Abstract
Treatment of water at the household level offers a promising approach to combat the global burden of diarrheal diseases. In particular, chlorination of drinking water has been a widely promoted strategy due to persistence of residual chlorine after initial treatment. However, the degree to which chlorination can reduce microbial levels in a controlled setting (efficacy) or in a household setting (effectiveness) can vary as a function of chlorine characteristics, source water characteristics, and household conditions. To gain more understanding of these factors, we carried out an observational study within households in rural communities of northern coastal Ecuador. We found that the efficacy of chlorine treatment under controlled conditions was significantly better than its effectiveness when evaluated both by ability to meet microbiological safety standards and by log reductions. Water treated with chlorine achieved levels of microbial contamination considered safe for human consumption after 24 hours of storage in the household only 39 – 51% of the time, depending on chlorine treatment regimen. Chlorine treatment would not be considered protective against diarrheal disease according to WHO log reduction standards. Factors that explain the observed compromised effectiveness include: source water turbidity, source water baseline contamination levels, and in-home contamination. Water in 38% of the households that had low turbidity source water (< 10 NTU) met the safe water standard as compared with only 17% of the households that had high turbidity source water (> 10 NTU). A 10 MPN/100mL increase in baseline E. coli levels was associated with a 2.2% increase in failure to meet the E. colistandard. Higher mean microbial contamination levels in 54% of household samples in comparison to their matched controls, which is likely the result of in-home contamination during storage. Container characteristics (size of the container mouth) did not influence chlorine effectiveness. We found no significant differences between chlorine treatment regimens in ability to meet the safe water standards or in overall log reductions, although chlorine dosage did modify the effect of source conditions. These results underscore the importance of measuring both source water and household conditions to determine appropriate chlorine levels, as well as to evaluate the appropriateness of chlorine treatment and other point-of-use water quality improvement interventions.
Keywords: Water quality, household water treatment, chlorination, sodium hypochlorite, Ecuador, diarrhea
1. INTRODUCTION
Consumption of contaminated water is a major cause diarrheal diseases, which each year claim the lives of 700,000 children under five (Walker et al. 2013). Many global regions lack the infrastructure to provide clean piped water to their entire population and water treatment at the household level is therefore a common practice (Rosa and Clasen 2010). This drinking water is often stored in the home prior to consumption, which has been shown to be associated with in-home contamination due to poor storage and hygiene practices (Wright et al. 2004). Water treatment at the point-of-use (POU) combined with safe water storage practices have therefore increasingly been promoted worldwide as a promising intervention strategy (Rosa and Clasen 2010, Clasen 2009, Mintz et al. 1995). Household water treatment and safe storage interventions can lead to reductions in diarrheal disease (Gundry et al. 2004).
Treatment of water by chlorination is low-cost, widely available, and simple to use. As a POU option it eliminates the majority of waterborne pathogens and can limit in-home contamination through the persistence of free chlorine in stored water (WHO 2008). Chlorination is promoted by the World Health Organization (WHO) and the United States Centers for Disease Control and Prevention (CDC) for household water treatment, and comprises the most common form of water treatment, after boiling, in areas lacking clean piped water (Rosa and Clasen 2010).
While chlorination is highly efficacious in reducing microbial contamination of water under laboratory conditions, its ability to improve water safety within a village setting can vary as a function of source water characteristics (e.g. turbidity, pH and temperature), and chlorine characteristics (e.g. concentration and dose) (WHO 2008, Berry et al. 2008, Lantagne 2008, LeChevallier et al. 1981). In addition, effectiveness of chlorination may vary under household conditions as a function of local cultural practices and storage methods.
We carried out an observational study of POU water treatment with sodium hypochlorite (NaOCl) solution (common household bleach) in order to understand factors that affect its ability to improve microbial water quality. The objective of this study was to compare efficacy (defined as microbial reductions under controlled conditions) versus effectiveness (defined as microbial reductions under household conditions) of chlorination after 24 hours of storage. This is important because the ultimate impact of an intervention depends on how effective it is in practice, not just how efficacious it is in a laboratory setting.
The effectiveness of POU interventions is often assessed through intervention trials, which are considered the gold standard for study design. However, because such intervention trials are designed to compare outcomes in households with and without an intervention they are seldom powered to isolate the effects of household conditions that may lead to reduced effectiveness among those with the intervention. In this observational study we compared chlorine effectiveness in households in three different treatment arms, and concurrently compare these values for household effectiveness versus those for laboratory efficacy under controlled conditions (a field laboratory within the village setting). Thus, the same water was evaluated under both control and household conditions. In addition, sampling of drinking water sources for each household in real-time allowed us to understand source-level factors impacting effectiveness of chlorine treatment in the household.
This research follows a previous study that we carried out in the same region focused on household chlorine use in which we observed no significant differences in log reductions of Escherichia coli between drinking water of households that reported chlorination of their water and those that reported no water treatment (McLaughlin et al. 2009). However, in that study we relied on self-reported treatment and chlorine dosage levels were not measured. In a commentary, Lantagne (2010) pointed out that without quality-control testing of the locally available bleach and an appropriate dosage regime, it was not possible to discern whether the lack of chlorine residual and microbiologic improvement in study households’ treated water was due to poor quality product, incorrect dosage, or inaccurate household reporting. Therefore, in this study we 1) tested chlorine concentrations 2) directly observed or controlled household dosing practices, and 3) gathered information on source water characteristics and household water storage practices.
