Abstract
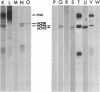
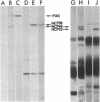
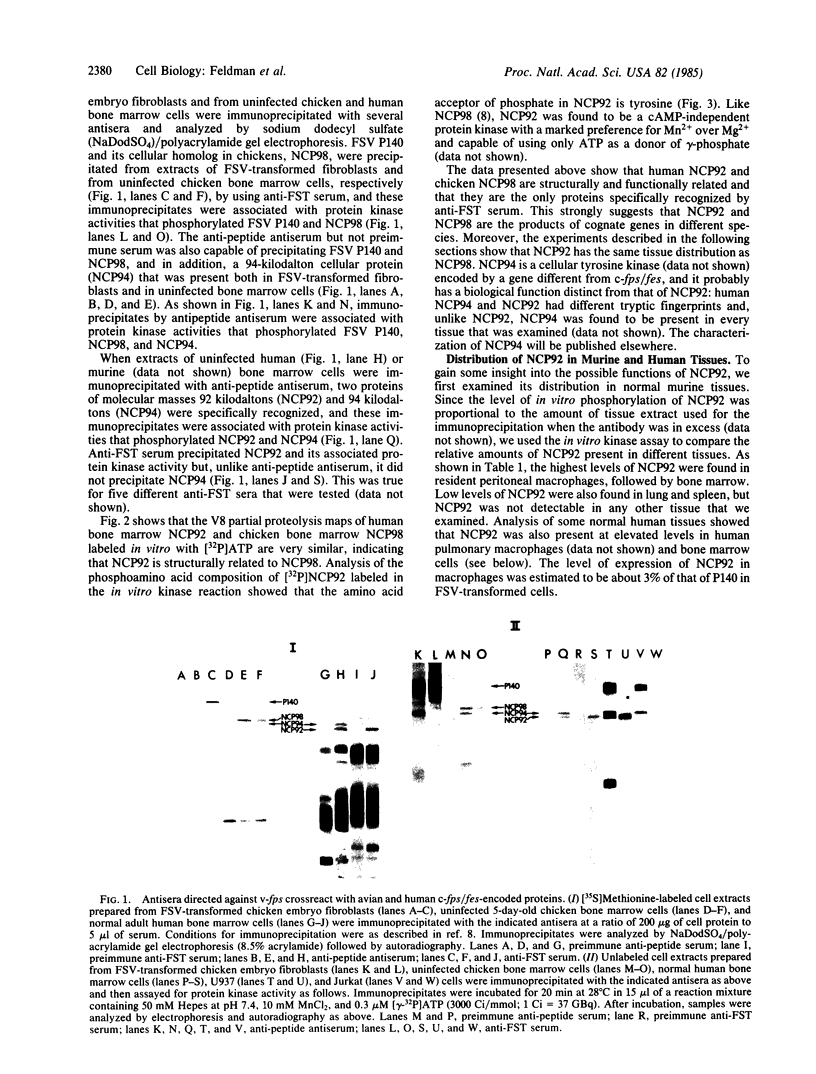
We have found that both an antibody directed against a synthetic peptide representing an amino acid sequence of the conserved kinase domain of transforming protein P140 of Fujinami sarcoma virus and a regressing tumor antiserum recognized the products of the c-fps/fes genes of both avian and mammalian cells. The anti-peptide antibody also recognized a 94-kilodalton protein that was related to but distinct from the c-fps/fes product in structure and in tissue distribution. A 92-kilodalton protein, NCP92, was found to be the mammalian counterpart of the previously identified avian c-fps/fes protein NCP98 by its structural similarity to NCP98, its associated tyrosine kinase activity, and its similar tissue distribution. The highest levels of NCP92 were found in tissue macrophages and in bone marrow. In bone marrow NCP92 expression was restricted to cells of the monocyte/macrophage and granulocyte lineages. That the expression of NCP92 is limited to these cell types was confirmed by the analysis of murine and human hematopoietic tumors representing different cell lineages: NCP92 was positive in leukemic cells of granulocytic and monocytic origin but not in B-lymphocytic, T-lymphocytic, or erythroid tumor cells. The expression of NCP92 seems to be related to the capacity of myeloid cells to differentiate and to respond to certain colony-stimulating factors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M., Beemon K., Devare S. G. Origin and functional properties of the major gene product of the Snyder-Theilen strain of feline sarcoma virus. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5158–5162. doi: 10.1073/pnas.77.9.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Dexter T. M. Blood cell development. The message in the medium. 1984 Jun 28-Jul 4Nature. 309(5971):746–747. doi: 10.1038/309746a0. [DOI] [PubMed] [Google Scholar]
- Feldman R. A., Hanafusa T., Hanafusa H. Characterization of protein kinase activity associated with the transforming gene product of Fujinami sarcoma virus. Cell. 1980 Dec;22(3):757–765. doi: 10.1016/0092-8674(80)90552-8. [DOI] [PubMed] [Google Scholar]
- Feldman R. A., Wang L. H., Hanafusa H., Balduzzi P. C. Avian sarcoma virus UR2 encodes a transforming protein which is associated with a unique protein kinase activity. J Virol. 1982 Apr;42(1):228–236. doi: 10.1128/jvi.42.1.228-236.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Metcalf D. Expression of myb, myc and fos proto-oncogenes during the differentiation of a murine myeloid leukaemia. Nature. 1984 Jul 19;310(5974):249–251. doi: 10.1038/310249a0. [DOI] [PubMed] [Google Scholar]
- Groffen J., Heisterkamp N., Shibuya M., Hanafusa H., Stephenson J. R. Transforming genes of avian (v-fps) and mammalian (v-fes) retroviruses correspond to a common cellular locus. Virology. 1983 Mar;125(2):480–486. doi: 10.1016/0042-6822(83)90219-2. [DOI] [PubMed] [Google Scholar]
- Hanafusa T., Mathey-Prevot B., Feldman R. A., Hanafusa H. Mutants of Fujinami sarcoma virus which are temperature sensitive for cellular transformation and protein kinase activity. J Virol. 1981 Apr;38(1):347–355. doi: 10.1128/jvi.38.1.347-355.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T., Wang L. H., Anderson S. M., Karess R. E., Hayward W. S., Hanafusa H. Characterization of the transforming gene of Fujinami sarcoma virus. Proc Natl Acad Sci U S A. 1980 May;77(5):3009–3013. doi: 10.1073/pnas.77.5.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Growth factors: mechanism of action and relation to oncogenes. Cell. 1984 May;37(1):9–20. doi: 10.1016/0092-8674(84)90296-4. [DOI] [PubMed] [Google Scholar]
- Kawai S., Yoshida M., Segawa K., Sugiyama H., Ishizaki R., Toyoshima K. Characterization of Y73, an avian sarcoma virus: a unique transforming gene and its product, a phosphopolyprotein with protein kinase activity. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6199–6203. doi: 10.1073/pnas.77.10.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeffler H. P., Billing R., Lusis A. J., Sparkes R., Golde D. W. An undifferentiated variant derived from the human acute myelogenous leukemia cell line (KG-1). Blood. 1980 Aug;56(2):265–273. [PubMed] [Google Scholar]
