBDEs, but focus on characterization and validation of the system. To validate biological aspects of the exposure system, we chose the viability and intracellular ROS generation of lung cells in responses to air delivered particle-bound PBDEs as initial endpoints. Selecting endpoints related to the changes of signaling pathways is, therefore, needed for future work since PBDEs exert their toxicity through interfering with intracellular signaling pathways. Statistically significant decreases in cell viability in response to PBDEs under solution cell culture conditions were of limited biological importance since reduction ranged only from 6 to 9%. The pattern of cytotoxic effects caused by PBDEs in NCI-H358 cells was similar under both exposure conditions, indicating a lower sensitivity of this cell line to these compounds. Exposure of lung cells from each cell line to 5 μM to 80 μM PBDE (congeners 35, 47 or 99) in the solution exposure method was not associated with a dose-dependent effect on cytotoxicity or intracellular ROS. The main reason for this observation is likely the partitioning of PBDEs into the medium under solution culture conditions. A recent investigation (Barber et al. 2006) of the partitioning of PBDEs from media into MCF-7 cells under solution cell culture conditions reported that only 5.5 and 1.9 % of BDE47 and 99, respectively, partitioned into the cells. They suggested that large losses occur during incubation with PBDEs, apparently adsorbed onto the plastic, resulting in the cells being exposed to lower doses than intended. Given these issues with the solution culture method, the biological effects of particle-bound PBDEs would not be accurately evaluated using this method. The ALI delivery method developed in this work may have the additional advantage of circumventing this problem.
Overall ROS was a more sensitive endpoint than cytotoxicity and A549 cells (epithelial cells) seemed to be more sensitive than NCI-H358 cells (Clara cells) to PBDEs in this study. BDE35 induced toxicity in A549 and ROS in NCI-H358 cells only if delivered as particles, while BDE99 induced cytotoxicity in both cell types only if applied in solution. These results indicate that there are cell types, test compounds and delivery specific effects, which can be further employed to obtain insights into physicochemical and biological determinants of in vitro toxicity. Disparities in cellular responses between lung cell lines and primary lung cells are common (Courcot et al. 2012; Melis et al. 1996; Nirmalanandhan et al. 2010). Lung surfactants and metabolic capacities of lung epithelial cells may alter toxicity of the inhaled particles and cell lines used in this study have specific cellular features such as high levels of glutathione and gene expression of heme oxygenase-1 (A549) (Carmichael et al. 1988; Speit and Bonzheim 2003) and expression of lung surfactant associated proteins and cytochrome P-450 enzymes (NCI-H358) (Gazdar et al. 1990; Hasenpusch et al. 2011). These differences may represent a possible explanation as to why A549 cells were more vulnerable to toxicity of PBDE-bound particles compared to NCI-H358 cells, but this warrants further investigation. Indeed, a recent study (Courcot et al. 2012) reported that A549 and NCI-H358 cell lines exhibited distinct differences in gene expression patterns (Pearson's correlation coefficient, r=0.75; the r value represents the strengths of the linear relationship between two sets of comparative components, a greater number indicating higher similarity). For example, A549 had a higher expression of SLCO1B1 (solute carrier organic anion transporter family, member B1), ARNT2 (nuclear receptor), BLMH (bleomycin hydrolase) and MT1A (metallothionein 1A), while NCI-H358 did not express these genes, indicating that A549 may differ in uptake, gene induction, metabolism, and toxic stress response. We may therefore assume that A549 is an appropriate cell model for investigating the level of intracellular ROS generated by particle-bound PBDEs. However, BEAS-2B cells can be also considered as a second cell model due to the highest similarity to primary lung cells (Courcot et al. 2012).
