Abstract
The mechanism by which tolerance is induced via systemic administration of high doses of aqueous antigen has been analyzed by using mice transgenic for a T-cell receptor specific for the influenza virus hemagglutinin (HA) peptide comprising amino acids 126-138. After intravenous injection of 750 (but not 75) micrograms of HA peptide, a state of hyporesponsiveness was rapidly induced. In the thymus, in situ apoptosis in the cortex and at the corticomedullary junction was responsible for a synchronous and massive deletion of CD4+ CD8+ thymocytes. In secondary lymphoid organs, HA-reactive T cells were initially activated but were hyporesponsive at the single cell level. After 3 days, however, those cells were rapidly deleted, at least partially, through an apoptotic process. Therefore, both thymic and peripheral apoptosis, in addition to T-cell receptor desensitization, contribute to high-dose tolerance.
Full text
PDF
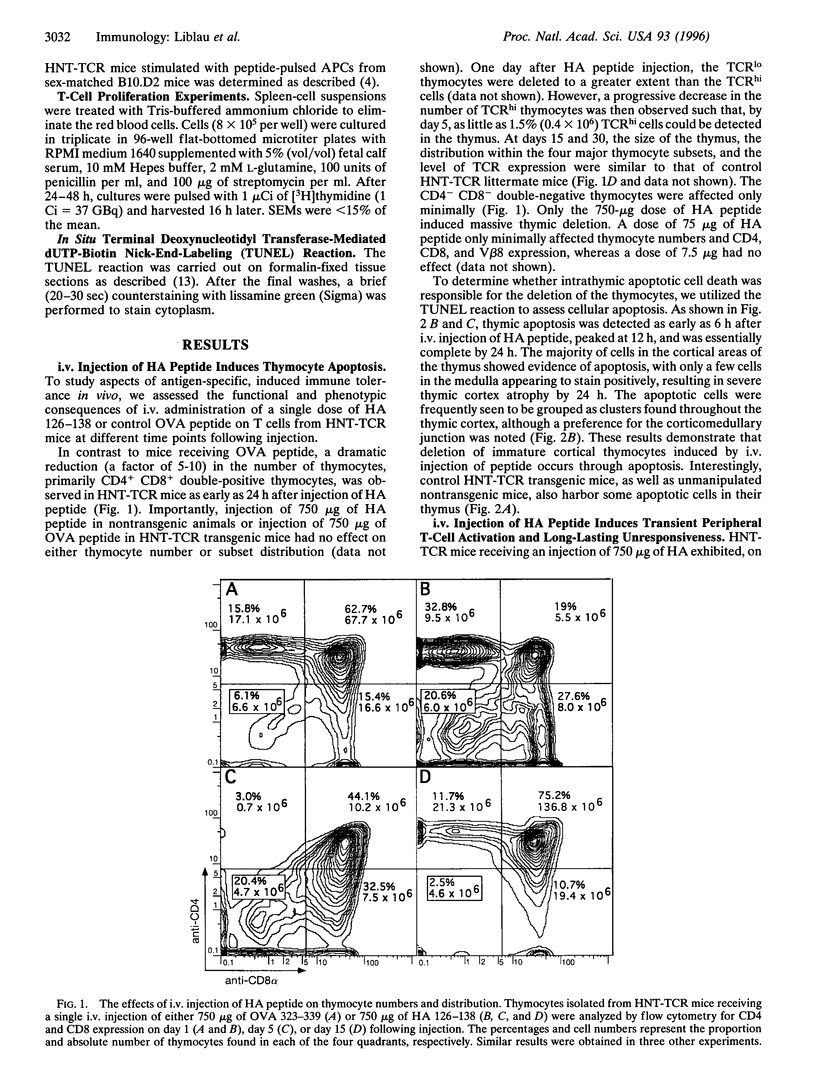
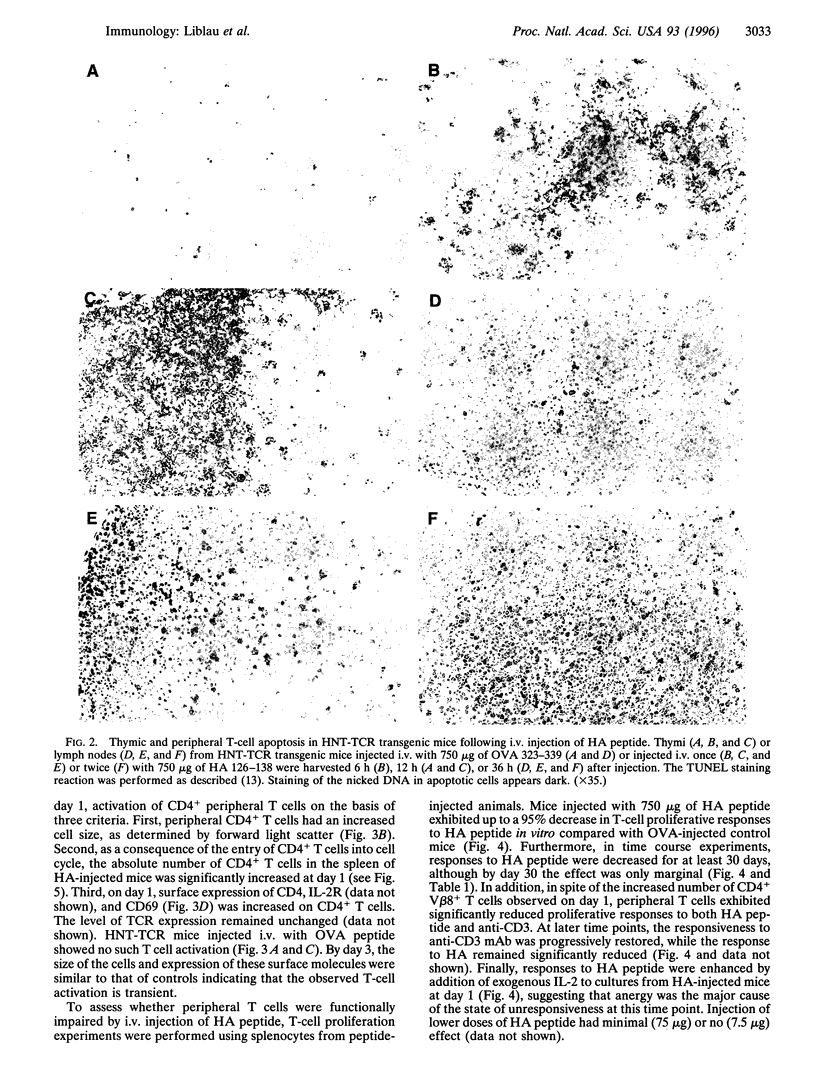
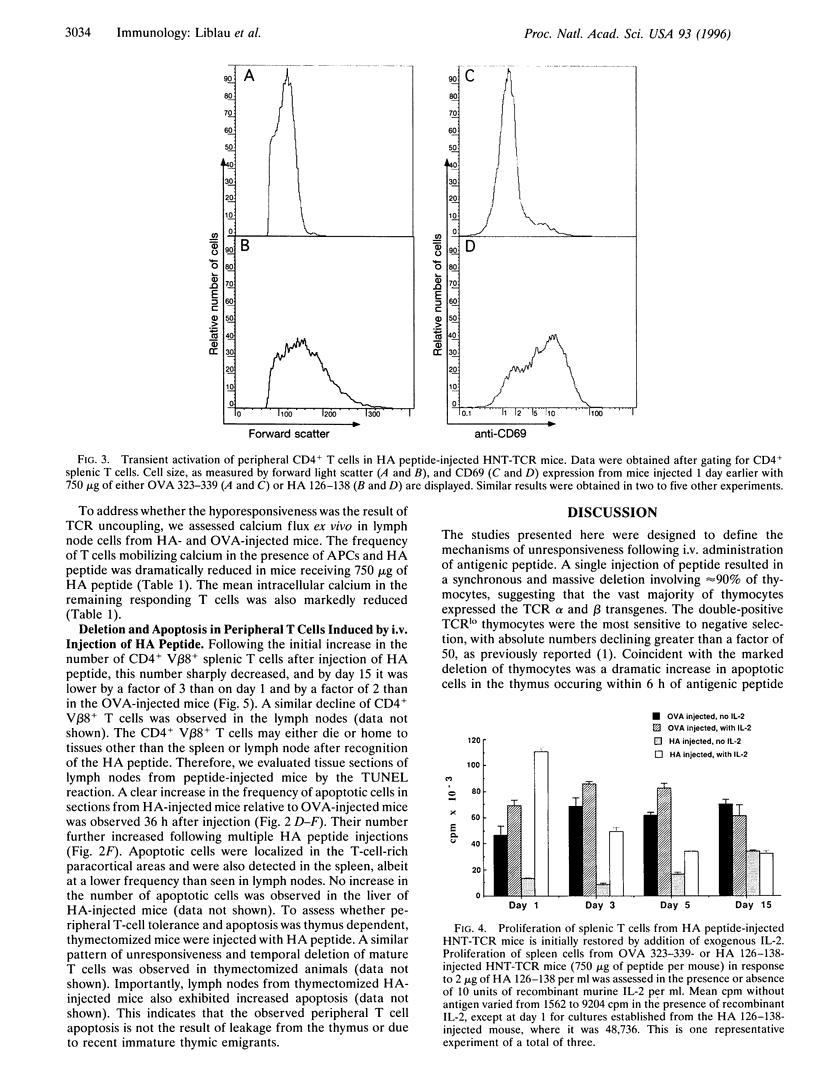
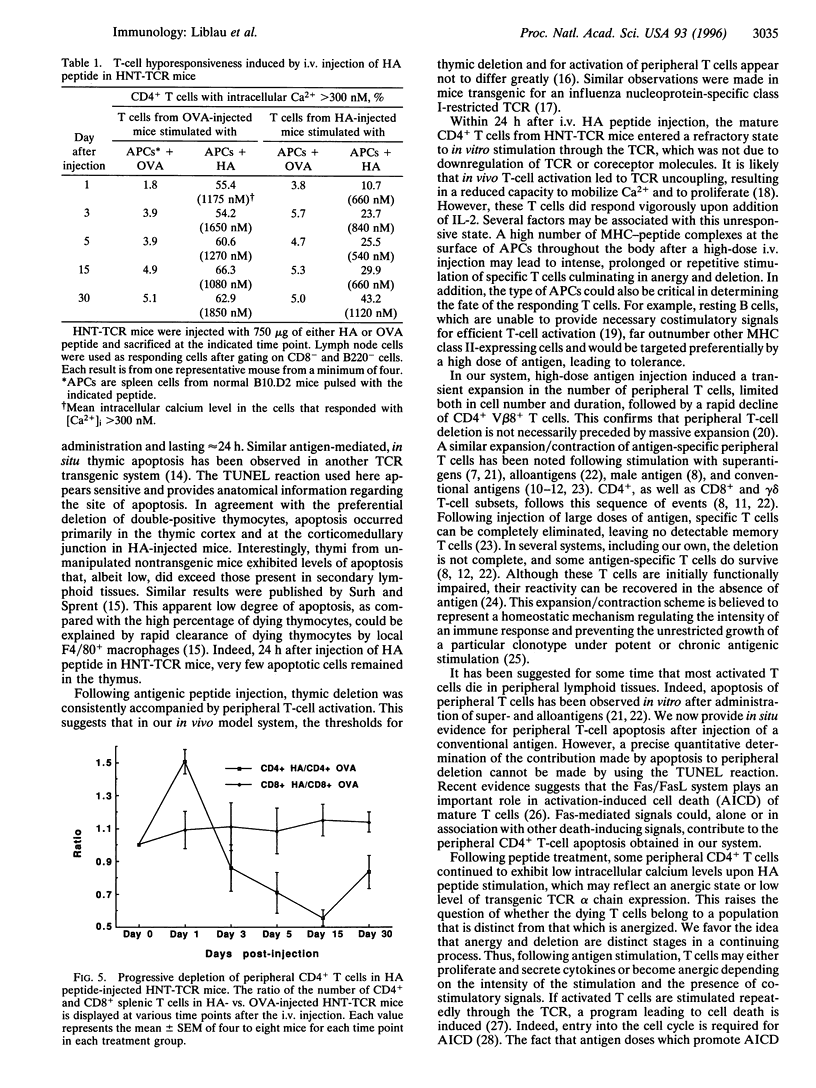

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackman M. A., Finkel T. H., Kappler J., Cambier J., Marrack P. Altered antigen receptor signaling in anergic T cells from self-tolerant T-cell receptor beta-chain transgenic mice. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6682–6686. doi: 10.1073/pnas.88.15.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme S. A., Lenardo M. J. Propriocidal apoptosis of mature T lymphocytes occurs at S phase of the cell cycle. Eur J Immunol. 1993 Jul;23(7):1552–1560. doi: 10.1002/eji.1830230724. [DOI] [PubMed] [Google Scholar]
- Burkly L. C., Lo D., Kanagawa O., Brinster R. L., Flavell R. A. T-cell tolerance by clonal anergy in transgenic mice with nonlymphoid expression of MHC class II I-E. Nature. 1989 Nov 30;342(6249):564–566. doi: 10.1038/342564a0. [DOI] [PubMed] [Google Scholar]
