Abstract
African-American (AA) women have higher rates of breast cancer (BCa) mortality than Caucasian women, and a recent study using data from the Surveillance, Epidemiology and End Results (SEER) registry suggests that this disparity may be due, in part, to the poorer health status of AAs at diagnosis and not treatment related issues. Randomized controlled trials involving supervised aerobic and resistance exercise have shown improved body composition and improvement in cancer-related biomarkers in BCa patients and may lead to improved recurrence and survival rates; however, most trials have focused on Caucasians and many have been conducted in academic- and clinic-based settings. We evaluated the feasibility of conducting a 20-week, supervised, resistance training, group exercise intervention coupled with a support group and home walking program utilizing facilities and personnel at a community cancer support center (The Gathering Place, Beachwood, Ohio) in AA Stage I–III BCa survivors who were within 12 months of completing treatment (surgery, chemotherapy, and/or breast irradiation); and, evaluated the potential effects of this intervention on physical measures and cancer-related biomarkers. 27 patients provided informed consent and 19 participated in the program. On average, attendance rates were 70.0% ± 19.1% for the exercise sessions and 63.1% ± 13.8% for the support group. We observed a significant decrease in circulating C-peptide levels (B: 893.9 ± 399.1 pg/mL; EOI: 723.9 ± 319.0 pg/mL; p=0.01). Although we did not observe a significant decrease in weight in the entire sample, there was a significant decrease in waist circumference and percent total body fat among those who attended 70% or more of the exercise sessions. In summary, we demonstrated that conducting lifestyle interventions in AA BCa survivors in a community setting is feasible. Future interventions should invoke strategies to enhance adherence and include a structured dietary intervention to enable greater weight loss.
Keywords: Breast Cancer, Exercise, African-American, Community-Based, Biomarkers
I. INTRODUCTION
Breast cancer (BCa) is the leading cancer among women in the U.S. with 232,340 new cases expected to be diagnosed in 2013 and, is the leading cause of cancer death in women with over 40,000 deaths annually [1]. Compared to non-Hispanic Whites, African-American BCa patients have a poorer prognosis when diagnosed at similar age and stage [2]. Furthermore, African-American BCa patients are more likely to die from comorbidities associated with obesity such as hypertension and diabetes [3, 4]. This racial disparity is even greater in Cuyahoga County, Ohio where the mortality rates of BCa in African-American women are greater than those observed in Caucasian women and, interestingly, where the incidence and mortality rates in both ethnic groups exceed national averages [5].
Factors that may contribute to this racial disparity in BCa mortality rates include socioeconomic status, access to health care and molecular and pathologic mechanisms [6]. A recent study using data from the Surveillance, Epidemiology and End Results (SEER) registry suggests that this disparity may be due, in part, to the poorer health status of AAs at diagnosis and not treatment related differences [7]. It has been well documented that African-American women have the highest rates of obesity in the U.S. [8]. In addition, African-American BCa survivors have lower rates of physical activity and tend to gain more weight compared to non-Hispanic Whites, which may contribute to their poorer survival [9]. Taken together, there is an urgent need to further explore the potential role that modifiable behavioral risk factors such as diet and physical activity play in explaining and potentially resolving this disparity.
Randomized control trials (RCTs) involving supervised aerobic and resistance exercise training have been shown to improve body composition and overall quality of life in BCa survivors [10, 11]. Exercise may also favorably modify hormones, growth factors, adipokines and other cytokine biomarkers in BCa survivors [12]. In addition, exercise may improve BCa recurrence and survival rates but larger, longer-term studies are needed [13].
The majority of RCTs involving supervised exercise in BCa patients have not focused on African-Americans and many of these trials have been conducted in academic- and clinic-based settings. Because African-Americans may prefer community-based culturally sensitive programs [9], we aimed to evaluate the feasibility of conducting a group exercise intervention coupled with a support group program at a community cancer support center (The Gathering Place, Beachwood, Ohio) in African-American women who were within one year of completion of their treatment for Stage I–III BCa. The primary goal of this pilot study was to determine the feasibility of conducting an exercise and support group program in African-American BCa survivors which engages academic, clinical and community partners; and, secondarily, to determine if this 20-week intervention could improve physical measures as well as putative cancer-related biomarkers. We hypothesize that the intervention will be feasible and lead to improvements in physical measures and circulating biomarkers of cancer progression.
II. MATERIALS AND METHODS
A. Reporting Guidelines
Our study involved a one-arm (intervention group), non-randomized trial design. Therefore, we followed the Transparent Reporting of Evaluations with Non-Randomized Designs (TREND) Statement guidelines for reporting [14].
B. Study Participants and Recruitment
Patients were African-American BCa survivors, 18 years of age and older, who were within 12 months of completing treatment (surgery, chemotherapy and/or breast irradiation) for Stage I–III disease at University Hospitals Case Medical Center, Cleveland Clinic, or MetroHealth Medical Center. Collaborating oncologists approached potentially eligible patients. Patients who expressed interest in the study and who provided informed consent were subsequently screened with the American Heart Association/American College of Sports Medicine (ACSM) exercise pre-participation questionnaire. All clinically eligible patients who consented to the study were required to undergo a stress test administered on a treadmill per ACSM guidelines [15] to ensure it was safe for the patients to participate in the exercise intervention. Any patient who did not pass the stress test (and did not receive clearance from the cardiologist) was deemed ineligible to participate in the intervention and, the patient was recommended to consult their referring physician for further care and follow-up. All patients included in this study provided informed written consent and all study protocols and procedures were approved by the Institutional Review Board of University Hospitals.