- Lee W. H., Bister K., Pawson A., Robins T., Moscovici C., Duesberg P. H. Fujinami sarcoma virus: an avian RNA tumor virus with a unique transforming gene. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2018–2022. doi: 10.1073/pnas.77.4.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathey-Prevot B., Hanafusa H., Kawai S. A cellular protein is immunologically crossreactive with and functionally homologous to the Fujinami sarcoma virus transforming protein. Cell. 1982 Apr;28(4):897–906. doi: 10.1016/0092-8674(82)90069-1. [DOI] [PubMed] [Google Scholar]
- Minowada J., Koshiba H., Sagawa K., Kubonishi I., Lok M. S., Tatsumi E., Han T., Srivastava B. I., Ohnuma T. Marker profiles of human leukemia and lymphoma cell lines. J Cancer Res Clin Oncol. 1981;101(1):91–100. doi: 10.1007/BF00405069. [DOI] [PubMed] [Google Scholar]
- Oliff A., Oliff I., Schmidt B., Famulari N. Isolation of immortal cell lines from the first stage of murine leukemia virus-induced leukemia. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5464–5467. doi: 10.1073/pnas.81.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Guyden J., Kung T. H., Radke K., Gilmore T., Martin G. S. A strain of Fujinami sarcoma virus which is temperature-sensitive in protein phosphorylation and cellular transformation. Cell. 1980 Dec;22(3):767–775. doi: 10.1016/0092-8674(80)90553-x. [DOI] [PubMed] [Google Scholar]
- Pelus L. M., Bockman R. S. Increased prostaglandin synthesis by macrophages from tumor-bearing mice. J Immunol. 1979 Nov;123(5):2118–2125. [PubMed] [Google Scholar]
- Pontén J., Saksela E. Two established in vitro cell lines from human mesenchymal tumours. Int J Cancer. 1967 Sep 15;2(5):434–447. doi: 10.1002/ijc.2910020505. [DOI] [PubMed] [Google Scholar]
- Ralph P., Kishimoto T. Tumor promoter phorbol myristic acetate stimulates immunoglobulin secretion correlated with growth cessation in human B lymphocyte cell lines. J Clin Invest. 1981 Oct;68(4):1093–1096. doi: 10.1172/JCI110332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M., Hanafusa H., Balduzzi P. C. Cellular sequences related to three new onc genes of avian sarcoma virus (fps, yes, and ros) and their expression in normal and transformed cells. J Virol. 1982 Apr;42(1):143–152. doi: 10.1128/jvi.42.1.143-152.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M., Hanafusa H. Nucleotide sequence of Fujinami sarcoma virus: evolutionary relationship of its transforming gene with transforming genes of other sarcoma viruses. Cell. 1982 Oct;30(3):787–795. doi: 10.1016/0092-8674(82)90283-5. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., deKernion J. B., Verma I. M., Cline M. J. Expression of cellular oncogenes in human malignancies. Science. 1984 Apr 20;224(4646):256–262. doi: 10.1126/science.6538699. [DOI] [PubMed] [Google Scholar]
- Takeya T., Feldman R. A., Hanafusa H. DNA sequence of the viral and cellular src gene of chickens. 1. Complete nucleotide sequence of an EcoRI fragment of recovered avian sarcoma virus which codes for gp37 and pp60src. J Virol. 1982 Oct;44(1):1–11. doi: 10.1128/jvi.44.1.1-11.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tertian G., Yung Y. P., Guy-Grand D., Moore M. A. Long-term in vitro culture of murine mast cells. I. Description of a growth factor-dependent culture technique. J Immunol. 1981 Aug;127(2):788–794. [PubMed] [Google Scholar]
- Welte K., Platzer E., Lu L., Gabrilove J. L., Levi E., Mertelsmann R., Moore M. A. Purification and biochemical characterization of human pluripotent hematopoietic colony-stimulating factor. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1526–1530. doi: 10.1073/pnas.82.5.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. W., Goldberg A. R. Synthetic peptide fragment of src gene product inhibits the src protein kinase and crossreacts immunologically with avian onc kinases and cellular phosphoproteins. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7412–7416. doi: 10.1073/pnas.78.12.7412. [DOI] [PMC free article] [PubMed] [Google Scholar]