- Burstein H. J., Abbas A. K. In vivo role of interleukin 4 in T cell tolerance induced by aqueous protein antigen. J Exp Med. 1993 Feb 1;177(2):457–463. doi: 10.1084/jem.177.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchfield J. M., Racke M. K., Zúiga-Pflücker J. C., Cannella B., Raine C. S., Goverman J., Lenardo M. J. T cell deletion in high antigen dose therapy of autoimmune encephalomyelitis. Science. 1994 Feb 25;263(5150):1139–1143. doi: 10.1126/science.7509084. [DOI] [PubMed] [Google Scholar]
- Eynon E. E., Parker D. C. Small B cells as antigen-presenting cells in the induction of tolerance to soluble protein antigens. J Exp Med. 1992 Jan 1;175(1):131–138. doi: 10.1084/jem.175.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämmerling G. J., Schönrich G., Ferber I., Arnold B. Peripheral tolerance as a multi-step mechanism. Immunol Rev. 1993 Jun;133:93–104. doi: 10.1111/j.1600-065x.1993.tb01511.x. [DOI] [PubMed] [Google Scholar]
- Kawabe Y., Ochi A. Programmed cell death and extrathymic reduction of Vbeta8+ CD4+ T cells in mice tolerant to Staphylococcus aureus enterotoxin B. Nature. 1991 Jan 17;349(6306):245–248. doi: 10.1038/349245a0. [DOI] [PubMed] [Google Scholar]
- Kearney E. R., Pape K. A., Loh D. Y., Jenkins M. K. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994 Jul;1(4):327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Blüthmann H., Staerz U. D., Steinmetz M., von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988 Jun 23;333(6175):742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- Kyburz D., Aichele P., Speiser D. E., Hengartner H., Zinkernagel R. M., Pircher H. T cell immunity after a viral infection versus T cell tolerance induced by soluble viral peptides. Eur J Immunol. 1993 Aug;23(8):1956–1962. doi: 10.1002/eji.1830230834. [DOI] [PubMed] [Google Scholar]