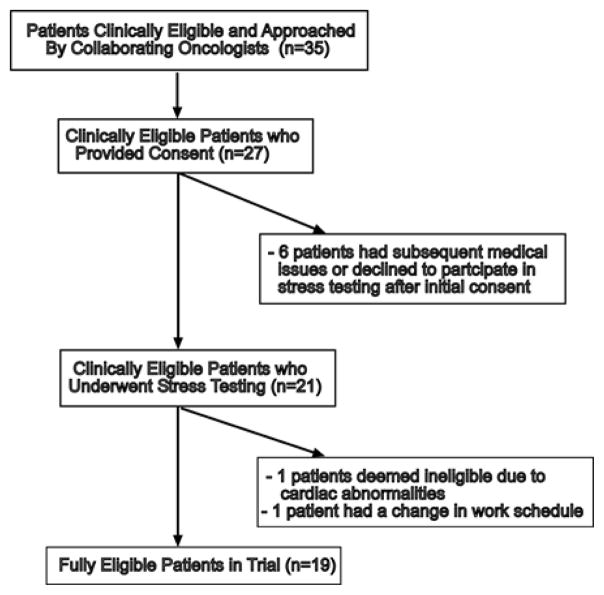
A consort diagram is shown in Fig. 1. Between August, 2011 and February, 2012, 35 patients who were deemed potentially eligible by collaborating oncologists were approached and informed about the study requirements by the referring oncologist. Of those who were approached, 27 provided informed written consent to participate in the study. Four of these patients had subsequent issues which prohibited further participation (surgery unrelated to cancer, uncontrolled hypertension, inability to go off of beta-blockers for stress test, cancer progression) and two patients subsequently declined to undergo the pre-screening stress test. Twenty-one patients completed the baseline stress testing. One of these patients was deemed ineligible because of cardiac abnormalities identified in the stress test and another patient had a change in schedule which prohibited her participation. Nineteen patients were fully eligible and consented to the trial; however, one of these patients did not complete the additional body composition and biomarker baseline testing (described in further detail below).
Fig. 1.
African-American breast cancer survivors (ABCs) consort diagram
C. Intervention Procedures
A single-arm, 20-week exercise and support group intervention was conducted in collaboration with The Gathering Place (TGP) located at 23300 Commerce Park Drive in Beachwood, Ohio. TGP is a non-profit, community-based cancer support center whose mission is to support, educate and empower individuals and families touched by cancer through a variety of programs and services, offered free of charge, that addresses their social, emotional, spiritual and physical needs. All support group sessions were held at TGP under the supervision of a licensed social worker and employed by TGP. All exercise sessions were conducted at TGP under the supervision of exercise specialists and ACSM Oncology Certified exercise trainers employed by TGP. The intervention was delivered in two small groups consisting of eight and eleven patients each during the evenings (Tuesday/Thursday and Monday/Wednesday, respectively). Transportation was provided to those in need both to and from the TGP and to and from the hospital for baseline and end of intervention testing.
The exercise intervention consisted of strength training sessions approximately 1 hour in duration conducted 2 times per week for 20 weeks. Patients were also asked to perform moderate intensity walking (3.0–3.5 mph) at home for 30 minutes a day on five days of the week (150 minutes/week) for 20 weeks based upon the current recommended guidelines for cancer patients [16].
Prior to initiating any exercise, a licensed physical therapist (PT) employed by TGP assessed patients for lymphedema using multiple measures including a self-reported personal history, visual inspection of limbs and circumferential measurements. In the involved arm, measurements were taken by the PT at three locations: mid-bicep, mid-forearm and mid-palm. The PT required any patient who had a history of lymphedema to wear a compression sleeve and the PT provided instructions on preventative lymphatic system decongestive exercises [17], which were completed as part of the warm-up to the exercise sessions. Patients were queried by the PT for subjective symptomatic changes throughout the intervention [17]. If a patient reported any concern, the patient was evaluated by the PT at TGP. Any patient found to have exacerbation of lymphedema (defined as greater than a 2 cm increase) was referred to as their primary lymphedema therapist and, the patient was asked to refrain from conducting upper body exercises in the intervention program until the patient received clearance from their primary lymphedema therapist. Any patient with potential exacerbation of lymphedema symptoms was encouraged to continue to perform the lower body strength training at the facility and the walking program at home.
The resistance training protocol was developed following the Resistance Training Strategies for Individuals with Cancer (RTSFIC) protocol [18] and ACSM guidelines for cancer survivors [16]. First, a range of motion (ROM) assessment without using weights was completed followed by a familiarization period with the weight machines. Then, we conducted baseline strength testing for major muscle groups. Although ACSM guidelines in healthy populations advocate for using 1 repetition maximum (RM) to 3 RM measures to assess muscular strength [15], we performed 5 RM testing and estimated the 1 RM [19] in only the lower extremity muscle group exercises to help prevent the potential onset of secondary lymphedema. Exercise training sessions began with 50% of 1 RM with 2 sets of 8 to 12 repetitions and progressed gradually to 80% of 1 RM with 2 sets of 8 to 12 repetitions based upon the progress of the individual patient. In general, the exercise specialist applied a 2–10% increase in weight when the patient could perform the prescribed workload at 12 repetitions in two consecutive training sessions. Muscle groups targeted included: upper legs (quadriceps, hamstrings); lower legs (soleus, gastronemius), upper and middle back (trapezius, latissimus dorsi); chest (pectorals); shoulders (deltoids); arms (biceps, triceps); and, abdominals. Specific exercises conducted include the leg extension, leg curl, leg press, chest press and lat pull-down machines and, 5, 8 and 10 lb dumbbells (depending on the progress of the patient) were used for bicep curls and triceps kickbacks. Extreme care was taken on all upper body exercises to minimize the potential for developing secondary lymphedema. Adherence to resistance training sessions was recorded using attendance records administered by the exercise specialists employed by TGP.
For the Support Group, 1–1.5 hour meetings were offered to patients on the first night of each week just prior to one of the exercise sessions (i.e., Monday or Tuesday) on a weekly basis for 20 weeks. The support group offered an opportunity for the African-American BCa survivors to come together with others of similar background for mutual support and education on topics including stress management, coping with fear and uncertainty, body image, sexuality and spirituality [20]. All of the sessions were led by an African-American licensed social worker at TGP. Adherence to support group sessions was recorded using attendance records administered by the support group leader employed by TGP.
D. Demographic and Clinical Measures
Baseline demographic and clinical information was abstracted from medical charts (e.g., age, cancer stage, treatment). In addition, a self-administered questionnaire was utilized to obtain general information on other demographic and risk factors (e.g., education, income, smoking). Eighteen patients completed the baseline general information questionnaire.