It is important to recognize that toxicological studies at physiologically- and environmentally-relevant concentrations are essential for risk assessment of PBDEs. However, a major aspect of the work described in present study is developmental work, i.e. evaluating our in vitro exposure system by comparing data obtained with literature results from traditional in vitro studies via medium exposure. Those in vitro toxicity studies often employed high concentrations (from 5 to 100 μM) (Barber et al. 2006; Zhang et al. 2013). The in vitro doses of particle-bound PBDEs used in this study ranged from 10 to 22 nmol/cm2 (approximately equivalent to 50 - 100 μM in traditional liquid in vitro methods) which is in the range of PBDE exposure concentrations used in most in vitro studies. Despite the need of toxicological studies at environmentally and biologically relevant concentrations, little is known about such exposure levels of PBDEs for in vitro investigations and only a few studies (Barber et al. 2006; Li et al. 2012; Napoli et al. 2013; Wei et al. 2010) have examined the effects of PBDEs in vitro at lower concentrations (10 to 100 nM). In particular, a recent study provided an approximate reference for setting environmentally relevant exposure concentrations (Wei et al. 2010), suggesting approximately 10 nM as highest environmentally relevant PBDE concentration for cells (MCF-7, HepG2, H295R and PC12) in medium. However, since under these assay conditions, only 2-5% of PBDEs is expected to partition into the cells (Barber et al. 2006), the cells would be exposed to lower doses of PBDEs than intended. Thus, the environmentally relevant exposure concentration should be adjusted to approximately 200 nM for cells in medium. In addition, those cells are derived from internal organs (breast, liver, adrenal gland) which are not the first site of contact. The one-time exposure doses used in our exploratory study are relatively higher, but lower doses of PBDEs and repeated dosing mimicking chronic exposure of lung cells to air contaminants are therefore planned for our future work.
In vitro exposure systems employing an ALI to assess the toxicity of inhaled particles have received considerable attention in recent years (Kim et al. 2013; Lenz et al. 2009; Lichtveld et al. 2012; Rothen-Rutishauser et al. 2009; Volckens et al. 2009). Many of these studies have illustrated limitation and problems of in vitro exposure of submerged cells to particles. Results by Lichtveld et al., using a direct in vitro sampling and exposure method (DSEM) demonstrate that the resuspended particulate matter (PM) collected on the filter into liquid significantly modified its toxicity compared to direct deposition due to the changes in chemical composition of the PM prior to the delivery of PM to the cellular surface (Lichtveld et al. 2012). Although submerged in vitro testing methods for assessing particle toxicity have beneficial advantages compared to ALI in vitro systems, results obtained from solution cell culture methods may represent only a lower bound on PM toxicity and it should, therefore, be used with caution (Lichtveld et al. 2012).
We recognize that directly comparing in vitro results to in vivo measurements is difficult for a number of reasons, including the fact that there are over 40 cell types in the lung. Cell lines have often lost the specific functional properties of their in vivo equivalents and do not always provide full and stable phenotypes (Prieto et al. 2006). There are a number of differences between in vitro and in vivo studies (e.g., different species, exposure doses and times). Despite these limitations the direct comparison of in vitro and in vivo findings is informative. It is very important to select relevant endpoints in the comparisons of particle toxicity in vitro and in vivo. Since cytotoxicity is a major inducer of inflammation for particles in vivo and in vitro, cell viability indicating cytotoxicity in in vitro studies and neutrophil recruitment as an indicator of inflammation in animal studies were selected to assess pulmonary toxicity of particle-bound PBDEs. Our comparative in vivo studies showed that PBDE particles produced increased recruitment of macrophages and neutrophils and particle phagocytosis in the lungs of rats. These data demonstrate the use of our exposure model for particle-bound contaminants in a manner that represents cellular responses of the pulmonary epithelium in vivo. This method and these observations can be used as guidance in the development of future ALI experiments with mixed cell cultures to explore mechanisms, interaction effects and structure-activity relationships.
Acknowledgements
This study was supported by the National Institute of Environmental Health Sciences (NIEHS) through Grants NIH P42 ES013661 (Iowa Superfund Research Program) and NIH P30 ES005605. The authors gratefully thank Dr. Andrea Adamcakova-Dodd for the animal insufflation exposures, Dr. Kai Wang for statistical analyses and Dr. Wanda Haschek-Hock for the histopathology assessments. Dr. Gregor Luthe gratefully acknowledges the Alexander von Humboldt Foundation (Feodor Lynen Program) for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest.