In our assessment of household water quality, we evaluated both log reductions and ability to achieve WHO safe water standards after 24 hours of storage because the two provide distinct pieces of information. Safety levels at the household ultimately determine whether individuals have the potential to become exposed to waterborne pathogens, whereas log reductions provide a measure of the effectiveness of the intervention. Both outcomes are important to understand, and provide different insights about the impact of POU treatment.
2. MATERIAL & METHODS
2.1 Study Area Description and Village Selection
Research for this study was conducted in seven villages along the Santiago-Cayapas-Onzole river system on the northern coast of Ecuador, in Esmeraldas Province. Villages were selected to maximize the quantity of households that had reported past chlorine use in an ongoing study of diarrheal disease transmission in the region. Characteristics of each of the study villages are shown in Table 1.
Table 1.
Characteristics of Study Communities. HH = Household; NT = No Treatment; LC = Local Chlorine; CC = Commercial Chlorine. Protocol changes between field sessions A and B are described in the text.
| Communit y Number |
Population | Total # HHs in Community |
% of HHs Previously Reporting Chlorine Use |
Water Sources Used |
# of HHs in Treatment Group |
Field Session |
|---|---|---|---|---|---|---|
| 1 | 94 | 18 | 19.0% | Rain (100%) | NT=1 LC=2 CC=2 |
A |
| 2 | 332 | 67 | 11.9% | Tap (93%) River (7%) |
NT=12 LC=3 |
A |
| 3 | 316 | 74 | 17.4% | Rain (47%) River (37%) Well (17%) |
NT=16 LC=9 CC=5 |
A |
| 4 | 148 | 33 | 14.3% | River (62%) Rain (38%) |
NT=6 LC=2 CC=1 |
A |
| 5 | 453 | 102 | 18.8% | River (100%) | NT=11 LC=3 CC=4 |
B |
| 6 | 942 | 212 | 30.5% | River (30%) Tap (10%) Well (60%) |
NT=8 LC=9 CC=3 |
B |
| 7 | 371 | 77 | 30.8% | River (100%) | NT=12 LC=13 CC=16 |
B |
2.2 Household Selection and Treatment Group Assignment
Households in the study villages were assigned to one of three study arms: (1) No water treatment; (2) Water treatment with locally available chlorine; and (3) Water treatment with commercially available chlorine. We included two different treatment arms to differentiate between the issues of poor quality product and/or incorrect dosing (Group 2) versus reduced effectiveness due to other household factors (Group 3).
Households in Group 2, the "local chlorine" arm, either used chlorine they had available at home or chlorine purchased from local vendors that we provided them. These vendors purchase concentrated NaOCl, which they dilute and re-sell in plastic containers. In this group, members of the household or the local health promoter determined the dosage, without any instruction from the research team. Households in Group (3), the "commercial chlorine" arm, used NaOCl that we provided to them (Ajax brand), with a dosage of 1.875 mg/L, as recommended by the CDC Safe Water System program for non-turbid waters (Lantagne 2008). We calculated the appropriate dosage based on the volume of water in the container, and used a pipette to measure the chlorine product and dose the water. NaOCl concentrations were determined for both local and commercially available chlorine with a Hach Digital Titrator (Loveland, CO). All equipment was calibrated prior to each field session. Information on concentration, dosing, and residual levels of NaOCl in the two treatment arms is shown in Table 2.
Table 2.
Sodium Hypochlorite (NaOCl) Concentrations, Dosage, and Residual in Local versus Commercial Chlorine Treatment Study Arms. P-values report on two-sided t-ests comparing local versus commercial study arms.
| Mean [NaOCl] of product (%) |
Mean vol. of H2O dosed (L) |
Mean vol. of NaOCl used (mL) |
Mean NaOCl dosage (mg/L) [range] |
Mean NaOC1 residual after 24 hrs of storage (Household) (mg/L) |
Mean NaOC1 residual after 24 hrs of storage (Control) (mg/L) |
|
|---|---|---|---|---|---|---|
| Local | 2.2 ± 0.73 | 32.5 ± 52.4 | 4.44 ± 7.16 | 4.52 ± 3.75 [0.9-15] |
Free: 1.31 ± 2.93 Total: 1.64 ± 3.80 |
Free: 1.67 ± 2.64 Total: 1.89 ± 2.98 |
| Commercial | 4.5 ± 0.00 | 16.6 ± 16.3 | 0.81 ± 0.78 | 2.24 ± 0.87 [1.95-6.75] |
Free: 0.22 ± 0.16 Total: 0.33 ± 0.21 |
Free: 0.34 ± 0.53 Total: 0.40 ± 0.54 |
| p<0.0001 | p=0.1166 | p=0.0076 | p=0.0023 | Free: p=0.0476
Total: p=0.0645 |
Free: p=0.0109
Total: p=0.0113 |
To assign households to study arms, we utilized data from household surveys carried out in an ongoing study between 2003-2009. Households that had previously reported treating their water with chlorine were assigned to one of the two treatment groups, and households that had never reported chlorine use were assigned to the control group. In smaller communities we enrolled all willing households according this scheme, and in larger communities, we employed block randomization to select control households for enrollment, to ensure spatial distribution of enrolled households across the village. Unfortunately, previous reports of past chlorine usage did not always align with use of chlorine as a form of water treatment at the time of study, and some households opposed their group assignment. We therefore altered treatment group assignments in order to successfully enroll study households. Households that had previously reported chlorine use that did not want to treat their water with chlorine were enrolled instead in the no-treatment arm of the study. Households that had not reported past chlorine use but had recently used chlorine as a form of water treatment were assigned to the local chlorine arm. Households that expressed interest in chlorine treatment but did not have chlorine at home or a working knowledge of correct dosage were enrolled into the commercial chlorine arm. Lack of randomized sampling is a limitation of the study, but we compared household-level characteristics and found no significant differences between treatment groups with respect to percent of households with access to an improved water source, improved sanitation, education, household crowding, job security, or socioeconomic status (all p-values>0.05).