E. Body Composition and Fitness Measures
Body composition was measured at baseline (B) and at the end of the 20-week intervention (EOI) period and, 17 patients completed the body composition testing at baseline and the EOI. Patients were weighed without shoes on a scale and weight was recorded to the nearest 0.1 kg. Height was measured without shoes on a stadiometer and recorded to the nearest 0.1 cm. Waist and hip circumference were measured at the top of the iliac crest and the widest point of the hips, respectively, using a cloth tape. Percent body fat and lean mass were measured using a Lunar iDXA™ (GE Healthcare, Madison, WI). Fitness was assessed using the 6 Minute Walk Test (6MWT), which measures the distance a patient walks on a level, indoor surface in 6 minutes and has been shown to be highly correlated with the 12 Minute Walk Test (12MWT) upon which it was based [21, 22].
F. Biomarker Measures
Blood was collected via venipuncture by an experienced phlebotomist at baseline and at the EOI. Patients were asked to fast for 12 hours prior to having their blood drawn, which occurred between approximately 7:00 AM to 10:00 AM. We were not able to obtain a blood sample from one patient due to complications from prior chemotherapy treatment; thus, only 17 patients had their blood drawn at baseline and EOI. Serum and plasma were separated and aliquoted in 1.5 mL vials with EDTA, as applicable, and stored at −80°C until analysis. At the end of the intervention, serum and plasma samples were shipped to the University of Vermont Laboratory for Clinical Biochemistry Research (Colchester, VT). All samples were assayed by the lab within 12 months of initial collection and analyzed on one occasion using kits with the same numbers to minimize potential alterations from repeated freeze-thaw cycling and potential of variation due to batch effects. Total adiponectin was measured using Milliplex (Millipore Corporation, Billerica, MA) adipokine panel A (HADK1-MAG-61K; Lot 2090647) and leptin and tumor necrosis factor (TNF)-α were measured using Milliplex adipokine panel B (HADS2-MAG-62K; Lot 090648). C-Peptide and satiety hormones, pancreatic peptide (PP) and peptide YY (PYY), were measured with Milliplex endocrine panel (HADK2-MAG-62K; Lot 2090646). Insulin-like growth factor (IGF)-1, IGF-binding protein (IGFBP)-3 and interleukin (IL)-6 were measured using ELISAs with kits (DG100, DGB300, HS600B) from R&D Systems (Minneapolis, MN). Insulin was measured using kits (#1201547122; Lot 166168) from Roche Diagnostics Elecsys® (Indianapolis, IN).
G. Statistical Analyses
Variables were evaluated for normality using visual inspection of histograms and QQ plots and, using goodness of fit (e.g., Kolmorgoriv-Smirnov and Cramer-von Mises) tests [23]. Variables with goodness of fit tests that provided evidence of being statistically significantly different from a normal distribution were natural log transformed to help improve normality. Changes in outcome variables in individuals from baseline to the end of the intervention (EOI) were analyzed using paired sample t-tests (two-sided; significance: α ≤ 0.05). For body composition and biomarker outcomes, we explored stratification by adherence to the exercise sessions above and below the mean (70%), which resulted in 12 patients in the ‘More Adherent’ (≥70% exercise session attendance) group and 6 patients in the ‘Less Adherent’ (<70% exercise session attendance) group. We used two-sample t-tests to compare changes in means between those in the ‘More Adherent’ group to those in the ‘Less Adherent’ group and between changes in those who had a decrease in percent body fat (n=8) compared to those whose percent body fat increased (n=9). Due to the exploratory nature of this feasibility study, we did not correct for multiple testing. All analyses were conducted using SAS v9.2 (SAS Institute Inc., Cary, NC).
III. RESULTS
A. Study Population Characteristics
On average, the patients were 56.5 ± 11.0 years old and 83.3% had received chemotherapy (Table 1). The majority of patients were employed (65.9%) and most had received some college education (76.5%). The average travel time to the intervention facility was 16.9 ± 6.0 minutes. Attendance rates were, on average, 70.0% ± 19.1% for the exercise sessions and 63.1% ± 13.8% for the support group sessions. Five (23%) of the patients utilized the transportation services to attend sessions at TGP (99 total roundtrips) and to attend baseline or EOI testing at the hospital (11 total roundtrips). Eight (42.1%) patients reported having a history of lymphedema.
TABLE 1.
CHARACTERISTICS OF THE ‘ABCS’ STUDY POPULATION AT BASELINE
| Characteristic | Mean (s.d.) or Number (%) |
|---|---|
| Age at Diagnosis (years) | 56.5 (11.0) |
| Stage I | 5 (27.8%) |
| Stage II | 11 (61.1%) |
| Stage III | 2 (11.1%) |
| Surgery: Mastectomy | 8 (50%) |
| Surgery: Lumpectomy | 8 (50%) |
| Chemotherapy | 15 (83.3%) |
| Radiation | 10 (55.6%) |
| Hormone Therapy | 12 (66.7%) |
| Grade 2 | 7 (46.7%) |
| Grade 3 | 8 (53.3%) |
| ER + | 12 (66.7%) |
| PR + | 8 (44.4%) |
| HER2 − | 15 (83.3%) |
| Previous Lymphedema Diagnosis | 8 (42.1%) |
| Previously Treated for Lymphedema | 6 (31.6%) |
| Post-Menopausal | 13 (76.5%) |
| Married or Living With Partner | 8 (47.1%) |
| Education | |
| High School or GED | 4 (23.5%) |
| Some college but No Degree | 7 (41.2%) |
| Bachelor’s Degree | 2 (11.8%) |
| Some Graduate School | 4 (23.5%) |
| Income | |
| Less than $20,000 | 4 (25.0%) |
| $20,000–$39,999 | 4 (25.0%) |
| $40,000–$59,999 | 4 (25.0%) |
| $60,000–$99,999 | 1 (6.2%) |
| Over $100,000 | 3 (18.75%) |
| Employed: Full or Part Time | 9 (58.9%) |
| Ever Smoker | 11 (64.7%) |
| Alcohol | |
| Never | 4 (23.5%) |
| <1 drink/week | 5 (29.4%) |
| 1–2 drinks/week | 6 (35.3%) |
| 3–5 drinks/week | 2 (11.8%) |
| Hypertension | 3 (17.6%) |
| Arthritis | 9 (52.9%) |
| Time to Intervention Facility (minutes) | 16.9 (6.0) |
| Readiness to Exercise | |
| Contemplation Phase | 9 (52.9%) |
| Preparation Phase | 7 (41.2%) |
| Relapse Phase | 1 (5.9%) |
B. Body Composition, Strength and Fitness Measure Results
At baseline, on average, patients had a body mass index (BMI) of 32.46 ± 6.30 kg/m2 and a waist circumference of 95.70 ± 13.08 cm (Table 2), which would classify them, on average, to be ‘obese’ (i.e., BMI ≥ 30 kg/m2) and at ‘substantially increased risk for metabolic complications’ (i.e., waist circumference ≥ 88 cm) per the World Health Organization (WHO) [24]. In terms of baseline fitness, the average distance walked in 6 minutes (6 Minute Walk Test; 6MWT) was less than 400 meters, which is a level that has been associated with an increased risk of cardiovascular disease and mortality [25].