References
- Allen J, Mcclean M, Stapleton H, Nelson J, Sanchez G, Fraser A, Webster T. PBDE Levels in Indoor Air and Dust Collected From US Urban Residences. Epidemiology. 2006;17:S375. [Google Scholar]
- Allen JG, McClean MD, Stapleton HM, Webster TF. Linking PBDEs in House Dust to Consumer Products using X-ray Fluorescence. Environ. Sci. Technol. 2008;42:4222–4228. doi: 10.1021/es702964a. [DOI] [PubMed] [Google Scholar]
- Barber JL, Walsh MJ, Hewitt R, Jones KC, Martin FL. Low-dose treatment with polybrominated diphenyl ethers (PBDEs) induce altered characteristics in MCF-7 cells. Mutagenesis. 2006;21:351–360. doi: 10.1093/mutage/gel038. [DOI] [PubMed] [Google Scholar]
- Batterman SA, Chernyak S, Jia C, Godwin C, Charles S. Concentrations and Emissions of Polybrominated Diphenyl Ethers from U.S. Houses and Garages. Environmental Science & Technology. 2009;43:2693–2700. doi: 10.1021/es8029957. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, Staskal DF. Brominated flame retardants: cause for concern? Environ Health Perspect. 2004;112:9–17. doi: 10.1289/ehp.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterle E, Karg E, Schroeppel A, Kreyling WG, Tippe A, Ferron GA, Schmid O, Heyder J, Maier KL, Hofer T. Dose-controlled exposure of A549 epithelial cells at the air-liquid interface to airborne ultrafine carbonaceous particles. Chemosphere. 2006;65:1784–1790. doi: 10.1016/j.chemosphere.2006.04.035. [DOI] [PubMed] [Google Scholar]
- Brown J, Gordon T, Price O, Asgharian B. Thoracic and respirable particle definitions for human health risk assessment. Particle and Fibre Toxicology. 2013;10:12. doi: 10.1186/1743-8977-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael J, Mitchell JB, Friedman N, Gazdar AF, Russo A. Glutathione and related enzyme activity in human lung cancer cell lines. British journal of cancer. 1988;58:437–440. doi: 10.1038/bjc.1988.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansson A, Hovander L, Athanassiadis I, Jakobsson K, Bergman Å. Polybrominated diphenyl ethers in aircraft cabins – A source of human exposure? Chemosphere. 2008;73:1654–1660. doi: 10.1016/j.chemosphere.2008.07.071. [DOI] [PubMed] [Google Scholar]
- Courcot E, Leclerc J, Lafitte J-J, Mensier E, Jaillard S, Gosset P, Shirali P, Pottier N, Broly F, Lo-Guidice J-M. Xenobiotic Metabolism and Disposition in Human Lung Cell Models: Comparison with In Vivo Expression Profiles. Drug Metabolism and Disposition. 2012;40:1953–1965. doi: 10.1124/dmd.112.046896. [DOI] [PubMed] [Google Scholar]
- de Wit CA. An overview of brominated flame retardants in the environment. Chemosphere. 2002;46:583–624. doi: 10.1016/s0045-6535(01)00225-9. [DOI] [PubMed] [Google Scholar]
- Desantes JM, Margot X, Gil A, Fuentes E. Computational study on the deposition of ultrafine particles from Diesel exhaust aerosol. Journal of Aerosol Science. 2006;37:1750–1769. [Google Scholar]
- Dingemans MM, van den Berg M, Westerink RH. Neurotoxicity of brominated flame retardants: (in)direct effects of parent and hydroxylated polybrominated diphenyl ethers on the (developing) nervous system. Environmental health perspectives. 2011;119:900–907. doi: 10.1289/ehp.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA, U.S. An Exposure Assessment of Polybrominated Diphenyl Ethers (Final Report) U.S. Environmental Protection Agency; Washington, DC: 2010. [Google Scholar]
- Fatisson J, Quevedo IR, Wilkinson KJ, Tufenkji N. Physicochemical characterization of engineered nanoparticles under physiological conditions: Effect of culture media components and particle surface coating. Colloids and Surfaces B: Biointerfaces. 2012;91:198–204. doi: 10.1016/j.colsurfb.2011.10.056. [DOI] [PubMed] [Google Scholar]
- Frederiksen M, Vorkamp K, Thomsen M, Knudsen LE. Human internal and external exposure to PBDEs – A review of levels and sources. International Journal of Hygiene and Environmental Health. 2009;212:109–134. doi: 10.1016/j.ijheh.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Gao P, He P, Wang A, Xia T, Xu B, Xu Z, Niu Q, Guo L, Chen X. Influence of PCB153 on oxidative DNA damage and DNA repair-related gene expression induced by PBDE-47 in human neuroblastoma cells in vitro. Toxicological sciences : an official journal of the Society of Toxicology. 2009;107:165–170. doi: 10.1093/toxsci/kfn224. [DOI] [PubMed] [Google Scholar]