- Lenardo M. J. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 1991 Oct 31;353(6347):858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- Mamalaki C., Norton T., Tanaka Y., Townsend A. R., Chandler P., Simpson E., Kioussis D. Thymic depletion and peripheral activation of class I major histocompatibility complex-restricted T cells by soluble peptide in T-cell receptor transgenic mice. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11342–11346. doi: 10.1073/pnas.89.23.11342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. E., Callahan J. E., Kappler J., Marrack P. C. Profound deletion of mature T cells in vivo by chronic exposure to exogenous superantigen. J Immunol. 1993 May 1;150(9):3785–3792. [PubMed] [Google Scholar]
- Moskophidis D., Lechner F., Pircher H., Zinkernagel R. M. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993 Apr 22;362(6422):758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- Murphy K. M., Heimberger A. B., Loh D. Y. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990 Dec 21;250(4988):1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- Nagata S., Golstein P. The Fas death factor. Science. 1995 Mar 10;267(5203):1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- Ohashi P. S., Oehen S., Buerki K., Pircher H., Ohashi C. T., Odermatt B., Malissen B., Zinkernagel R. M., Hengartner H. Ablation of "tolerance" and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991 Apr 19;65(2):305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- Pircher H., Bürki K., Lang R., Hengartner H., Zinkernagel R. M. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989 Nov 30;342(6249):559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- Pircher H., Rohrer U. H., Moskophidis D., Zinkernagel R. M., Hengartner H. Lower receptor avidity required for thymic clonal deletion than for effector T-cell function. Nature. 1991 Jun 6;351(6326):482–485. doi: 10.1038/351482a0. [DOI] [PubMed] [Google Scholar]
