Abstract
Adverse reactions may occur with any of the medications prescribed or administered in dental practice. Most of these reactions are somewhat predictable based on the pharmacodynamic properties of the drug. Others, such as allergic and pseudoallergic reactions, are less common and unrelated to normal drug action. This article will review the most common adverse reactions that are unrelated to drug allergy.
Key Words: Adverse drug reactions, Drug side effects, Dentistry
Adverse drug reactions occur in 10–20% of hospitalized patients and in approximately 7% of those in the ambulatory setting.1 By convention, adverse drug reactions are categorized as type A or type B. Type B reactions were addressed in a previous continuing education article in this journal and consist mostly of idiosyncrasy or drug allergy.2 Type A reactions are the focus of this article. They are more common and are generally attributable to known pharmacological or toxic effects of the drug. The typical pharmacopeia in dental practice is relatively small, consisting primarily of sedatives, local anesthetics, analgesics, and antibiotics. The most common adverse effects will be addressed for each of these classes.
SEDATIVES, OPIOIDS, AND GENERAL ANESTHETICS
Respiratory depression is the most significant side effect of all drug classes used for procedural sedation and general anesthesia. However, before embarking on this discussion, it is important to distinguish respiratory depression from anatomical airway obstruction. Most of the drug classes used for procedural sedation and general anesthesia produce relaxation of glossopharyngeal musculature and thereby reduce pharyngeal patency. Even mild degrees of respiratory depression may be intense enough to prevent patients from overcoming the obstruction, but patients will generally breathe if airway patency is improved. It is always sound practice to position the patient as upright as possible, tilt the head upward, and protrude the mandible when necessary.
Respiratory depression proceeds in a dose-response manner, and the intensity is further increased when the various classes are used in combination. Minimal to moderate sedation carries little risk for clinically significant respiratory depression, but deep sedation and general anesthesia introduce substantial risk for both respiratory depression and airway obstruction. (See Table 1.) The individual drug classes differ in their predilection for depressing central hypercapnic drive or peripheral hypoxemic drive. For example, the opioids primarily depress central hypercapnic drive whereas the potent inhalation agents depress hypoxemic drive. At higher doses there is less selectivity and both drives become depressed.
Table 1.
General Influences of Sedation Levels and General Anesthesia3

Potent inhalation anesthetics depress tidal volume while generally increasing respiratory rate; their net influence on minute ventilation varies. In contrast, the intravenous sedatives and opioids usually depress both tidal volume and respiratory rate. Benzodiazepines produce the least intensity of respiratory depression, but this increases when they are combined with other drugs or high doses are administered. For example, doses intended to induce unconsciousness may produce transient episodes of apnea.
In addition to respiratory influences, these drug classes may also lower arterial blood pressure and heart rate. Cardiovascular influences are rarely significant at doses intended for minimal to deep sedation, but with doses and combinations intended for general anesthesia the risk for hypotension may become more substantial. A summary of respiratory and cardiovascular influences of the most commonly used drugs is provided in Table 2.
Table 2.
Relative Respiratory and Cardiovascular Influences of Selected Drugs4

Benzodiazepines and propofol produce anterograde amnesia when administered at sedative dosages.5 This is an inability to recall events that occur while conscious but under the influence of a medication. The term is not applicable during general anesthesia because the patient is unconscious. Although anterograde amnesia is an attractive effect during unpleasant procedures, it may also be problematic should a need arise to alter a dental treatment plan. These issues must be taken into account prior to the procedure and the vested escort educated regarding the patient's lack of judgment during recovery at home.
ANTIHISTAMINES AND ANTIEMETICS
The antihistamines and related antiemetics are commonly used in regimens for procedural sedation but also are indicated for minor allergic reactions and nausea or vomiting. When used alone they have little influence on respiration, but they may potentiate respiratory depression produced by opioids and other sedatives. All of these agents have anticholinergic action, but peripheral side effects are rarely if ever encountered, although mouth dryness might be a nuisance to some patients. Central cholinergic blockade can be another matter, however. Cognition and memory are functions of cholinergic neurotransmission, and degeneration of cholinergic neurons is the key component of Alzheimer's dementia. Drugs having central anticholinergic actions should probably be avoided in elderly patients, particularly those having evidence of dementia.6 Moreover, high doses of anticholinergic drugs can result in a “central anticholinergic syndrome” that includes delirium and combativeness. During lengthy treatment under sedation it is important to not exceed conventionally suggested doses for these agents.