2.3 Sampling Methods
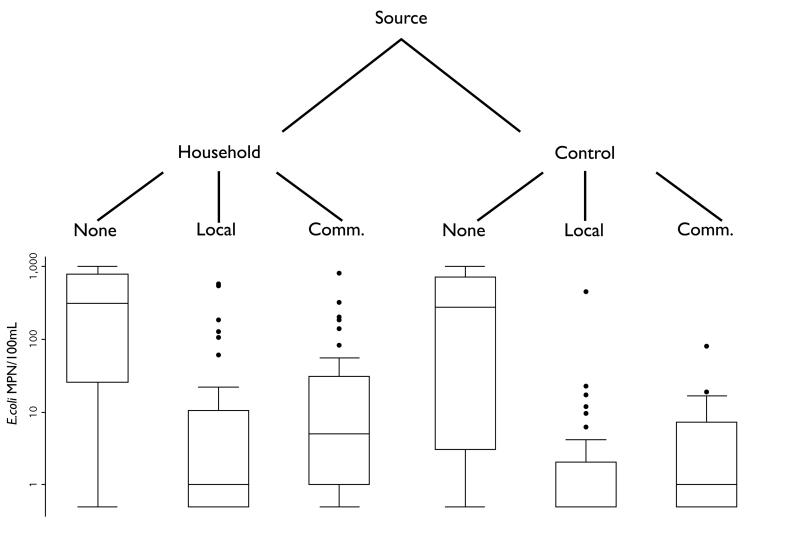
Each household was visited three times, first for household enrollment, second at the time of source water collection, and third for a follow-up visit after 24 (±3) hours of storage. At the time water was collected from the source during the second visit, at which time we also filled a container (20L plastic jerry can) at the same source from which the household members filled their water containers. These control containers were filled in a manner consistent with the household's collection method and stored in our field laboratory, where conditions were similar to household conditions, yet capped and left undisturbed. If the household was assigned to either chlorine arm of the study the household and control containers were dosed comparably and simultaneously. Households were instructed to use the water as they normally would but to save some for the research team to sample on the following day. After 24 (±3) hours we collected a sample from each container of stored water (household and control) after agitating the container, either by shaking or stirring with a sterilized metal pole (when containers were too large to pick up). Figure 1 shows a schematic of the study design.
Figure 1.
E. coli MPN/100mL after 24 (±3) hours of storage (log-scale axis) under the different treatments of the study. Water was collected at the source and stored in either household or control conditions. Stored water received one of three treatments: None, locally available chlorine dosed by the household member (“Local”), or commercial chlorine dosed by the researchers (“Comm”).
Data collection took place over two field sampling sessions: (A) June 5 – June 20, and (B) July 3 – July 20, 2010. During Field Session A, 10L of control water was collected from river and tap water sources; but for rainwater sources only 3-10L (mean: 5L) of rainwater was collected, due to the limited amount of rainwater that households were storing. Because of the limited volumes available, for Field Session B, 20L of control water was collected and households that could not give 20L of water for a control were excluded. This excluded all rainwater sources.
Households were excluded if they did not store water in the home for at least 24 hours, if they could not provide locally collected water for the control container, if they were unavailable for the three consecutive days needed to participate in the study, if water was added to the container after collection, or if insufficient water was left in the container after 24 hours. In Field Session A this level was defined as <100 mL and in Field Session B as less than half of the original volume of water in the container.
After samples were taken from the control storage containers the contents were drained and the jerry cans were washed with detergent and available water. The control containers were also washed with chlorine between villages.
2.4 Data Collection on Covariates of Interest
We collected data on covariates of interest during household visits for enrollment, source water collection, and 24-hour follow-up. Container-level characteristics included size of opening of mouth, presence of lid, and visible contamination of the container. Household-level characteristics included socioeconomic status, education, job security, and household crowding. Source-level characteristics included turbidity, temperature, pH, and conductivity. Further details on these variables is provided in the supplemental online material. All interaction with human subjects was approved by the Institutional Review Board at the University of Michigan, Emory University, and Universidad San Francisco de Quito.
