Abstract
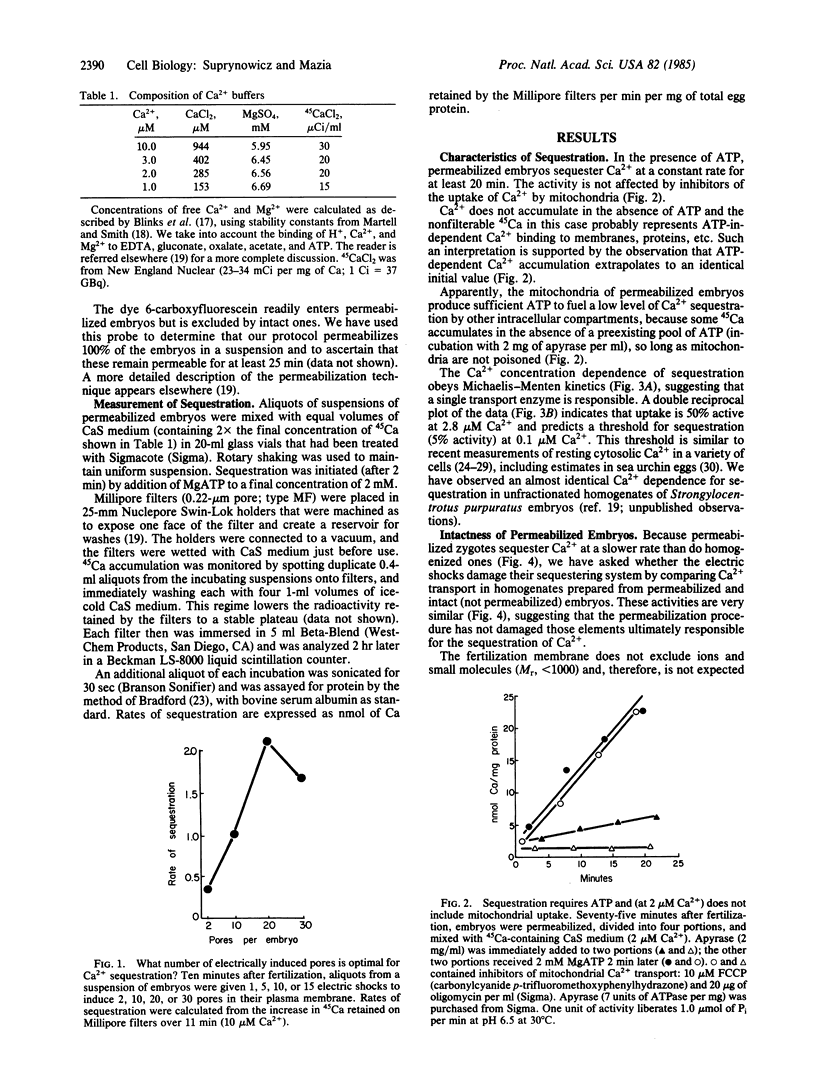
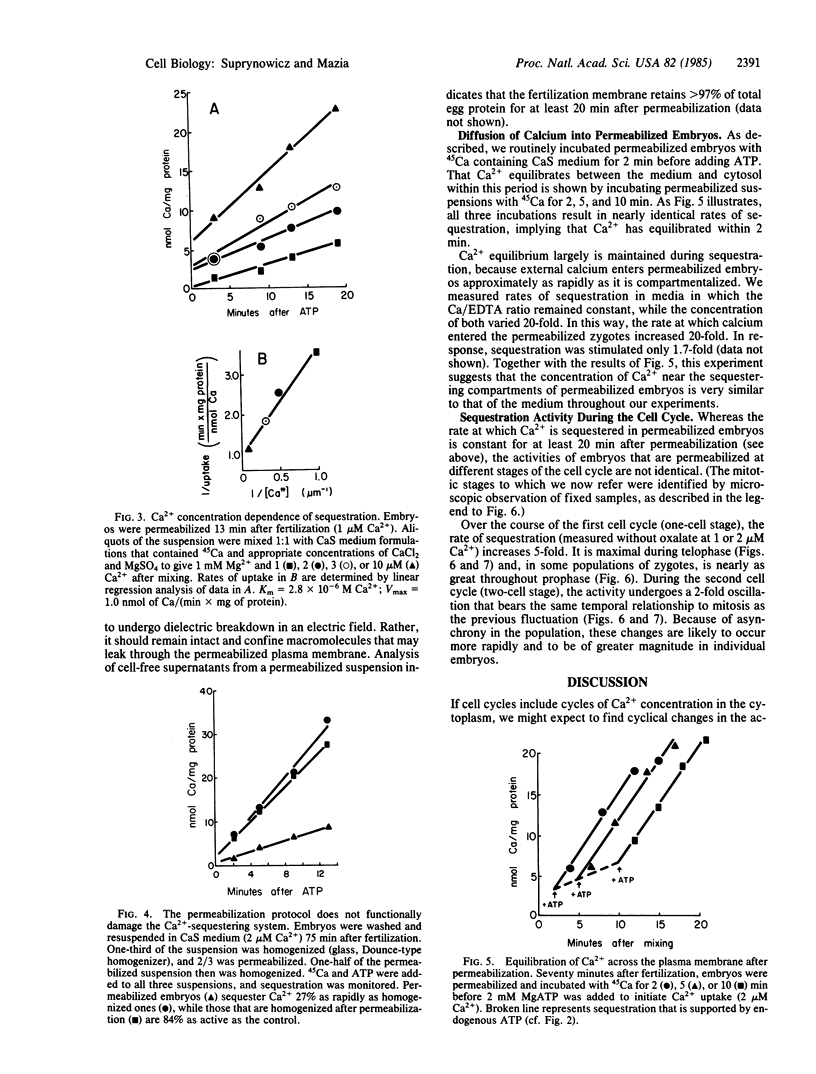
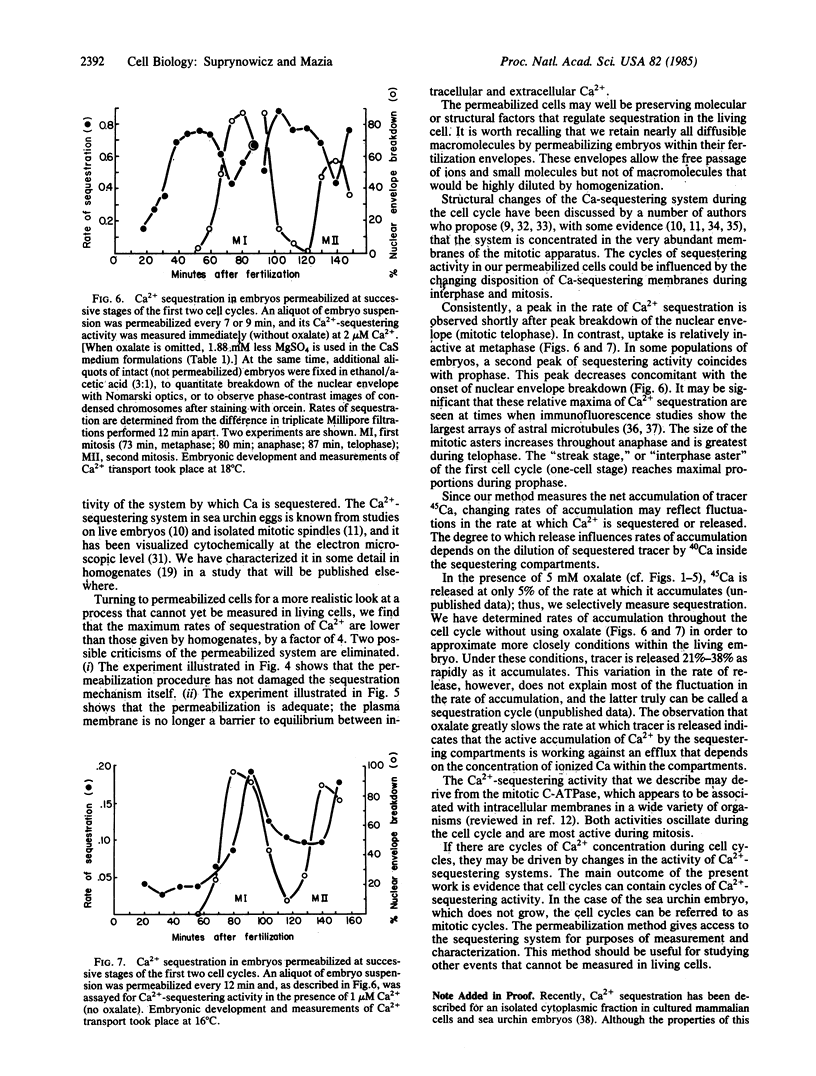
We have followed the sequestration of Ca2+ by intracellular compartments in sea urchin embryos through the first cell cycles. To gain biochemical access to these compartments, the embryos were permeabilized by brief exposure to an intense electric field. Sequestration was determined as the retention of tracer, 45Ca, after filtration of aliquots on Millipore filters. The permeabilized cells sequester Ca2+ at a constant rate for at least 20 min, with the following characteristics: (i) ATP is required. (ii) Sequestration occurs at Ca2+ levels corresponding to those estimated in vivo. (iii) The Ca2+ concentration dependence of sequestration and its insensitivity to mitochondrial poisons imply that the activity derives from a single, nonmitochondrial transport system. The Ca2+-sequestering activities of embryos that are permeabiized at