Unlike diphenhydramine or hydroxyzine, promethazine (Phenergan) also acts as a dopamine receptor antagonist, an action shared by other antiemetic drugs such as prochlorperazine (Compazine) and droperidol (Inapsine). Although dopamine receptor blockade within the chemoreceptor trigger zone provides an added antiemetic mechanism, this identical action within the basal ganglia introduces the risk for extrapyramidal syndromes. This is a collective term for several conditions including acute dystonia, akathisia, and Parkinsonism. Acute dystonias generally present as spasms of the tongue, facial, and neck muscles, whereas akathisia presents as a subjective feeling of restlessness and a compelling need to move about. These behaviors may be mistaken for agitation, and their distinction is critical to avoid an inclination to further sedate the patient. Although extrapyramidal symptoms are bizarre, and generally frighten the patient and practitioner alike, they are never life threatening. The added anticholinergic action of diphenhydramine is useful for countering acute episodes should they occur.7
A final note on promethazine is worth mention. Like other phenothiazine as well as butyrophenone derivatives, it has antagonist actions on vascular alpha receptors, which increases risk for postural hypotension, especially in the elderly.
LOCAL ANESTHETICS
Local anesthetics are remarkably safe when used in proper doses and concentrations, but they are certainly capable of producing both local and systemic toxicity. Ischemic necrosis of tissues may follow injections of local anesthetics. This can be due to the irritating nature of a solution, pressure from large volumes, or constriction of the vasculature by vasopressors. This concern is greatest when injecting into attached mucosa such as the hard palate. There is also mounting concern regarding direct neurotoxicity related to formulations containing high concentrations, such as 4% articaine and prilocaine.
Local anesthetics can produce direct toxicity to nerve trunks, leading to persistent paresthesias. Although the dental community has been slow to reach consensus regarding this issue, it should be appreciated that medical anesthesia literature is emphatic in claiming that greater concentration of local anesthetic solutions increases risk for direct neurotoxicity to nerve trunks:
“All the clinically used local anesthetics can produce direct toxicity to nerves if they achieve sufficiently high intraneural concentrations. Clinicians should be aware that the concentrations of formulated local anesthetic solutions are neurotoxic per se and that their dilution, in situ or in tissue, is essential for safe use.”8
Local anesthetic concentrations of 2% or 3% carry little risk, but 4% articaine and prilocaine formulations most certainly introduce added risk. Haas and Lennon first reported an increased incidence of paresthesias in Canada following the introduction of articaine in the mid-1980s.9 When 4% articaine was first submitted for approval to the Food and Drug Administration in the United States, it was identified as having a higher risk for paresthesia than 2% lidocaine.
More recently, Garisto et al10 reviewed claims of paresthesia in the United States during the period of November 1997 through August 2008 and found 248 cases of paresthesia following dental procedures. Most cases (∼95%) involved mandibular nerve blocks, and in 89% of these the lingual nerve was affected. Compared to other local anesthetics, paresthesia was found to be 7.3 times more likely with 4% articaine and 3.6 times more likely with 4% prilocaine. Similar findings from reports of paresthesia in Denmark were published by Hillerup et al11 and even more convincing is their demonstration of greater neural toxicity for 4% compared to 2% articaine in sciatic nerve preparations.12 As with all drugs, each practitioner needs to perform a risk-benefit analysis before using a medication. Only if the benefit of using a 4% concentration outweighs the risk for a patient should it be considered for use. It might be wise to limit the use of 4% concentrations for infiltration and avoid their use for nerve blocks, opting instead for agents formulated in lower concentrations.10,11
As local anesthetics are absorbed from the injection site, their concentration in the bloodstream rises and the peripheral and central nervous systems are depressed in a dose-dependent manner.8 (See Figure 1.) Low serum concentrations are used clinically for suppressing cardiac arrhythmias and status seizures, but as their concentration rises, local anesthetics produce drowsiness. At higher concentrations, convulsive seizures occur and are the initial life-threatening consequence of local anesthetic overdose. This is presumably due to selective depression of cortical inhibitory tracts allowing unopposed activity of excitatory pathways.8,13 This selectivity is lost as serum concentrations rise even further and all pathways are inhibited, resulting in coma, respiratory arrest, and eventually cardiovascular collapse. Evidence of lidocaine toxicity may commence at serum concentrations >5 μg/mL, but convulsive seizures generally require concentrations >10 μg/mL. If maximum recommended doses published in conventional references are adhered to, excessive serum concentrations are unlikely to occur.