2.5 Laboratory Analysis
Samples were collected in WhirlPak bags (Nasco, Fort Atkinson, WI; bags pre-packed with sodium thiosulfate tablets were used for chlorine-treated water), placed on ice and analyzed within eight hours of collection. For water treated with NaOCl an additional 50mL sample was taken to test both free and total residual chlorine, performed with a LaMotte 1200 Colorimeter (Chestertown, MD).
Samples were processed in a field laboratory set up inside a health dispensary or a resident's home, depending on the community. E. coli MPN per 100mL was assessed using the IDEXX Quantitray 2000 system (Westbrook, ME). Trays were incubated for at least 24h at 37.5°C (+/−3°C). Serial dilutions were not possible, and an upper limit of detection of 1011.2 MPN/100mL was used because the large cell on the Quantitray was not counted. The lower limit of detection was 1 MPN/100mL. Laboratory quality control procedures are described in the supplementary online material.
2.6 Data Management and Statistical Analysis
Both the U.S. Environmental Protection Agency (US EPA) and the WHO recommend that E. coli and thermotolerant coliform bacteria must not be detectable in any 100mL sample of drinking water (USEPA 2006, WHO 2011a). We therefore used logistic regression models to evaluate factors affecting whether a given sample failed to achieve safe levels (E. coli <1 MPN/100mL) after 24 hours. In addition to understanding whether household drinking water is achieving safe levels of microbial contamination, it is important to understand the reduction in microbial loads resulting from chlorine treatment. We therefore calculated log reductions between the source and storage container and modeled the impact of the same factors described above on log reduction outcomes, using linear regression.
Because of the limitations of the IDEXX system and our logistical ability to carry out multiple dilutions on our samples, our results included a number of non-detect values (75/418, 17.9%) and values at the upper limit of detection (43/418, 10.3%). This left- and right-censoring affects the accuracy of our log reduction calculations.
A value of 0.5 MPN/100mL, halfway between zero and the lower detection limit of 1 MPN/100mL, was used in the place of zero values in order to calculate log differences. All analyses were carried out in Stata 10.1 (Stata Corp, College Station, TX).
3. RESULTS
We analyzed a total of 418 samples from 138 households in seven communities. Of the households sampled, 66 were in Group 1 (no chlorine treatment), 41 were in Group 2 (“local chlorine” treatment), and 31 were in Group 3 (“commercial chlorine” treatment). The source waters displayed a range of turbidities and baseline contamination levels across the different sources sampled (Table S1).
3.1 Efficacy and Effectiveness of Water Treatment
After 24 hours of storage, the overall concentrations of E. coli in water were significantly different between the non-treatment and treatment groups in both household and control settings (p<0.0001) (Table 3 & Figure 1).
TABLE 3.
Water Sample Characteristics after 24 hours of storage. P-values report on paired one-sided t-tests comparing the E. coli Most Probable Number (MPN)/100mL for household versus control samples.
| Sample Type | Geometric mean E. coli MPN/100mL (95% CI) |
Average E. coli
log reduction from source (Range) |
% of Samples <1 MPN/l00mL E. coli |
% of Samples with free chlorine residual 0.2-2.0 ppm |
|---|---|---|---|---|
| NO TREATMENT GROUP (n=66) | ||||
| Source | 111.4 (57.4-216.1) |
N/A | 13.6% | N/A |
| Household | 121.5 (67.9-217.2) |
N/A | 9.1% | N/A |
| Control | 59.4 (29.5-119.9) |
N/A | 18.2% | N/A |
| p-value | 0.0765 | N/A | N/A | N/A |
| LOCAL CHLORINE TREATMENT GROUP (n=41) | ||||
| Source | 154.6 (67.8-352.6) |
N/A | 12.2% | 2.2% |
| Household | 3.1 (1.5-6.4) |
1.70 (−0.91 – 3.31) |
51.2% | 35.0% |
| Control | 1.1 (0.7-1.9) |
2.15 (−1.65 - 3.31) |
73.2% | 44.7% |
| p-value | 0.0241* | 0.0012* | ||
| COMMERCIAL CHLORINE TREATMENT GROUP (n=31) | ||||
| Source | 273.8 (116.2-644.9) |
N/A | 6.5% | 0% |
| Household | 6.9 (3.0-15.8) |
1.53 (−1.69 – 3.00) |
38.7% | 53.3% |
| Control | 2.1 (1.2-3.7) |
2.06 (−2.21-3.31) |
51.6% | 42.9% |
| p-value | 0.0268* | 0.0008* | ||
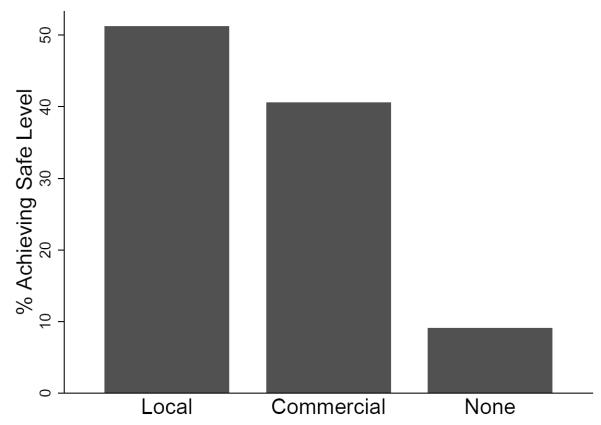
A greater proportion of samples from water stored in households in the local chlorine group versus the commercial chlorine group achieved safe (<1 E. coli MPN/100mL) status after 24 hours of storage in the household (51% vs. 39%; p=0.291) and control (73% vs. 52%; p=0.059) settings, but overall contamination levels of stored water and log reductions achieved were not significantly different between the local and commercial chlorine treatment groups (Table 3, Figure 2a).