TABLE 2.
BODY COMPOSITION AND STRENGTH MEASURES AT BASELINE AND END OF INTERVENTION (EOI)
| Measure | Baseline Mean (s.d.) | EOI Mean (s.d.) | p-value |
|---|---|---|---|
| Body Composition | |||
| Weight (kg) | 86.12 (15.65) | 86.67 (16.92) | 0.44 |
| BMI (kg/m2) | 32.46 (6.30) | 32.69 (6.81) | 0.40 |
| Waist Circumference (cm) | 95.70 (13.08) | 94.95 (13.33) | 0.23 |
| Hip Circumference (cm) | 119.23 (14.63) | 118.70 (15.92) | 0.42 |
| Waist/Hip Ratio | 0.80 (0.06) | 0.80 (0.06) | 0.61 |
| Body Fat (%) | 47.21 (6.70) | 46.91 (8.18) | 0.55 |
| Lean Mass (kg) | 43.60 (4.34) | 43.92 (4.62) | 0.35 |
| Fitness | |||
| 6 Minute Walk Test (meters) | 389.46 (75.02) | 524.64 (122.61) | 0.01 |
| Strength | |||
| Leg Press (1 RM; lbs)1 | 122.71 (39.40) | 140.07 (47.16) | 0.12 |
| Leg Extension (1 RM; lbs)1 | 73.36 (37.57) | 102.29 (39.46) | 0.01 |
| Leg Curl (1 RM; lbs)1 | 63.00 (24.57) | 84.43 (28.11) | 0.01 |
1 Repetition Maximum (RM) estimated using 5 RM test
We observed no statistically significant changes in any of the body composition measures when comparing means at baseline to the end of the intervention (EOI) (Table 2). However, we found a significant increase in fitness using the 6MWT (B: 389.5 ± 75.0 meters; EOI: 524.6 ± 122.6 meters; p=0.01). In addition, we observed significant post-intervention compared to baseline mean increases in strength for the leg extension (B: 73.4 ± 37.6 lbs; EOI: 102.3 ± 39.5 lbs; p=0.01) and leg curl (B: 63.0 ± 24.6 lbs; EOI: 84.4 ± 28.1 lbs; p=0.01) exercises.
C. Lymphedema Measures Results
On average, the arm circumference measurements at the end of the intervention were lower than those observed at baseline in the involved arm in patients reporting a history of lymphedema; however, the changes were not statistically significant. For the mid-bicep, the circumference decreased by 0.20% (B: 37.3 ± 5.7 cm; EOI: 37.2 ± 6.0 cm; p=0.78). For the mid-forearm, the circumference decreased by 0.16% (B: 23.8 ± 2.4 cm; EOI: 23.7 ± 2.2 cm; p=0.78); and, the mid-palm circumference decreased by 0.16% (B: 19.6 ± 1.4 cm; EOI: 19.5 ± 1.3 cm; p=0.79). One patient experienced an exacerbation of lymphedema.
D. Biomarker Measures Results
We found a significant decrease in C-peptide levels after the intervention (B: 893.9 ± 399.1 pg/mL; EOI: 723.9 ± 319.0 pg/mL; p=0.01) (Table 3). There were no statistically significant changes in the other adipokine, hormone and growth factor biomarkers examined (Table 3).
TABLE 3.
CIRCULATING BIOMARKERS AT BASELINE AND END OF INTERVENTION (EOI)
| Measure | Baseline Mean (s.d.) | EOI Mean (s.d.) | p-value |
|---|---|---|---|
| Adipokines and Cytokines | |||
| Adiponectin (μg/mL)1 | 9.42 (0.88) | 9.37 (0.99) | 0.60 |
| Leptin (ng/mL) | 30.69 (14.83) | 31.65 (18.66) | 0.66 |
| IL-6 (pg/mL) | 2.88 (1.26) | 2.94 (1.17) | 0.33 |
| TNF-α (pg/mL) | 2.41 (1.13) | 2.20 (1.19) | 0.24 |
| Hormones | |||
| Insulin (μIU/mL)1 | 2.53 (0.48) | 2.35 (0.58) | 0.15 |
| C-peptide (pg/mL) | 893.88 (399.11) | 723.86 (318.98) | 0.01 |
| PP (pg/mL) | 38.32 (22.12) | 27.30 (29.01) | 0.15 |
| PYY (pg/mL) | 46.07 (17.05) | 47.28 (17.56) | 0.34 |
| Growth Factors | |||
| IGF-1 (ng/mL) | 72.63 (28.03) | 73.19 (26.58) | 0.90 |
| IGFBP-3 (ng/mL)1 | 7.64 (0.20) | 7.64 (0.17) | 0.98 |
geometric mean
E. Exploratory Analyses: Changes in Body Composition and Strength by Adherence
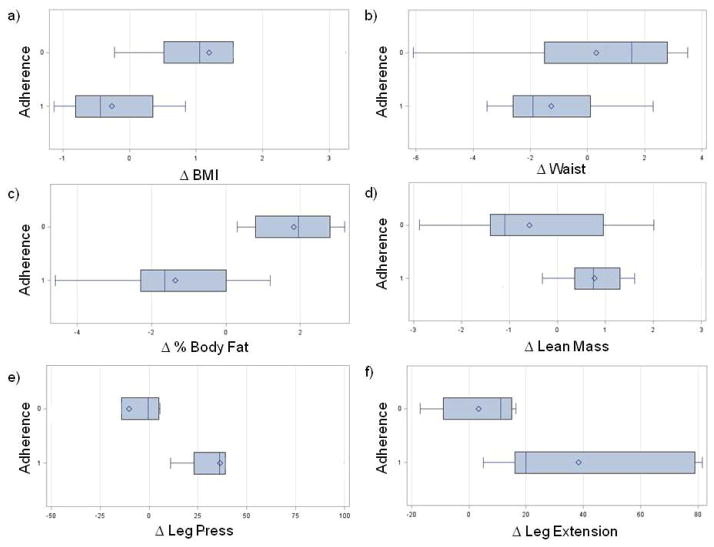