Figure 1.

Approximate serum concentrations and systemic influences of lidocaine.
It is essential that local anesthetics be respected as central nervous system depressants, and they potentiate any respiratory depression associated with sedatives and opioids. Furthermore, serum concentrations required to produce seizures are lower if hypercarbia (elevated carbon dioxide) is present. This is the case when respiratory depression is produced by concurrent administration of sedatives and opioids. Goodson and Moore have documented catastrophic consequences of this drug interaction in pediatric patients receiving procedural sedation, along with excessive dosages of local anesthetics.14
Although all local anesthetics carry similar risk for central nervous system toxicity, it should be noted that bupivacaine exhibits greater potential for direct cardiac toxicity than other agents.8,13 The explanation is not fully established but is thought to be related to the fact that bupivacaine has greater affinity for the inactive and resting sodium channel configurations and dissociates from these channels more slowly. This delays recovery from action potentials, rendering cardiac tissues susceptible to arrhythmias. This concern is relevant for certain medical procedures during which bupivacaine is administered in very high doses. It has never been found to occur with doses up to the maximum recommended in dental anesthesia.
When considering the toxicity of any drug class, one should be mindful of metabolites, as well as the parent drug. A metabolite of prilocaine, 0-toluidine, can oxidize the iron in hemoglobin from ferrous (Fe2+) to ferric (Fe3+). Hemes so altered do not bind oxygen, and normal hemes on the same hemoglobin molecule do not readily release their oxygen. This form of hemoglobin is called methemoglobin, and when >1% of total hemoglobin is so altered, the condition is called methemoglobinemia. Patients appear cyanotic and become symptomatic when the proportion of methemoglobin exceeds 15%. Hemoglobin saturation by pulse oximetry will decline despite clinical evidence of adequate oxygenation and ventilation. At methemoglobin levels of up to 35%, oxygen saturation via pulse oximetry decreases by an amount proportional to the concentration of methemoglobin until the latter reaches approximately 35%. At higher methemoglobin levels, the oxygen saturation levels out at about 85%.15 The condition becomes life threatening when methemoglobin levels exceed 50–60%, and it is managed using intravenous methylene blue, which reduces the hemes to their normal state. Methemoglobinemia attributed to prilocaine is unlikely to follow the administration of recommended doses. Rarely, one may encounter a patient with hereditary methemoglobinemia, which contraindicates the use of prilocaine.
VASOCONSTRICTORS: EPINEPHRINE AND LEVONORDEFRIN
Vasoconstrictors are combined with local anesthetics to provide hemostasis in the operative field and to delay anesthetic absorption. This influence is mediated by activation of alpha-1 receptors on submucosal vasculature, but, following systemic absorption, cardiovascular influences can result from their activation of additional adrenergic receptors as well. The doses and cardiovascular influences of both epinephrine and levonordefrin have been thoroughly reviewed in previous continuing education articles in this journal.16,17 Epinephrine is the vasoconstrictor used most commonly, and produces the systemic cardiovascular effects illustrated in Figure 2. Even small doses of epinephrine produce these cardiovascular effects; this is unequivocal. At issue is the actual magnitude and whether or not these influences pose a significant risk to a particular patient. The most often cited guidelines suggest that a 2-cartridge limit be imposed for patients with cardiovascular disease, but this is naïve. Ultimately, the decision requires the dentist to exercise sound clinical judgment based on a thorough analysis of each patient under consideration.
Figure 2.
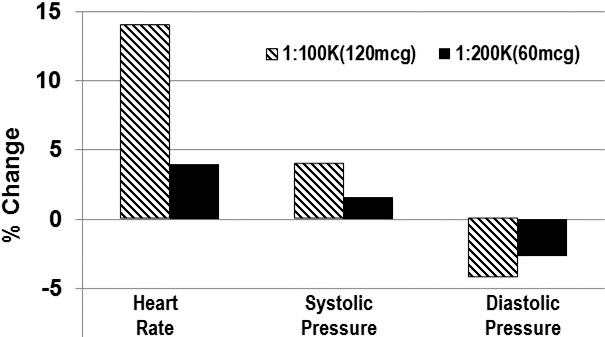
Cardiovascular influences of epinephrine. The graph is adapted from Hersh et al.18 Average of cardiovascular changes were recorded following injection of 7 cartridges (11.9 mL) of articaine containing either 1 : 100,000 or 1 : 200,000 concentrations of epinephrine (∼120 and 60 μg respectively). Although actual changes were mild, consider that all volunteers were healthy young adults taking no medications. Even so, 2 volunteers experienced palpitations. Also note confirmation of the dose-dependent responses for 60 versus 120 μg.