- Radvanyi L. G., Mills G. B., Miller R. G. Religation of the T cell receptor after primary activation of mature T cells inhibits proliferation and induces apoptotic cell death. J Immunol. 1993 Jun 15;150(12):5704–5715. [PubMed] [Google Scholar]
- Rocha B., Tanchot C., Von Boehmer H. Clonal anergy blocks in vivo growth of mature T cells and can be reversed in the absence of antigen. J Exp Med. 1993 May 1;177(5):1517–1521. doi: 10.1084/jem.177.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha B., von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991 Mar 8;251(4998):1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- Scott B., Liblau R., Degermann S., Marconi L. A., Ogata L., Caton A. J., McDevitt H. O., Lo D. A role for non-MHC genetic polymorphism in susceptibility to spontaneous autoimmunity. Immunity. 1994 Apr;1(1):73–83. doi: 10.1016/1074-7613(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Spaner D., Migita K., Ochi A., Shannon J., Miller R. G., Pereira P., Tonegawa S., Phillips R. A. Gamma delta T cells differentiate into a functional but nonproliferative state during a normal immune response. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8415–8419. doi: 10.1073/pnas.90.18.8415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh C. D., Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994 Nov 3;372(6501):100–103. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- Webb S., Morris C., Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990 Dec 21;63(6):1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- Weigle W. O. Immunological unresponsiveness. Adv Immunol. 1973;16:61–122. doi: 10.1016/s0065-2776(08)60296-5. [DOI] [PubMed] [Google Scholar]