We explored changes in body composition and strength in patients who attended 70% (the mean attendance) or more of the exercise sessions (‘More Adherent’ group) compared to those who attended less than 70% of the exercise sessions (‘Less Adherent’ group) (Fig. 2). We found that the mean change in BMI (Fig. 2a; Less Adherent: 1.2 ± 0.5 kg/m2; More Adherent:−0.3 ± 0.2 kg/m2; p=0.03) and percent body fat (Fig. 2c; Less Adherent: 1.8 ± 0.5%; More Adherent: −1.4 ± 0.5%; p<0.001) was statistically significant between the adherence groups. Changes in waist circumference (Fig. 2b; p=0.23) and lean mass (Fig. 2d; p=0.12) were not significantly different between adherence groups. When exploring changes within the individual adherence groups, we found a significant decrease in the waist circumference (B: 91.3 ± 11.6 cm; EOT: 90.0 ± 12.5 cm; p=0.04), hip circumference (B: 115.2 ± 13.2 cm; EOT: 113.1 ± 13.2 cm; p=0.01) and percent total body fat (B: 45.4 ± 7.1%; EOT: 44.0 ± 8.0%; p=0.01) and, a significant increase in lean mass (B: 42.4 ± 4.4 kg; EOT: 43.2 ± 4.9 kg; p=0.02) among those who attended 70% or more of the exercise sessions. In the Less Adherent group, we observed a significant increase in percent body fat (B: 51.8 ± 3.9%; EOT: 54.0 ± 4.8%; p=0.01) and, a trend towards an increase in hip circumference (B: 128.7 ± 16.4 cm; EOT: 130.9 ± 17.6 cm) but this was not statistically significant (p=0.07).
Fig. 2.
Box and whisker plots of changes in body composition and strength by adherence (<70%=0; ≥70%=1): a) BMI (kg/m2; p=0.01); b) waist circumference (cm; p=0.24); c) body fat (%; p=0.001); d) lean mass (kg; p=0.05); e) leg press, 1 RM (lbs; p=0.01); f) leg extension, 1 RM (lbs; p=0.03).
In terms of strength, we found that the mean change in the estimated 1 RM for the leg press (Fig. 2e; Less Adherent: −10.2 ± 9.8 lbs; More Adherent: 36.2 ± 8.6 lbs; p=0.01) and the estimated 1 RM for the leg extension (Fig. 2f: Less Adherent: 3.3 ± 6.8 lbs; More Adherent: 38.3 ± 9.1 lbs; p=0.03) were significantly different between adherence groups. The mean change in the estimated 1 RM for the leg curl trend towards a difference between adherence groups but this was not statistically significant (data not shown; Less Adherent: 8.8 ± 6.2 lbs; More Adherent: 29.4 ± 6.8 lbs; p=0.08). When exploring changes within individual adherence groups, we found a significant increase in the More Adherent group for the leg press (B: 123.1 ± 36.5 lbs; EOT: 148.4 ± 43.1 lbs; p=0.03), leg extension (B: 74.5 ± 38.7 lbs; EOT: 110.4 ± 34.9 lbs; p=0.01) and leg curl (B: 65.0 ± 26.1 lbs; EOT: 88.7 ± 27.6 lbs; p=0.01) exercises. No statistically significant changes were observed in the Less Adherent group.
F. Exploratory Analyses: Changes in Biomarkers by Body Fat
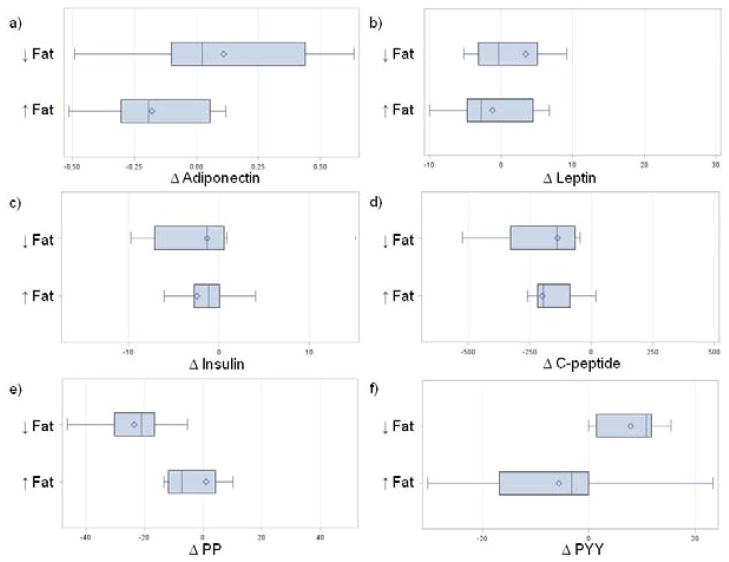
We explored changes in biomarkers in those who had a decrease in body fat versus those for whom body fat increased (Fig. 3). The mean change in the satiety hormone PYY (Fig. 3f: Decreased Fat: 7.9 ± 5.8 pg/ml; Increased Fat: −5.6 ± 6.2 pg/ml; p=0.05) was significantly different between those with decreased versus increased body fat. In addition, there was a trend towards a difference in the mean change between body fat groups in adiponectin (Fig. 3a; Decreased Fat: 0.1 ± 0.1; Increased Body Fat: −0.2 ± 0.1; p=0.08) and in the satiety hormone, PP (Fig. 3e: Decreased Fat: −0.2 ± 0.2; Increased Fat: −0.7 ± 0.1; p=0.09) but these were not statistically significantly different. No other mean changes in any of the other biomarkers including leptin (Fig. 3b; p=0.30), insulin (Fig. 3c; p=0.77), C-peptide (Fig. 3d; p=0.63), TNF-α (not shown; p=0.52) and IL-6 (not shown; p=0.23) were statistically significantly different between the body fat groups. When exploring changes within each body fat group separately, we found a significant increase in PYY (B: 44.2 ± 18.8 pg/ml; EOI: 53.9 ± 22.3 pg/ml; p=0.01) and a significant decrease in C-peptide (980.3 ± 409.0 pg/ml; EOI: 781.1 ± 290.7 pg/ml; p=0.02) after the intervention compared to baseline among those whose percent body fat decreased. In those whose percent body fat increased, we found a significant decrease in adiponectin (geometric mean; B: 9.1 ± 0.8; EOI: 8.9 ± 0.6; p=0.05).