Generally, the hemodynamic influences of epinephrine are witnessed within 5 minutes of injection and have completely subsided in 10–15 minutes. If for any reason the medical status of a patient is in question, a sensible protocol is to record baseline heart rate and blood pressure preoperatively and again following every 20–40 μg administered (∼1–2 cartridges containing a 1 : 100,000 epinephrine concentration). Virtually any ambulatory patient can tolerate the cardiovascular influences of this amount. If the patient's vital signs remain stable for 5 minutes following injection, additional doses may be administered and followed by a similar pattern of reassessing vital signs. One should also consider using lower concentrations of epinephrine. Despite the popularity of epinephrine 1 : 100,000, concentrations greater than 1 : 200,000 (5 μg/mL) offer little if any advantage. Greater concentrations do not provide better onset or duration for inferior alveolar nerve block.19,20 Nor do higher concentrations reduce local anesthetic serum concentrations.21
ANALGESICS
Nonsteroidal Anti-Inflammatory Drugs
The most frequent adverse effects of nonsteroidal anti-inflammatory drugs (NSAIDs) relate to their gastrointestinal (GI) toxicity: mucosal erosion and ulceration. Parenteral administration does not preclude this risk because inhibition of normally protective prostaglandins occurs regardless of the route of administration. It is important to distinguish dyspepsia (upset stomach) from GI toxicity, which reflects actual mucosal damage. The incidence of dyspepsia attributed to NSAIDs does not correlate with mucosal injury. For example, buffered aspirin is less likely to produce gastric upset but carries similar risk for mucosal damage as regular aspirin.22 Patients receiving antithrombotic therapy with either antiplatelet or anticoagulant drugs are at risk for more significant bleeding from NSAID-induced mucosal injury. NSAIDs increase the risk for GI bleeding twofold to threefold in patients medicated with clopidogrel (Plavix) and fourfold to fivefold in those taking warfarin.23 Concerns regarding NSAID-induced mucosal injury are particularly important for older patients.
Actual antiplatelet influences for most NSAIDs are generally overstated. Although nonaspirin NSAIDs all prolong bleeding times to some degree, this does not correlate with significant clinical bleeding following minor surgical procedures.24 However, they are generally withheld prior to major thoracic, abdominal, or orthopedic procedures.
Prostaglandins play an essential role in renal perfusion, and their inhibition is believed to account for reported cases of nephrotoxicity following chronic NSAID use. In the healthy patient, nephrotoxicity attributed to NSAIDs requires high doses for extended periods, eg, a year or more.25 However, a patient with compromised renal function relies more heavily on prostaglandins for adequate function, and acute renal failure can occur within 24 hours of NSAID administration. NSAIDs must never be prescribed for patients having compromised or questionable renal function.
Acetaminophen
Acetaminophen has virtually no adverse effects when administered at conventional doses in healthy patients.26 Hepatotoxicity is the principal adverse effect associated with excessive dosages. Formerly the maximum recommended dose was 4 g daily, but recently the Food and Drug Administration has reduced the daily maximum to 3 g in an effort to curtail excessive use. Despite this recommendation, it is still acceptable to prescribe 4 g daily for healthy adults when managing brief periods of postoperative pain (3–5 days). In healthy adults, hepatotoxicity may occur after ingestion of a single dose of 7.5–10 g (≥150 mg/kg in children), and 20–25 g or more is potentially fatal.27 These amounts can be considerably lower in patients with hepatic compromise.
Hepatic injury from acetaminophen is due mainly to a single toxic metabolite, N-acetyl-p-benzoquinone imine, which is formed by oxidation of the drug. With therapeutic doses in healthy subjects, oxidation is a minor metabolic pathway, and glutathione conjugates and inactivates the toxic metabolite.27 (See Figure 3.)
Figure 3.

Acetaminophen toxicity. The major portion of acetaminophen is metabolized to nontoxic metabolites excreted in urine. Only 5–15% is oxidized by cytochrome P450 (CYP 450) enzymes to a potentially toxic metabolite, N-acetyl-p-benzoquinone imine (NAPQI). The normally small amounts of this metabolite are readily converted to harmless mercapturic acid conjugates by glutathione. When high doses of acetaminophen are consumed, glutathione can be depleted, allowing NAPQI to accumulate and produce hepatic necrosis. Also, normal biotransformation is diminished with compromised liver function, including that associated with malnutrition and alcohol abuse. Toxicity can be further accentuated by ethanol consumption, which induces CYP 450 activity, leading to greater portions of acetaminophen converted to NAPQI. Emergency management of acetaminophen overdose consists of administering high doses of acetylcysteine, which replenishes glutathione.