Figure 2.
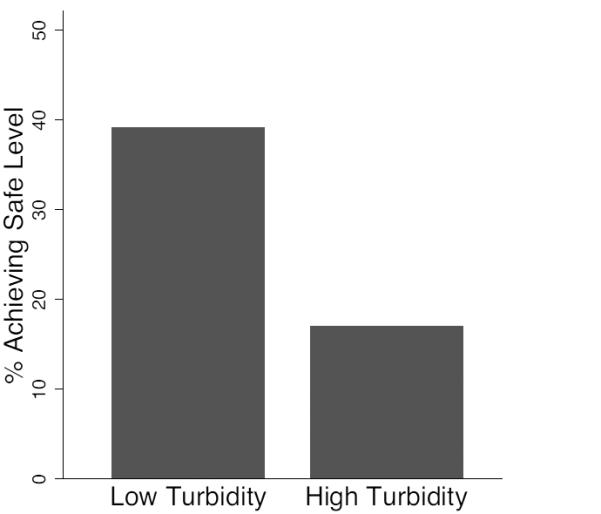
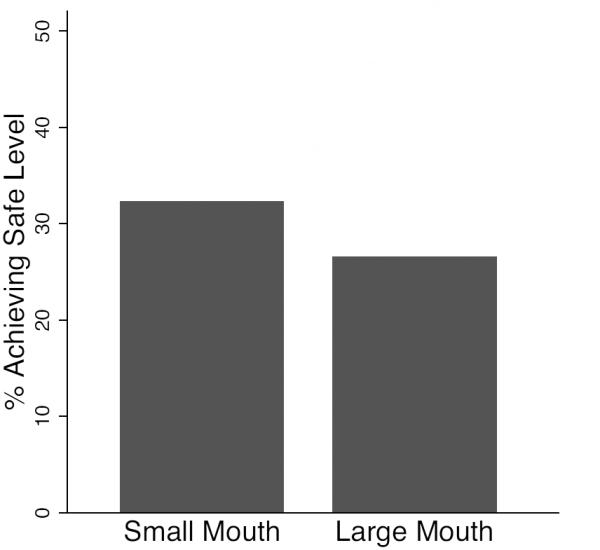
Proportion of samples achieving safe or low risk status after 24 (±3) hours of household storage, comparing samples from a) chlorine treatment groups, and b) high (≥10 nephelometric turbidity units - NTU) vs. low (<10 NTU) turbidity source water, and c) small (≤8cm) versus large (>8cm) mouthed containers. ≥10 NTU
Concentrations of the commercial chlorine product were on average twice as high as the local chlorine product. However, dosage was estimated to be approximately twice as high in the local chlorine treatment arm on average per volume of treated water. Individuals in households likely dosed at higher concentrations in the presence of observers, so this bias may result in a higher estimated effectiveness of chlorine treatment in the household. The mean chlorine residual for both groups was within the recommended guidelines of 0.2-2.0 mg/L, but was substantially higher in the local chlorine group (Table 2).
Log reductions of E. coli averaged 1.70 (−0.91 – 3.31) for the local chlorine treatment group and 1.53 (−1.69 – 3.00) for the commercial chlorine treatment group. The WHO (2011b) recommends that POU disinfection achieve ≥4 log10 reduction or ≥2 log10 reduction in bacteria to be “highly protective” or “protective” in meeting the health-based targets of 10−6 DALY or 10−4 DALY per person per year, respectively. According to these criteria, POU water treatment with chlorine in this study would therefore not be considered protective in limiting drinking water disease burden.
For both treatment groups, geometric mean concentrations of E. coli were significantly higher, log reductions were significantly lower, and fewer samples met safe water status in the household versus the control setting (Table 3). Higher variance was also observed in the household setting (Figure 1), likely as a result of in-home contamination (defined as control – household sample > 0 E .coli MPN/100mL), which occurred in 54% of households.
3.2 Factors Affecting Treatment Effectiveness
For household water treatment, the WHO classifies high turbidity as >10 nephelometric turbidity units (NTU), and recommends a double-dosage of chlorine at these levels (WHO 2011a, p. 141). Water stored in households achieved a safe outcome more frequently for source waters with low (<10 NTU) versus high ≥10 NTU) turbidity (p=0.007) (Figure 2b).
A greater proportion of samples from water stored in containers with small (≤8cm) openings (31%) versus large (>8cm) openings (26%) achieved safe status after 24 hours of storage, but the difference was not statistically significant (p=0.355) (Figure 2c). In-home contamination was apparent in 47% of households using small-mouthed containers versus 60% of households using large-mouthed containers, but again this difference was not statistically significant (p=0.123).