successive stages of the first cell cycle (one-cell stage) progressively increase to 5 times the initial level. The rate of sequestration is maximal during telophase and, in some populations of zygotes, is nearly as great throughout prophase. Over the course of the second cell cycle (two-cell stage), the activity undergoes a 2-fold oscillation that bears the same temporal relationship to mitosis as the previous fluctuation.
Keywords: dielectric breakdown, mitosis
Full text
PDF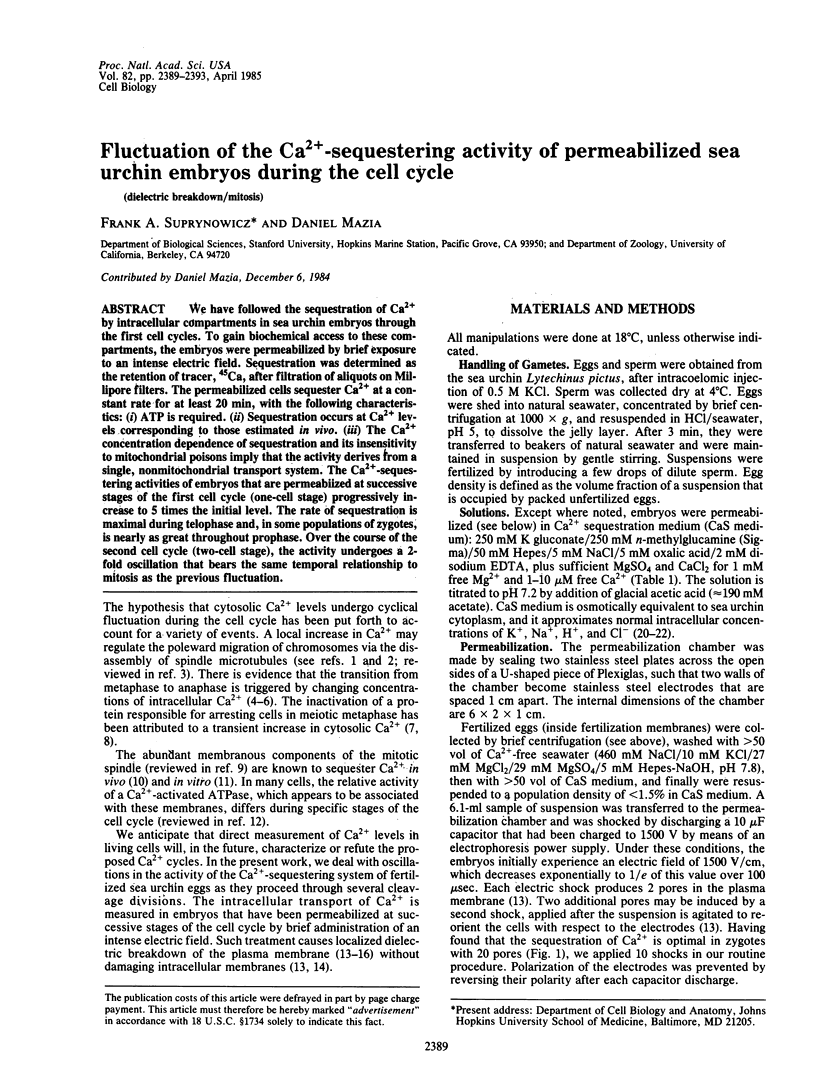

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez-Leefmans F. J., Rink T. J., Tsien R. Y. Free calcium ions in neurones of Helix aspersa measured with ion-selective micro-electrodes. J Physiol. 1981 Jun;315:531–548. doi: 10.1113/jphysiol.1981.sp013762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Wier W. G., Hess P., Prendergast F. G. Measurement of Ca2+ concentrations in living cells. Prog Biophys Mol Biol. 1982;40(1-2):1–114. doi: 