Opioids
As addressed earlier in this article, respiratory depression is a significant side effect of opioids included in sedation and anesthesia regimens but is rarely a concern at the conventional doses prescribed for postoperative pain. Analgesic doses are more commonly associated with constipation and nausea. The inhibitory effect of opioids on GI motility is highly variable among patients, but for those susceptible, a stool softener should be suggested. Patients having a history of nausea and vomiting with opioids should be encouraged to remain stationary for an hour or so following each dose. Vestibular influences potentiate opioid actions within the vomiting center and chemoreceptor trigger zone of the medulla.28,29
Fear of dependence and addiction often results in underprescribing of opioids for severe acute, chronic, and even terminal pain. This unfortunate practice is due to poor understanding of these terms. Dependence occurs when the body accommodates to the influences of a drug and, upon sudden discontinuation, the patient experiences a withdrawal syndrome that generally includes reactions opposite those produced by the particular drug. For example, opioids produce sedation, lethargy, and constipation. A patient experiencing opioid withdrawal becomes excited, and experiences abdominal cramping and diarrhea. If opioid doses are tapered gradually, a dependent patient will not experience withdrawal. Patients consuming opioids regularly for more than a week can develop some degree of dependence. This may require gradual tapering of the dosage to avoid withdrawal symptoms, which can be confused as an exacerbation of their painful condition. However, this does not mean the patient has become addicted.29,30
Following repeated administration, patients develop tolerance to opioids. This is to say that greater doses are required to produce the same intensity of effect formerly provided by a smaller dose. Tolerances to analgesia, sedation, and respiratory depression occur simultaneously, but curiously, there is no tolerance to the constipating effects of opioids. This is problematic for the patient with chronic or terminal pain. Although staggering doses may be required to control pain and will generally not jeopardize the patient's respiratory status, constipation can become extremely severe. Similar doses, if administered to patients who have not developed tolerance, ie, opioid-naive patients, can be lethal. Issues regarding tolerance and dependence must be considered when managing dental pain for patients who are medically dependent as well as those who are chronic opioid abusers.
Addiction is distinct from dependence or tolerance. It is a compulsive behavior centered on seeking a drug and its effects for nonmedical reasons, generally for pleasure. It is a complex psychiatric phenomenon, but it should not be considered an adverse effect of the drug per se. Addictive behavior can be reinforced by an opioid, but it is not a pharmacodynamic property. For example, an opioid-dependent patient who lacks addictive behavior can be easily weaned from opioid dosages without fear of precipitating addictive behavior. In contrast, an addicted patient will seek the drug despite having no remaining evidence of dependence or medical need for the drug. Opioids should not be withheld on the presumption that the patient will become addicted, but they must be prescribed cautiously for patients who demonstrate addictive personality. Patients who are dependent on opioids for medical reasons or exhibit addictive behavior should not be prescribed or administered agonist-antagonists such as pentazocine or nalbuphine or weak agonists such as tramadol or tapentadol because they may precipitate withdrawal.
ANTIBIOTICS
There are surprisingly few complications associated with antibiotic therapy other than drug allergies addressed previously2 and tetracycline staining of developing teeth. Otherwise, their principal complications are opportunistic yeast infections or GI complications such as nausea, diarrhea, and colitis.
Opportunistic Yeast Infection
Candida albicans is a fungus also referred to as yeast because of its manner of growth. It is a normal component of oropharyngeal and vaginal flora. Overgrowth is ordinarily prevented by resident bacteria, but this control may be lost when patients are immunocompromised or treated with antibiotics. It is not uncommon for female patients to experience opportunistic vulvovaginitis following antibiotic therapy. For susceptible patients, the use of probiotics should be encouraged, as they repopulate the colon and cross the perineum into the vaginal tract. When infection occurs, the patient can obtain an over-the-counter antifungal, or the dentist may prescribe a course of fluconazole.