We were primarily interested in the impact of source water characteristics, including baseline contamination levels and turbidity of source water. These two variables exhibited a high degree of collinearity (Pearson Correlation Coefficient = 0.614, p-value <0.0001), and we found no evidence for confounding when including them together in a multivariate model, so we considered the effects of each independently. Results of logistic regression models showed that both higher baseline E. coli concentrations and higher turbidity levels at the source were associated with increased odds of failing to meet safe water quality standards after 24 hours of storage (Table 4). Every 10-unit increase in baseline E. coli MPN/100mL at the source was associated with a 2.2% (95% CI: 0.9 - 3.6%) increase in failure to meet the E. coli standard. Every one-unit increase in turbidity at the source was associated with a 4.0% (95% CI: 1.1-7.1%) increase in failure to meet the E. coli standard.
TABLE 4.
Results of univariate logistic regression models testing the effect of factors associated with the odds of NOT meeting the safe water standard of E. coli <1 MPN/l00mL. Results are presented collectively and stratified by chlorine treatment group. MPN = Most Probable Number; NTU = Nephelometric Turbidity Units.
| OR | Lower 95% C.I. |
Upper 95% C.I. |
p-value | |
|---|---|---|---|---|
|
Baseline E. coli
(unit increase of 10 MPN/l00mL) | ||||
| Overall (n=70) | 1.022 | 1.009 | 1.036 | 0.001 * |
|
Local Chlorine
(n=40) |
1.013 | 0.996 | 1.029 | 0.134 |
|
Commercial
Chlorine (n=29) |
1.037 | 1.012 | 1.062 | 0.003 * |
|
Turbidity
(unit increase of 1 NTU) | ||||
| Overall (n=72) | 1.040 | 1.010 | 1.071 | 0.009 * |
|
Local Chlorine
(n=41) |
1.017 | 0.984 | 1.052 | 0.309 |
|
Commercial
Chlorine (n=31) |
1.093 | 1.021 | 1.170 | 0.010 * |
We also explored the impact of the size of the opening of the container, because of previous work suggesting that this is an important factor for in-home contamination (Mintz et al. 1995, Levy et al. 2008). In addition, we examined the effect of other covariates of interest by adding them one by one to the primary baseline E. coli and turbidity models. We examined both size of container opening and chlorine treatment group as effect modifiers of the relationship of source water conditions and failure to meet the safe water standards. We did not find evidence for effect modification when stratifying our analysis by small versus large opening (Table S2), but we did find that chlorine treatment group modified the outcome. When stratified by treatment group, the effect of both baseline contamination levels and turbidity of source water increased for the commercial chlorine group, whereas the effect decreased and was not statistically significant for the local chlorine group (Table 4). None of the other covariates considered modified the results.
Results of linear regression models showed that higher baseline E. coli concentrations at the source were associated with increased log reductions after 24 hours of storage in the household, but higher turbidity levels were not. Every 10-unit increase in baseline E. coli MPN/100mL at the source was associated with a 1.1% (95% CI: 0.05 – 1.79%) increased log reduction of E. coli. When stratified by treatment group, the effect size of baseline contamination levels increased for the local chlorine group, whereas the effect size decreased and was not statistically significant for the commercial chlorine group (Table 5).
TABLE 5: Results of univariate linear regression models testing the effect of factors associated with log reductions in E. coli concentrations between the source and the point of use after 24 (±3) hours of storage. Results are presented collectively and stratified by chlorine treatment group. MPN = Most Probable Number; NTU = Nephelometric Turbidity Units.
| Coeff | Lower 95% C.I. |
Upper 95% C.I. |
p-value | |
|---|---|---|---|---|
|
Baseline E. coli
(unit increase of 10 MPN/100mL) | ||||
| Overall (n=68) | 0.011 | 0.005 | 0.018 | 0.001 * |
|
Local Chlorine
(n=39) |
0.0160 | 0.007 | 0.025 | 0.001 * |
|
Commercial
Chlorine (n=29) |
0.007 | −0.003 | 0.017 | 0.145 |
|
Turbidity
(unit increase of 1NTU) | ||||
| Overall (n=67) | 0.006 | −0.009 | 0.213 | 0.424 |
|
Local Chlorine
(n=39) |
0.010 | −0.010 | 0.031 | 0.319 |
|
Commercial
Chlorine (n=28) |
0.002 | −0.024 | 0.028 | 0.872 |
4. DISCUSSION
This controlled observational study demonstrates that the effectiveness of chlorination can be compromised under household conditions. We found that the efficacy of chlorine treatment under controlled conditions was significantly better than its effectiveness when evaluated both by log reductions and by ability to meet microbiological safety standards (Table 3, Figure 1). After 24 hours of storage in the household, safe water was achieved in only 39-51% of households (Figure 2a, Table 3), and households achieved recommended residual levels of chlorine only 35-53% of the time. Chlorine treatment would not be considered protective against diarrheal disease according to WHO log reduction standards.
The results also underscore the importance of evaluating source water in addition to household conditions when implementing chlorine treatment and other point-of-use water quality improvement interventions. Factors related to compromised household effectiveness include both in-home contamination and source water conditions, including baseline contamination levels and turbidity.