- Gazdar AF, Linnoila RI, Kurita Y, Oie HK, Mulshine JL, Clark JC, Whitsett JA. Peripheral airway cell differentiation in human lung cancer cell lines. Cancer research. 1990;50:5481–5487. [PubMed] [Google Scholar]
- Gomes A, Fernandes E, Lima JLFC. Fluorescence probes used for detection of reactive oxygen species. Journal of Biochemical and Biophysical Methods. 2005;65:45–80. doi: 10.1016/j.jbbm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Harrad S, Hazrati S, Ibarra C. Concentrations of Polychlorinated Biphenyls in Indoor Air and Polybrominated Diphenyl Ethers in Indoor Air and Dust in Birmingham, United Kingdom: Implications for Human Exposure. Environ. Sci. Technol. 2006;40:4633–4638. doi: 10.1021/es0609147. [DOI] [PubMed] [Google Scholar]
- Hasenpusch G, Pfeifer C, Aneja MK, Wagner K, Reinhardt D, Gilon M, Ohana P, Hochberg A, Rudolph C. Aerosolized BC-819 Inhibits Primary but Not Secondary Lung Cancer Growth. PLoS ONE. 2011;6:e20760. doi: 10.1371/journal.pone.0020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P, Wang AG, Xia T, Gao P, Niu Q, Guo LJ, Xu BY, Chen XM. Mechanism of the neurotoxic effect of PBDE-47 and interaction of PBDE-47 and PCB153 in enhancing toxicity in SH-SY5Y cells. Neurotoxicology. 2009;30:10–15. doi: 10.1016/j.neuro.2008.10.004. [DOI] [PubMed] [Google Scholar]
- He W, He P, Wang A, Xia T, Xu B, Chen X. Effects of PBDE-47 on cytotoxicity and genotoxicity in human neuroblastoma cells in vitro. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2008;649:62–70. doi: 10.1016/j.mrgentox.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Huwe JK, Hakk H, Smith DJ, Diliberto JJ, Richardson V, Stapleton HM, Birnbaum LS. Comparative Absorption and Bioaccumulation of Polybrominated Diphenyl Ethers following Ingestion via Dust and Oil in Male Rats. Environ. Sci. Technol. 2008;42:2694–2700. doi: 10.1021/es702644k. [DOI] [PubMed] [Google Scholar]
- Hwang H-M, Park E-K, Young TM, Hammock BD. Occurrence of endocrine-disrupting chemicals in indoor dust. Science of The Total Environment. 2008;404:26–35. doi: 10.1016/j.scitotenv.2008.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman S, Song Y, Verkman AS. Airway Surface Liquid Osmolality Measured using Fluorophore-encapsulated Liposomes. J. Gen. Physiol. 2001;117:423–430. doi: 10.1085/jgp.117.5.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S, Yang F, Hui Y, Xu Y, Lu Y, Liu J. Cytotoxicity and apoptosis induction on RTG-2 cells of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) and decabrominated diphenyl ether (BDE-209) Toxicology in Vitro. 2010;24:1190–1196. doi: 10.1016/j.tiv.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Kameyama S, Kondo M, Takeyama K, Nagai A. Air Exposure Causes Oxidative Stress In Cultured Bovine Tracheal Epithelial Cells and Produces A Change In Cellular Glutathione Systems. Experimental Lung Research. 2003;29:567–583. doi: 10.1080/01902140390240113. [DOI] [PubMed] [Google Scholar]
- Kim JS, Peters TM, O’Shaughnessy PT, Adamcakova-Dodd A, Thorne PS. Validation of an in vitro exposure system for toxicity assessment of air-delivered nanomaterials. Toxicology in Vitro. 2013;27:164–173. doi: 10.1016/j.tiv.2012.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klo□sener J, Peters TM, Adamcakova-Dodd A, Teesch LM, Thorne PS, Robertson LW, Luthe G. Innovative Application of Fluoro Tagging To Trace Airborne Particulate and Gas-Phase Polybrominated Diphenyl Ether Exposures. Chemical Research in Toxicology. 2008;22:179–186. doi: 10.1021/tx8003032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Lu M, Gao X, Wu H, Cai Z. New evidence for toxicity of polybrominated diphenyl ethers: DNA adduct formation from quinone metabolites. Environ Sci Technol. 2011;45:10720–10727. doi: 10.1021/es203068f. [DOI] [PubMed] [Google Scholar]