Antibiotic-Associated Diarrhea
The incidence of diarrhea attributed to those antibiotics commonly used in dentistry ranges from 2 to 10%, and may be as high as 25% with amoxicillin/clavulanic acid (Augmentin).31 In general, diarrhea is related to an imbalance in the normal intestinal flora favoring opportunists. The use of probiotics to prevent or manage antibiotic-associated diarrhea remains controversial. Nevertheless, current evidence suggests they are indeed effective and should be suggested for particularly frail patients or those who have experienced diarrhea with antibiotic regimens in the past.32
Clinically, the challenge when managing a patient with diarrhea is to distinguish so-called nuisance diarrhea from that associated with Clostridium difficile disease. Although C difficile infection accounts for only 10–20% of nuisance cases, it is the principal culprit in the vast majority of colitis cases. Mild symptoms (1 or 2 unformed stools per day) in patients who have previously experienced diarrhea with antibiotics favor nuisance diarrhea and may be managed using antiperistaltics and changing the antibiotic to a narrower spectrum if possible.31,33
C difficile Disease
C difficile is an anaerobic, spore-forming bacillus. It is not regarded as a normal component of intestinal flora; it is present in only 1–4% of the general population. However, it can be found in >20% of patients admitted for a week or more to health care facilities where it resides as a nosocomial pathogen.33,34 Nevertheless, community sources for C difficile are on the rise.
Normal intestinal flora will typically prevent colonization by C difficile, but antibiotics can diminish this protection. If a patient either harbors or comes into contact with C difficile, colonization may supervene. Two recent meta-analyses revealed the odds ratios for C difficile infection to be 16.8–20.4 for clindamycin, 4.5–5.7 for cephalosporins, and 2.7–3.2 for penicillins. Neither study found an increased risk for tetracyclines.35,36 It should be clarified that colonization alone does not necessarily result in C difficile infection. Risk for actual infection depends on the interaction of several additional factors, including virulence of the particular strain and patient-related factors such as age, immune status, and the concurrent use of acid-reduction GI drugs, eg, proton pump inhibitors. Actual consequences of C difficile infection range from diarrhea to pseudomembranous colitis.
In a patient who normally tolerates antibiotics but experiences diarrhea that is florid (≥3 unformed stools per day for ≥2 days) and complains of abdominal pain, C difficile infection should be suspected, and the following protocol is suggested.31,33,37
-
1.
Avoid antiperistaltics. Accumulation of toxin can worsen the infection.
-
2.
Stop the current antibiotic and prescribe metronidazole 500 mg TID × 10–14 days.
-
3.
If there is no improvement after 2–3 days (based on severity), or it subsides and recurs, refer the patient to his or her family physician, who will evaluate fluid/electrolyte status. For severe cases the physician may switch metronidazole to oral vancomycin, which is not absorbed but provides its action locally within the colon. However, oral vancomycin is shockingly expensive and will be initiated only in extreme cases.
It is significant that diarrhea from C difficile infection generally occurs within 5–10 days of commencing an antibiotic, but it has been reported to occur as late as 6–8 weeks following clindamycin use. However, this complication is unheard of following abbreviated use of clindamycin for prophylaxis of infective endocarditis.
CONTINUING EDUCATION QUESTIONS
-
1.
Anterograde amnesia may occur with which of the following?
(1) minimal sedation (2) moderate sedation (3) general anesthesia
-
A.
1 and 2
-
B.
1 and 3
-
C.
2 and 3
-
D.
1, 2, and 3
-
2.
Extrapyramidal side effects may occur with which of the following?
-
A.
Diphenhydramine
-
B.
Hydroxyzine
-
C.
Midazolam
-
D.
Promethazine
-
3.
Opioids produce which of the following side effects?
(1) addiction (2) dependence (3) tolerance
-
A.
1 and 2
-
B.
1 and 3
-
C.
2 and 3
-
D.
1, 2, and 3
-
4.
Clostridium difficile colitis is most often associated with which antibiotic?
-
A.
Amoxicillin
-
B.
Cephalexin
-
C.
Clindamycin
-
D.