4.1 Source Water Baseline Contamination Levels
We hypothesized that the ability to effectively treat water with chlorine would be affected by baseline concentrations of E. coli because chlorine might be used up more quickly with more contaminated source water. We found that higher baseline E. coli concentrations at the source were associated with increased log reductions (Table 5) but decreased household drinking water safety. A 10 MPN/100mL increase in baseline E. coli levels was associated with a 2.2% increase in failure to meet the E. coli standard. Because source water conditions can impact the effectiveness of chlorination, it is important to consider these baseline conditions when implementing a POU chlorination intervention. The log reductions recommended for POU disinfection by the WHO (2011b) are based on assumptions of baseline water quality of one pathogenic bacterial organism per liter, but baseline water quality varies by location, season, and other conditions.
4.2 Source Water Turbidity
Turbidity is known to affect the action of chlorine (LeChevallier et al. 1981, Crump et al. 2004), so we also hypothesized that higher turbidity would be associated with reduced effectiveness of chlorine. We found that the water in 38% of the households that had low turbidity source water (< 10 NTU) met the safe water standard as compared with only 17% of the households that had high turbidity source water (> 10 NTU) (Figure 2b). Log reductions were not affected by source turbidity levels. While actions such as increasing chlorine dosage, pre-filtering, or using a flocculant may improve chlorine effectiveness in more turbid waters, it is important to note that turbidity levels of 10 NTU are not visible by the naked eye. In our source samples, turbidity values averaged approximately 20 NTU, with a range from 0.31-113 NTU (Table S1). In practice, knowing when to use a double dose of chlorine, especially for many members of rural communities, could be quite challenging, especially given that turbidity and microbial contamination conditions are highly variable for surface source waters (see, for example Levy et al. 2009).
4.3 In-Home Contamination
Higher mean microbial contamination levels in household samples in comparison to their matched controls occurred in 54% of households, which is likely the result of in-home contamination during storage in the household. This compares with in-home contamination rates found by Levy et al. (2008) of 46% for E. coli in a separate study in the same region; in that study the majority of households did not use any water treatment. The in-home contamination occurring in about half of the households is likely a major cause of the higher observed variance under household versus control conditions.
The reduced effectiveness of chlorine treatment in the household context might also explain why we previously were unable to detect significant differences in log reductions between drinking water of households that reported chlorination of their water and those that reported no water treatment in our previous study in this region (McLaughlin et al. 2009). In that study we did not employ controls, a necessary design feature to estimate recontamination.
Because size of container opening has been suggested as an important factor leading to in-home contamination in previous studies (Mintz et al. 1995, Levy et al. 2008), we examined the impact of container mouth size on our safety and effectiveness outcomes. While we found slightly elevated percentages of containers with small mouths meeting the safety standards (Figure 2c), these differences were not significant, and we found no direct effects of mouth size in univariate analysis nor evidence that container opening modified the effect of source water conditions (Tables S2-S3). We also found no direct effects of other household container-level factors examined (whether the container was covered, storage location, safety of extraction method, and presence of visible contamination) in univariate analyses.
4.4 Impact of Chlorine Dosage
We evaluated three different chlorine dosage regimes: no water treatment, water treatment with locally available chlorine as dosed by community members, and water treatment with commercial chlorine at a prescribed dose of 1.875 mg/L, as recommended by the CDC Safe Water System program.
We found no significant differences between the two treatment groups in ability to meet the safe water standards (Figure 2a) or in overall log reductions. However, chlorine treatment group did modify the effect of source conditions. For the log reduction models, the impact of higher baseline contamination levels was more marked for the local chlorine (higher dosage) treatment group (Table 5). This is consistent with the result for the combined data, that higher reductions are achieved with higher baseline contamination levels. Higher chlorine doses are associated with improved log reduction values at higher baseline contamination levels. For the water safety models, the effects of both baseline E. coli and turbidity levels were exacerbated for the commercial chlorine (lower dosage) group, but reduced the effect of source water quality for the local chlorine (higher dosage) group (Table 4).
While we did not follow recommended guidelines to double the NaOCl dosage for high turbidity (>10 NTU) waters (Lantagne 2008, WHO 2011a) for the commercial chlorine treatment group, the local chlorine group can roughly be considered to have provided this double dose (Table 2). This analysis therefore supports the suggestion for doubling the recommended chlorine dose when treating waters with high turbidity, and also suggests that a double dose should also be applied when treating source waters with high baseline contamination levels. However, doubling the dose raises issues of taste acceptability as well as concerns about chlorine disinfection byproducts at higher dosage levels. It is also important to note that a substantial proportion (27%) of the water in the local chlorine group did not achieve safe levels even under laboratory conditions (Table 3).
5. CONCLUSIONS
Our results emphasize the importance of considering source water conditions when implementing a chlorine water treatment or other POU water treatment intervention. In areas relying on surface water sources, additional measures should be considered, such as introducing a pre-filtration step or addition of a flocculent (e.g., Islam et al. 2011) to reduce turbidity levels of source water where they are known to be >10 NTU. In addition, the wide ranges of dosing volumes and concentrations of locally available chlorine we observed in this study indicate that education on proper dosages and provision of a reliable quality chlorine product are important factors in achieving successful POU chlorination.