- Lenz A, Karg E, Lentner B, Dittrich V, Brandenberger C, Rothen-Rutishauser B, Schulz H, Ferron G, Schmid O. A dose-controlled system for air-liquid interface cell exposure and application to zinc oxide nanoparticles. Particle and Fibre Toxicology. 2009;6:32. doi: 10.1186/1743-8977-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZH, Liu XY, Wang N, Chen JS, Chen YH, Huang JT, Su CH, Xie F, Yu B, Chen DJ. Effects of decabrominated diphenyl ether (PBDE-209) in regulation of growth and apoptosis of breast, ovarian, and cervical cancer cells. Environmental health perspectives. 2012;120:541–546. doi: 10.1289/ehp.1104051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtveld KM, Ebersviller SM, Sexton KG, Vizuete W, Jaspers I, Jeffries HE. In Vitro Exposures in Diesel Exhaust Atmospheres: Resuspension of PM from Filters versus Direct Deposition of PM from Air. Environmental Science & Technology. 2012;46:9062–9070. doi: 10.1021/es301431s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Johnson PI, Camann D, Hauser R. Polybrominated diphenyl ether (PBDE) concentrations in house dust are related to hormone levels in men. Science of The Total Environment. 2009;407:3425–3429. doi: 10.1016/j.scitotenv.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Spatafora M, Melodia A, Pace E, Gjomarkaj M, Merendino AM, Bonsignore G. ICAM-1 expression by lung cancer cell lines: effects of upregulation by cytokines on the interaction with LAK cells. The European respiratory journal. 1996;9:1831–1838. doi: 10.1183/09031936.96.09091831. [DOI] [PubMed] [Google Scholar]
- Napoli E, Hung C, Wong S, Giulivi C. Toxicity of the flame-retardant BDE-49 on brain mitochondria and neuronal progenitor striatal cells enhanced by a PTEN-deficient background. Toxicological sciences : an official journal of the Society of Toxicology. 2013;132:196–210. doi: 10.1093/toxsci/kfs339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirmalanandhan VS, Duren A, Hendricks P, Vielhauer G, Sittampalam GS. Activity of anticancer agents in a three-dimensional cell culture model. Assay and drug development technologies. 2010;8:581–590. doi: 10.1089/adt.2010.0276. [DOI] [PubMed] [Google Scholar]
- Paustenbach DJ, Finley BL, Long TF. The Critical Role of House Dust in Understanding the Hazards Posed by Contaminated Soils. International Journal of Toxicology. 1997;16:339–362. [Google Scholar]
- Prieto P, Baird AW, Blaauboer BJ, Castell Ripoll JV, Corvi R, Dekant W, Dietl P, Gennari A, Gribaldo L, Griffin JL, Hartung T, Heindel JJ, Hoet P, Jennings P, Marocchio L, Noraberg J, Pazos P, Westmoreland C, Wolf A, Wright J, Pfaller W. The assessment of repeated dose toxicity in vitro: a proposed approach. The report and recommendations of ECVAM workshop. 2006;56;34:315–341. doi: 10.1177/026119290603400307. Alternatives to laboratory animals : ATLA. [DOI] [PubMed] [Google Scholar]
- Ren XM, Guo LH, Gao Y, Zhang BT, Wan B. Hydroxylated polybrominated diphenyl ethers exhibit different activities on thyroid hormone receptors depending on their degree of bromination. Toxicology and applied pharmacology. 2013;268:256–263. doi: 10.1016/j.taap.2013.01.026. [DOI] [PubMed] [Google Scholar]
- Rothen-Rutishauser B, Grass RN, Blank F, Limbach LK, Mu□hlfeld C, Brandenberger C, Raemy DO, Gehr P, Stark WJ. Direct Combination of Nanoparticle Fabrication and Exposure to Lung Cell Cultures in a Closed Setup as a Method To Simulate Accidental Nanoparticle Exposure of Humans. Environmental Science & Technology. 2009;43:2634–2640. doi: 10.1021/es8029347. [DOI] [PubMed] [Google Scholar]
- Savi M, Kalberer M, Lang D, Ryser M, Fierz M, Gaschen A, Ric□ka J, Geiser M. A Novel Exposure System for the Efficient and Controlled Deposition of Aerosol Particles onto Cell Cultures. Environmental Science & Technology. 2008;42:5667–5674. doi: 10.1021/es703075q. [DOI] [PubMed] [Google Scholar]