Fig. 3.
Box and whisker plots of changes in biomarkers by a decrease or increase in percentage of total body Fat: a) adiponectin (ng/mL; p=0.08); b) leptin (μg/mL; p=0.30); c) insulin (μIU/mL; p=0.77); d) C-peptide (pg/mL; p=0.63); e) PP (pg/mL; p=0.09); and, f) PYY (pg/mL; p=0.05).
IV. DISCUSSION
We demonstrated the feasibility of conducting a 20-week exercise and support group intervention in African-American breast cancer survivors in a community setting, which is novel because most prior randomized controlled trials in these women have been conducted in academic- and clinic-based settings. Our exercise and support group program resulted in improvements in fitness and strength and, a significant decrease in circulating C-peptide levels after compared to before the intervention. For those patients who attended 70% or more of the exercise sessions, BMI and body fat significantly decreased.
Most randomized control trials (RCTs) involving aerobic and/or resistance training exercise in cancer survivors after treatment have observed significant improvements in strength and fitness but most have been conducted in academic- and clinic-based settings. A recent meta-analysis of 34 RCTs, 22 (65%) of which were conducted in BCa survivors and predominantly Caucasian, found that exercise significantly improved fitness, as measured by the 6MWT (summary effect: 29 (4–55) meters; p=0.03), and leg press strength, as measured by a 1 RM test (summary effect: 19 (9–28) kg; p<0.01) [10]. In our study, we found a significant improvement in fitness as measured by the 6MWT and in leg curl and leg extension strength; however, improvements in leg press strength were only observed in those who attended 70% or more of the exercise sessions.
Interestingly, although the aforementioned meta-analysis found significant improvements in BMI (summary effect: −0.4 (−0.6 to −0.2) kg/m2; p<0.01) and non-significant improvements in percent body fat (summary effect: −0.8 (−1.7 to 0.02)%; p=0.06) when all studies were evaluated collectively, the majority of the individual studies did not report significant improvements [10]. Similar findings were observed in another meta-analysis of 16 ‘high quality’ RCTs involving exercise in cancer survivors [11]. In our study involving African-American BCa survivors, we only observed significant improvements in BMI and percent body fat among those who attended 70% or more of the exercise sessions. Thus, our data appear consistent with individual study data from prior meta-analyses and suggest that larger samples may be required to observe significant improvements in BMI and percentage of total body fat.
Only a few RCTs involving aerobic and/or resistance training exercise have examined the effects of exercise on changes in putative cancer-related biomarkers in BCa survivors who have completed treatment. Insulin and IGF-1 act as growth factors that promote cell proliferation and inhibit apoptosis [26]. Some studies [27, 28] but not all [29, 30] have found a significant decrease in circulating insulin levels in BCa survivors, predominantly of Caucasian ethnicity. In addition, although many individual studies have failed to find a significant change in IGF-1 [30, 31], a recent meta-analysis involving 4 RCTs in BCa survivors found a significant decrease in IGF-1 [10]. A recent RCT involving a weight loss intervention at Curves® facilities in minority (79% Hispanic; 21% Black) BCa survivors found a significant decrease in insulin among those who lost 2% or more compared to less than 2% of their body fat [32]. Although we did not find a significant change in insulin or IGF-1, we did find a significant decrease in circulating C-peptide levels, which is a surrogate marker for pancreatic insulin secretion with a slower metabolic clearance. Prospective epidemiological studies have shown that lower fasting insulin and C-peptide levels are associated with improved BCa recurrence and survival rates [33, 34]. Thus, the decreased levels of C-peptide induced by exercise may lead to better recurrence and survival rates but larger and longer-term trials are needed to validate this hypothesis.
The adipokines, leptin and adiponectin have been shown to correlate positively and inversely, respectively, with the amount of fat mass and, may also play a role in obesity-related carcinogenesis [35]. Leptin is expressed in mammary tissue and may enhance breast tumor development [36–38]. Adiponectin may be involved in inhibiting cancer cell proliferation and differentiation and may also act indirectly by counteracting the pro-inflammatory effects of cytokines, TNF-α and IL-6. However, the effect of exercise on adipokines and cytokines has been less well studied and, most RCTs in BCa patients have not found significant changes in circulating leptin and adiponectin with exercise [39, 40]. We did not find a statistically significant change in any of the adipokines or cytokines examined; however, when we explored changes in the markers by change in body fat, there was a significant decrease in adiponectin after the intervention among those whose body fat increased. We also found a trend towards a difference in adiponectin levels between those whose body fat increased compared to those whose body fat decreased but this was not statistically significant (p=0.08). Our results coupled with those from previous exercise trials suggest that significant effects may only be observed in resistance training when substantial changes in body composition occur [41, 42].
We found a significant increase in the satiety hormone, PYY, among those who decreased body fat. PYY is a gut hormone that inhibits appetite and is believed to act on Y2 receptors in the hypothalamus [43]. Although PYY is known to be released into the circulation following a meal and is generally suppressed during fasting, PYY is also believed to play a role in longer-term energy regulation [43]. Thus, the increase in PYY we observed in the fasting state after the intervention compared to baseline may suggest an increase in longer-term satiety resulting from a decrease in fat afforded by exercise; however, additional larger studies in fasting and fed states are needed to confirm these suggestive findings.
The results from our exploratory analyses suggest that adherence to the exercise prescription is key to derive beneficial effects on body composition. Although retention in the trial was generally good, the adherence to the exercise was not optimal with the average attendance to the exercise sessions being about 70%. Motivating African-American BCa survivors to adhere to exercise programs may be particularly important because of their potential for increased weight gain [44] and, it is well-known that sustaining weight loss requires a long-term commitment to regular exercise in addition to having a healthy diet. Thus, future interventions should develop specific strategies to enhance adherence in African-American BCa survivors.