These results also underscore the need to evaluate POU water treatment techniques according to final safety levels of drinking water in the household, and not just by their potential to achieve log reductions in microbial contamination levels. Relying on log reduction data from laboratory studies (efficacy) likely overestimates the true impact of an intervention in the household context. In addition, using log reduction data from households (effectiveness) provides different information than that provided by ability to achieve safety standards.
Supplementary Material
HIGHLIGHTS.
The effectiveness of chlorine water treatment under household conditions was lower than laboratory efficacy
Only 39-51% of stored water was safe for consumption and only 35-53% achieved recommended residual levels of chlorine
Chlorine treatment was not protective against diarrhea by WHO standards
Point-of-use interventions should take source water conditions such as baseline contamination and turbidity into account
ACKNOWLEDGEMENTS
This work was supported by National Institute of Allergy and Infectious Diseases Grant #RO1AI050038, the Global Field Experiences program at the Emory University Rollins School of Public Health, and the Epidemiology program at the University of Michigan. Additional in-kind support was provided by a cooperative agreement between Emory University and the U.S. Centers for Disease Control and Prevention. We would like to thank Shannon Oliver and Denis David Tenorio for assistance with fieldwork. We would also like to thank the study participants, without whom this research would not have been possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- 1.Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, O'Brien KL, Campbell H, Black RE. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381:1405–1416. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosa G, Clasen T. Estimating the scope of household water treatment in low- and medium-income countries. Am J Trop Med Hyg. 2010;82(2):289–300. doi: 10.4269/ajtmh.2010.09-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright J, Gundry S, Conroy R. Household drinking water in developing countries: a systematic review of microbiological contamination between source and point-of-use. Tropical Medicine and International Health. 2004;9(1):106–117. doi: 10.1046/j.1365-3156.2003.01160.x. [DOI] [PubMed] [Google Scholar]
- 4.Clasen T. Scaling Up Household Water Treatment Among Low-Income Populations. World Health Organization; Geneva: 2009. [Google Scholar]
- 5.Mintz ED, Reiff FM, Tauxe RV. Safe Water Treatment and Storage in the Home. Journal of the American Medical Association. 1995;273(12):948–953. [PubMed] [Google Scholar]
- 6.Gundry S, Wright J, Conroy R. A systematic review of the health outcomes related to household water quality in developing countries. Journal of Water and Health. 2004;2(1):1–13. [PubMed] [Google Scholar]
- 7.WHO . Guidelines for Drinking-water Quality. Third World Health Organization; Geneva: 2008. [Google Scholar]
- 8.Berry D, Xi C, Raskin L. Effect of Growth Conditions on Inactivation of Escherichia coli with Monochloramine. Environmental Science & Technology. 2008;43:884–889. doi: 10.1021/es8017545. [DOI] [PubMed] [Google Scholar]
- 9.Lantagne D. Sodium hypochlorite dosage for household and emergency water treatment. Journal American Water Works Association. 2008;100(8):106–119. doi: 10.2166/wh.2017.012. [DOI] [PubMed] [Google Scholar]
- 10.LeChevallier MW, Evans TM, Seidler RJ. Effect of turbidity on chlorination efficiency and bacterial persistence in drinking water. Applied Environmental Microbiology. 1981;42(1):159–167. doi: 10.1128/aem.42.1.159-167.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLaughlin LA, Levy K, Beck NK, Shin G, Meschke SM, Eisenberg JN. An Observational Study of the Effectiveness of Point-Of-Use Chlorination. Journal of Environmental Health. 2009;71(8):48–53. [PMC free article] [PubMed] [Google Scholar]
- 12.Lantagne D. Comment on “An Observational Study of the Effectiveness of Point-Of-Use Chlorination”. Journal of Environmental Health. 2010;72(6):74–78. [PubMed] [Google Scholar]
- 13.USEPA List of Drinking Water Contaminants and MCLs: National Primary Drinking Water Regulations. 2006.
- 14.WHO . Guidelines for drinking-water quality. 4th Geneva, Switzerland: 2011a. [Google Scholar]
- 15.Levy K, Nelson KL, Hubbard A, Eisenberg JNS. Following the water: a controlled study of drinking water storage in northern coastal Ecuador. Environmental Health Perspectives. 2008;116(11):1533–1540. doi: 10.1289/ehp.11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO . Evaluating household water treatment options: Health-based targets and microbiological performance specifications. Geneva: 2011b. [Google Scholar]
- 17.Crump JA, Okoth GO, Slutsker L, Ogaja DO, Keswick BH, Luby SP. Effect of point-of-use disinfection, flocculation and combined flocculation-disinfection on drinking water quality in western Kenya. Journal of Applied MIcrobiology. 2004;97(1):225–231. doi: 10.1111/j.1365-2672.2004.02309.x. [DOI] [PubMed] [Google Scholar]
- 18.Levy K, Hubbard A, Nelson KL, Eisenberg JNS. Drivers of water quality variability in northern coastal Ecuador. Environmental Science & Technology. 2009;43(6):1788–1797. doi: 10.1021/es8022545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Islam MS, Mahmud ZH, Uddin MH, Islam K, Yunus M, Islam MS, Nair GB, Endtz HP, Sack DA. Purification of household water using a novel mixture reduces diarrhoeal disease in Matlab, Bangladesh. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2011;105:341–345. doi: 10.1016/j.trstmh.2011.03.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.