- Sayes CM, Reed KL, Warheit DB. Assessing Toxicity of Fine and Nanoparticles: Comparing In Vitro Measurements to In Vivo Pulmonary Toxicity Profiles. Toxicol. Sci. 2007;97:163–180. doi: 10.1093/toxsci/kfm018. [DOI] [PubMed] [Google Scholar]
- Schecter A, Pavuk M, Papke O, Ryan JJ, Birnbaum L, Rosen R. Polybrominated diphenyl ethers (PBDEs) in U.S. mothers' milk. Environmental health perspectives. 2003;111:1723–1729. doi: 10.1289/ehp.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoeib M, Harner T, Webster GM, Sverko E, Cheng Y. Legacy and current-use flame retardants in house dust from Vancouver, Canada. Environmental Pollution. 2012;169:175–182. doi: 10.1016/j.envpol.2012.01.043. [DOI] [PubMed] [Google Scholar]
- Speit G, Bonzheim I. Genotoxic and protective effects of hyperbaric oxygen in A549 lung cells. Mutagenesis. 2003;18:545–548. doi: 10.1093/mutage/geg028. [DOI] [PubMed] [Google Scholar]
- Stringer B, Imrich A, Kobzik L. Lung epithelial cell (A549) interaction with unopsonized environmental particulates: quantitation of particle-specific binding and IL-8 production. Exp Lung Res. 1996;22:495–508. doi: 10.3109/01902149609046038. [DOI] [PubMed] [Google Scholar]
- Tagliaferri S, Caglieri A, Goldoni M, Pinelli S, Alinovi R, Poli D, Pellacani C, Giordano G, Mutti A, Costa LG. Low concentrations of the brominated flame retardants BDE-47 and BDE-99 induce synergistic oxidative stress-mediated neurotoxicity in human neuroblastoma cells. Toxicology in Vitro. 2010;24:116–122. doi: 10.1016/j.tiv.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Timm Schreiber KG, Götz Christine, Hübenthal Ulrike, Moors Michaela, Krause Guido, Merk Hans F., Nguyen Ngoc-Ha, Scanlan Thomas S., Abel Josef, Rose Christine R., Fritsche Ellen. Polybrominated Diphenyl Ethers Induce Developmental Neurotoxicity in a Human in Vitro Model: Evidence for Endocrine Disruption. Environ. Health Perspect. 2010;118:572–578. doi: 10.1289/ehp.0901435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tippe A, Heinzmann U, Roth C. Deposition of fine and ultrafine aerosol particles during exposure at the air/cell interface. Journal of Aerosol Science. 2002;33:207–218. [Google Scholar]
- Volckens J, Dailey L, Walters G, Devlin RB. Direct Particle-to-Cell Deposition of Coarse Ambient Particulate Matter Increases the Production of Inflammatory Mediators from Cultured Human Airway Epithelial Cells. Environmental Science & Technology. 2009;43:4595–4599. doi: 10.1021/es900698a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorkamp K, Thomsen M, Frederiksen M, Pedersen M, Knudsen LE. Polybrominated diphenyl ethers (PBDEs) in the indoor environment and associations with prenatal exposure. Environment International. 2011;37:1–10. doi: 10.1016/j.envint.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Watkins DJ, McClean MD, Fraser AJ, Weinberg J, Stapleton HM, Sjödin A, Webster TF. Impact of Dust from Multiple Microenvironments and Diet on PentaBDE Body Burden. Environmental Science & Technology. 2011;46:1192–1200. doi: 10.1021/es203314e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei RG, Zhao YX, Liu PY, Qin ZF, Yan SS, Li Y, Qin XF, Xia XJ, Xu XB, Yan MC. Determination of environmentally relevant exposure concentrations of polybrominated diphenyl ethers for in vitro toxicological studies. Toxicology in vitro : an international journal published in association with BIBRA. 2010;24:1078–1085. doi: 10.1016/j.tiv.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Wilford BH, Thomas GO, Jones KC, Davison B, Hurst DK. Decabromodiphenyl ether (deca-BDE) commercial mixture components, and other PBDEs, in airborne particles at a UK site. Environment International. 2008;34:412–419. doi: 10.1016/j.envint.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Zhang S, Kuang G, Zhao G, Wu X, Zhang C, Lei R, Xia T, Chen J, Wang Z, Ma R, Li B, Yang L, Wang A. Involvement of the mitochondrial p53 pathway in PBDE-47-induced SH-SY5Y cells apoptosis and its underlying activation mechanism. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2013;62:699–706. doi: 10.1016/j.fct.2013.10.008. [DOI] [PubMed] [Google Scholar]