Metronidazole
REFERENCES
- 1.Gomes ER, Demoly P. Epidemiology of hypersensitivity drug reactions. Curr Opin Allergy Clin Immunol. 2005;5:309–316. doi: 10.1097/01.all.0000173785.81024.33. [DOI] [PubMed] [Google Scholar]
- 2.Becker DE. Drug allergies and implications for dental practice. Anesth Prog. 2013;60:188–197. doi: 10.2344/0003-3006-60.4.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96:1004–1017. doi: 10.1097/00000542-200204000-00031. [DOI] [PubMed] [Google Scholar]
- 4.Butterworth JF, IV, Mackey DC, Wasnick JS. Morgan & Mikhail's Clinical Anesthesiology. 5th ed. New York, NY: Lange Medical Books/McGraw Hill;; 2013. [Google Scholar]
- 5.Veselis RA, Reinsel RA, Feshchenko VA, Wronski M. The comparative amnestic effects of midazolam, propofol, thiopental, and fentanyl at equisedative concentrations. Anesthesiology. 1997;87:749–764. doi: 10.1097/00000542-199710000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Dierdorf SF, Walton JS. Anesthesia for patients with rare and co-existing diseases. In: Barash PG, BF Cullen, Stoelting RK, editors. Clinical Anesthesia. 5th ed. Philadelphia, PA: JB Lippincott Co;; 2006. In. eds. [Google Scholar]
- 7.Vinson DR, Drotts DL. Diphenhydramine for the prevention of akathisia induced by prochlorperazine: a randomized, controlled trial. Ann Emerg Med. 2001;37:125–131. doi: 10.1067/mem.2001.113032. [DOI] [PubMed] [Google Scholar]
- 8.Berde CB, Strichartz GR. Miller RD, Eriksson LI, Fleisher LA, et al, eds. Miller's Anesthesia. 7th ed. Philadelphia, PA: Elsevier, Churchill Livingstone;; 2009. Local anesthetics. In. [Google Scholar]
- 9.Haas DA, Lennon D. A. 21 year retrospective study of reports of paresthesia following local anesthetic administration. J Can Dent Assoc. 1995;61:319–330. [PubMed] [Google Scholar]
- 10.Garisto GA, Gaffen AS, Lawrence HP, Tenenbaum HC, Haas DA. Occurrence of paresthesia after dental local anesthetic administration in the United States. J Am Dent Assoc. 2010;141:836–844. doi: 10.14219/jada.archive.2010.0281. [DOI] [PubMed] [Google Scholar]
- 11.Hillerup S, Jensen RH, Ersboll BK. Trigeminal nerve injury associated with injection of local anesthetics: needle lesion or neurotoxicity? J Am Dent Assoc. 2011;142:531–539. doi: 10.14219/jada.archive.2011.0223. [DOI] [PubMed] [Google Scholar]
- 12.Hillerup S, Bakke M, Larsen JO, Thomsen CE, Gerds TA. Concentration-dependent neurotoxicity of articaine: an electrophysiological and stereological study of the rat sciatic nerve. Anesth Analg. 2011;112:1330–1338. doi: 10.1213/ANE.0b013e3182172a2e. [DOI] [PubMed] [Google Scholar]
- 13.Katzung BG, White PF. Local anesthetics. In: Katzung BG, Masters SB, Trevor AJ, editors. Basic and Clinical Pharmacology. 11th ed. New York, NY: The McGraw-Hill Companies Inc;; 2009. In. eds. [Google Scholar]
- 14.Goodson JM, Moore PA. Life-threatening reactions after pedodontic sedation: an assessment of narcotic, local anesthetic and antiemetic drug interactions. J Am Dent Assoc. 1983;107:239–245. doi: 10.14219/jada.archive.1983.0225. [DOI] [PubMed] [Google Scholar]
- 15.Barker SJ, Tremper KK, Hyatt J. Effects of methemoglobinemia on pulse oximetry and mixed venous oximetry. Anesthesiology. 1989;70:112–117. doi: 10.1097/00000542-198901000-00021. [DOI] [PubMed] [Google Scholar]
- 16.Becker DE, Reed KL. Local anesthetics: review of pharmacological considerations. Anesth Prog. 2012;59:90–102. doi: 10.2344/0003-3006-59.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becker DE. Basic and clinical pharmacology of autonomic drugs. Anesth Prog. 2012;59:159–168. doi: 10.2344/0003-3006-59.4.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hersh EV, Giannakopoulos H, Levin LM, et al. The pharmacokinetics and cardiovascular effects of high-dose articaine with 1 : 100,000 and 1 : 200,000 epinephrine. J Am Dent Assoc. 2006;137:1562–1571. doi: 10.14219/jada.archive.2006.0092. [DOI] [PubMed] [Google Scholar]