Although our findings suggest community-based interventions which can lead to improvements in strength, fitness, and circulating C-peptide levels in African-American BCa survivors, our study has several limitations. We had a small sample size which limited our statistical power to observe significant changes. For example, some of the trends towards a significant change within or difference between those attending 70% or more compared to those attending less than 70% of the exercise sessions such as the mean change in the leg curl estimated 1 RM between groups may have been statistically significant with a larger sample size. In addition, we did not have a control arm. Future studies should include an attention-control arm to determine the true efficacy of the exercise and support group intervention on physical, psychosocial and biomarker outcomes. Furthermore, the 20-week intervention was relatively short in duration and had moderate adherence rates, with an average attendance of around 70%. Moreover, we conducted many exploratory analyses and did not correct for multiple testing; thus, our significant findings, particularly those from the subgroup analyses, could be due to chance. Nevertheless, our results are generally consistent with previous exercise trials in Caucasian and minority breast cancer survivors [10, 32, 40].
V. CONCLUSIONS
Given the recent evidence that poorer survival in African-American BCa patients may be due, in part, to the poorer health status of AAs at diagnosis and not treatment related differences (7) and, the high rates of obesity in African-American women (8), there is an urgent need to address this disparity. Lifestyle interventions currently represent an underutilized means to improve physical, behavioral and clinically relevant outcomes in African-American breast cancer patients. Our findings demonstrate that conducting lifestyle interventions in African-American breast cancer survivors in a community setting is feasible. Further, the community-based approach described in this study may be more cost-effective and have greater outreach potential than academic- and clinic-based interventions. Nevertheless, future interventions should include strategies to enhance adherence and include a structured dietary component to enable greater weight loss and reduction in body fat, which may help to improve cancer-related biomarkers and cancer prognosis.
Acknowledgments
This work was supported, in part, by the American Cancer Society (ACS) [IRG-91-022-18 to N.L.N], the National Institutes of Health (NIH) [NCI K07CA129162 to N.L.N.; U54 CA116867 to N.A.B.] and, the Susan G. Komen Breast Cancer Foundation, Career Catalyst in Disparities Award [KG100319 to C.O.].
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012 Jan;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Facts and Figures for African-Americans 2011–2012. American Cancer Society; Atlanta, GA: 2011. [Google Scholar]
- 3.Eley JW, Hill HA, Chen VW, Austin DF, Wesley MN, Muss HB, et al. Racial differences in survival from breast cancer. Results of the National Cancer Institute Black/White Cancer Survival Study. JAMA. 1994 Sep;272(12):947–954. doi: 10.1001/jama.272.12.947. [DOI] [PubMed] [Google Scholar]
- 4.Tammemagi CM, Nerenz D, Neslund-Dudas C, Feldkamp C, Nathanson D. Comorbidity and survival disparities among black and white patients with breast cancer. JAMA. 2005 Oct;294(14):1765–1772. doi: 10.1001/jama.294.14.1765. [DOI] [PubMed] [Google Scholar]
- 5.Cuyahoga County Board of Health. The Cuyahoga County Comprehensive Cancer Report of 2011; A Detailed Look at Cancer Incidence and Mortality Data from 2002–2006. 2012. [Google Scholar]
- 6.Ademuyiwa FO, Groman A, O’Connor T, Ambrosone C, Watroba N, Edge SB. Impact of body mass index on clinical outcomes in triple-negative breast cancer. Cancer. 2011 Sep;117(18):4132–4140. doi: 10.1002/cncr.26019. [DOI] [PubMed] [Google Scholar]
- 7.Silber JH, Rosenbaum PR, Clark AS, Giantonio BJ, Ross RN, Teng Y, et al. Characteristics associated with differences in survival among black and white women with breast cancer. JAMA. 2013 Jul;310(4):389–397. doi: 10.1001/jama.2013.8272. [DOI] [PubMed] [Google Scholar]
- 8.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010 Jan;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 9.Stolley MR, Sharp LK, Wells AM, Simon N, Schiffer L. Health behaviors and breast cancer: experiences of urban African American women. Health Educ Behav. 2006 Oct;33(5):604–624. doi: 10.1177/1090198106290845. [DOI] [PubMed] [Google Scholar]
- 10.Fong DY, Ho JW, Hui BP, Lee AM, Macfarlane DJ, Leung SS, et al. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ. 2012;344:e70. doi: 10.1136/bmj.e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010 Jun;4(2):87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 12.Nock NL, Berger NA. Exercise Associated Regulation of Tumor Promoters, Hormones and Cytokines in Cancer Control. In: Ulrich C, Steindorf K, Berger NA, editors. In Exercise, Energy Balance and Cancer. New York: Springer; 2012. [Google Scholar]
- 13.Irwin ML. Benefits of Aerobic and Resistance Exercise in Cancer Survivors. In: Berger NA, Steindorf K, Ulrich C, editors. In Exercise, Energy Balance and Cancer. New York: Springer; 2012. [Google Scholar]
- 14.Des Jarlais DC, Lyles C, Crepaz N. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health. 2004 Mar;94(3):361–366. doi: 10.2105/ajph.94.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American College of Sports Medicine. ACSM resource manual for guidelines for exercise testing and prescription. 5. Baltimore, MD: 2005. [Google Scholar]
- 16.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010 Jul;42(7):1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 17.Sander A. Safe and effective upper extremity resistive exercise program for women post breast cancer treatment. Rehabilitation Oncology. 2008;26(2):3–10. [Google Scholar]
- 18.Swank AM, Hagerman P. In Resistant Training for Special Populations. Clifton Park, NY: DELMAR Cengage Learning; 2010. Resistance training strategies for individuals with cancer; pp. 305–324. [Google Scholar]
- 19.Cummings B, Fink K. Estimation of one repetition maximum bench press for untrained women. J Strength Conditioning Assoc. 2011;12:262–265. [Google Scholar]
- 20.Shapiro JP, McCue K, Heyman EN, Dey T, Haller HS. A naturalistic evaluation of psychosocial interventions for cancer patients in a community setting. J Psychosoc Oncol. 2010 Jan;28(1):23–42. doi: 10.1080/07347330903438891. [DOI] [PubMed] [Google Scholar]
- 21.Butland RJ, Pang J, Gross ER, Woodcock AA, Geddes DM. Two-, six-, and 12-minute walking tests in respiratory disease. Br Med J (Clin Res Ed) 1982 May;284(6329):1607–1608. doi: 10.1136/bmj.284.6329.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper KH. A means of assessing maximal oxygen intake. Correlation between field and treadmill testing. JAMA. 1968 Jan;203(3):201–204. [PubMed] [Google Scholar]
- 23.Stephens MA. EDF Statistics for Goodness of Fit and Some Comparisons. Journal of American Statistical Association. 1974;69:730–737. [Google Scholar]
- 24.World Health Organization (WHO) 2000 [Google Scholar]
- 25.Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006 May;295(17):2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 26.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004 Aug;4(8):579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 27.Irwin ML, Varma K, Alvarez-Reeves M, Cadmus L, Wiley A, Chung GG, et al. Randomized controlled trial of aerobic exercise on insulin and insulin-like growth factors in breast cancer survivors: the Yale Exercise and Survivorship study. Cancer Epidemiol Biomarkers Prev. 2009 Jan;18(1):306–313. doi: 10.1158/1055-9965.EPI-08-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ligibel JA, Campbell N, Partridge A, Chen WY, Salinardi T, Chen H, et al. Impact of a mixed strength and endurance exercise intervention on insulin levels in breast cancer survivors. J Clin Oncol. 2008 Feb;26(6):907–912. doi: 10.1200/JCO.2007.12.7357. [DOI] [PubMed] [Google Scholar]
- 29.Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Mackey JR. Effects of exercise training on fasting insulin, insulin resistance, insulin-like growth factors, and insulin-like growth factor binding proteins in postmenopausal breast cancer survivors: a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2003 Aug;12(8):721–727. [PubMed] [Google Scholar]
- 30.Schmitz KH, Ahmed RL, Hannan PJ, Yee D. Safety and efficacy of weight training in recent breast cancer survivors to alter body composition, insulin, and insulin-like growth factor axis proteins. Cancer Epidemiol Biomarkers Prev. 2005 Jul;14(7):1672–1680. doi: 10.1158/1055-9965.EPI-04-0736. [DOI] [PubMed] [Google Scholar]
- 31.McTiernan A, Sorensen B, Yasui Y, Tworoger SS, Ulrich CM, Irwin ML, et al. No effect of exercise on insulin-like growth factor 1 and insulin-like growth factor binding protein 3 in postmenopausal women: a 12-month randomized clinical trial. Cancer Epidemiol Biomarkers Prev. 2005 Apr;14(4):1020–1021. doi: 10.1158/1055-9965.EPI-04-0834. [DOI] [PubMed] [Google Scholar]
- 32.Greenlee HA, Crew KD, Mata JM, McKinley PS, Rundle AG, Zhang W, et al. A Pilot Randomized Controlled Trial of a Commercial Diet and Exercise Weight Loss Program in Minority Breast Cancer Survivors. Obesity (Silver Spring) 2012 Jun; doi: 10.1002/oby.20245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Madarnas Y, Hartwick W, Hoffman B, Hood N. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol. 2002 Jan;20(1):42–51. doi: 10.1200/JCO.2002.20.1.42. [DOI] [PubMed] [Google Scholar]
- 34.Irwin ML, Duggan C, Wang CY, Smith AW, McTiernan A, Baumgartner RN, et al. Fasting C-peptide levels and death resulting from all causes and breast cancer: the health, eating, activity, and lifestyle study. J Clin Oncol. 2011 Jan;29(1):47–53. doi: 10.1200/JCO.2010.28.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nock NL, Berger NA. Obesity and Cancer: Overview of Mechanisms. In: Berger NA, editor. In Cancer and Energy Balance, Epidemiology and Overview. New York: Springer; 2010. pp. 129–179. [Google Scholar]
- 36.Baratta M. Leptin--from a signal of adiposity to a hormonal mediator in peripheral tissues. Med Sci Monit. 2002 Dec;8(12):RA282–RA292. [PubMed] [Google Scholar]
- 37.Hu X, Juneja SC, Maihle NJ, Cleary MP. Leptin--a growth factor in normal and malignant breast cells and for normal mammary gland development. J Natl Cancer Inst. 2002 Nov;94(22):1704–1711. doi: 10.1093/jnci/94.22.1704. [DOI] [PubMed] [Google Scholar]
- 38.Hursting SD, Nunez NP, Varticovski L, Vinson C. The obesity-cancer link: lessons learned from a fatless mouse. Cancer Res. 2007 Mar;67(6):2391–2393. doi: 10.1158/0008-5472.CAN-06-4237. [DOI] [PubMed] [Google Scholar]
- 39.Ligibel JA, Giobbie-Hurder A, Olenczuk D, Campbell N, Salinardi T, Winer EP, et al. Impact of a mixed strength and endurance exercise intervention on levels of adiponectin, high molecular weight adiponectin and leptin in breast cancer survivors. Cancer Causes Control. 2009 Oct;20(8):1523–1528. doi: 10.1007/s10552-009-9358-3. [DOI] [PubMed] [Google Scholar]
- 40.Winzer BM, Whiteman DC, Reeves MM, Paratz JD. Physical activity and cancer prevention: a systematic review of clinical trials. Cancer Causes Control. 2011 Jun;22(6):811–826. doi: 10.1007/s10552-011-9761-4. [DOI] [PubMed] [Google Scholar]
- 41.Simpson KA, Singh MA. Effects of exercise on adiponectin: a systematic review. Obesity (Silver Spring) 2008 Feb;16(2):241–256. doi: 10.1038/oby.2007.53. [DOI] [PubMed] [Google Scholar]
- 42.de Salles BF, Simao R, Fleck SJ, Dias I, Kraemer-Aguiar LG, Bouskela E. Effects of resistance training on cytokines. Int J Sports Me. 2010 Jul;31(7):441–450. doi: 10.1055/s-0030-1251994. [DOI] [PubMed] [Google Scholar]
- 43.Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology. 2007 May;132(6):2116–2130. doi: 10.1053/j.gastro.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 44.Rock CL, McEligot AJ, Flatt SW, Sobo EJ, Wilfley DE, Jones VE, et al. Eating pathology and obesity in women at risk for breast cancer recurrence. Int J Eat Disord. 2000 Mar;27(2):172–179. doi: 10.1002/(sici)1098-108x(200003)27:2<172::aid-eat5>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]