- 19.Dagher FB, Yared GM, Machtou P. An evaluation of 2% lidocaine with different concentrations of epinephrine for inferior alveolar nerve block. J Endod. 1997;23:178–180. doi: 10.1016/S0099-2399(97)80271-3. [DOI] [PubMed] [Google Scholar]
- 20.Tofoli GR, Ramacciato JC, de Oliveira PC, et al. Comparison of effectiveness of 4% articaine associated with 1 : 100,000 or 1 : 200,000 epinephrine in inferior alveolar nerve block. Anesth Prog. 2003;50:164–168. [PMC free article] [PubMed] [Google Scholar]
- 21.Scott DB, Jebson PJR, Braid DP, et al. Factors affecting plasma levels of lignocaine and prilocaine. Br J Anaesth. 1972;44:1040–1049. doi: 10.1093/bja/44.10.1040. [DOI] [PubMed] [Google Scholar]
- 22.Kimmey MB. Cardioprotective effects and gastrointestinal risks of aspirin: maintaining the delicate balance. Am J Med. 2004;117(72s)(78s) doi: 10.1016/j.amjmed.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Delaney JA, Opatrny L, Brophy JM, Suissa S. Drug-drug interactions between antithrombotic medications and the risk of gastrointestinal bleeding. CMAJ. 2007;177:347–351. doi: 10.1503/cmaj.070186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldenberg NA, Jacobson MT, Manco-Johnson MJ. Duration of platelet dysfunction after a 7-day course of ibuprofen. Ann Intern Med. 2005;142:506–509. doi: 10.7326/0003-4819-142-7-200504050-00009. [DOI] [PubMed] [Google Scholar]
- 25.DeBroe ME, Elseviers MM. Analgesic nephropathy. N Engl J Med. 1998;338:446–452. doi: 10.1056/NEJM199802123380707. [DOI] [PubMed] [Google Scholar]
- 26.Abramowicz M. Acetaminophen safety—deja vu. Med Lett Drugs Ther. 2009;51:53. ed. [PubMed] [Google Scholar]
- 27.Grosser T, Smyth E, Fitzgerald GA. Anti-inflammatory, antipyretic, and analgesic agents; pharmacotherapy of gout. In: Brunton LL, Chabner BA, Knollmann BC, editors. Goodman and Gilman's the Pharmacological Basis of Therapeutics. 12th ed. New York, NY: McGraw-Hill Companies Inc;; 2011. In. eds. [Google Scholar]
- 28.Apfel CC. Miller RD, Eriksson LI, Fleisher LA, et al, eds. Miller's Anesthesia. 7th ed. Philadelphia, PA: Elsevier, Churchill Livingstone;; 2009. Postoperative nausea and vomiting. In. [Google Scholar]
- 29.Yaksh TL, Wallace MS. Opioids, analgesia, and pain management. In: Brunton LL, Chabner BA, Knollmann BC, editors. Goodman and Gilman's the Pharmacological Basis of Therapeutics. 12th ed. New York, NY: McGraw-Hill Companies Inc;; 2011. In. eds. [Google Scholar]
- 30.Abramowicz M. Drugs for pain. Treat Guidel Med Lett. 2007;5:23–32. ed. [PubMed] [Google Scholar]
- 31.Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med. 2002;346:334–339. doi: 10.1056/NEJMcp011603. [DOI] [PubMed] [Google Scholar]
- 32.Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959–1969. doi: 10.1001/jama.2012.3507. [DOI] [PubMed] [Google Scholar]
- 33.Gerding DN, Johnson S. Longo DL, Fauci AS, Kasper DL, et al, eds. Harrison's Principles of Internal Medicine. 18th ed. New York, NY: McGraw Hill;; 2012. Clostridium difficile-associated disease, including pseudomembranous colitis. In. [Google Scholar]
- 34.Kelly CP. A 76-year-old man with recurrent Clostridium difficile–associated diarrhea: review of C difficile infection. JAMA. 2009;301:954–962. doi: 10.1001/jama.2009.171. [DOI] [PubMed] [Google Scholar]
- 35.Brown KA, Khanafer N, Daneman N, Fisman DN. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob Agents Chemother. 2013;57:2326–2332. doi: 10.1128/AAC.02176-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deshpande A, Pasupuleti V, Thota P, et al. Community-associated Clostridium difficile infection and antibiotics a meta-analysis. J Antimicrob Chemother. 2013;68:1951–1961. doi: 10.1093/jac/dkt129. [DOI] [PubMed] [Google Scholar]
- 37.Abramowicz M. Treatment of Clostridium difficile infection. Med Lett Drugs Ther. 2011;1358:14–15. ed. [PubMed] [Google Scholar]