10.1016/0079-6107(82)90011-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cande W. Z., McDonald K., Meeusen R. L. A permeabilized cell model for studying cell division: a comparison of anaphase chromosome movement and cleavage furrow constriction in lysed PtK1 cells. J Cell Biol. 1981 Mar;88(3):618–629. doi: 10.1083/jcb.88.3.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cande W. Z. Nucleotide requirements for anaphase chromosome movements in permeabilized mitotic cells: anaphase B but not anaphase A requires ATP. Cell. 1982 Jan;28(1):15–22. doi: 10.1016/0092-8674(82)90370-1. [DOI] [PubMed] [Google Scholar]
- Epel D., Patton C., Wallace R. W., Cheung W. Y. Calmodulin activates NAD kinase of sea urchin eggs: an early event of fertilization. Cell. 1981 Feb;23(2):543–549. doi: 10.1016/0092-8674(81)90150-1. [DOI] [PubMed] [Google Scholar]
- Harris P., Osborn M., Weber K. Distribution of tubulin-containing structures in the egg of the sea urchin Strongylocentrotus purpuratus from fertilization through first cleavage. J Cell Biol. 1980 Mar;84(3):668–679. doi: 10.1083/jcb.84.3.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P. The role of membranes in the ogranization of the mitotic apparatus. Exp Cell Res. 1975 Sep;94(2):409–425. doi: 10.1016/0014-4827(75)90507-8. [DOI] [PubMed] [Google Scholar]
- Inoué S. Cell division and the mitotic spindle. J Cell Biol. 1981 Dec;91(3 Pt 2):131s–147s. doi: 10.1083/jcb.91.3.131s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izant J. G. The role of calcium ions during mitosis. Calcium participates in the anaphase trigger. Chromosoma. 1983;88(1):1–10. doi: 10.1007/BF00329497. [DOI] [PubMed] [Google Scholar]
- Kiehart D. P. Studies on the in vivo sensitivity of spindle microtubules to calcium ions and evidence for a vesicular calcium-sequestering system. J Cell Biol. 1981 Mar;88(3):604–617. doi: 10.1083/jcb.88.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinosita K., Jr, Tsong T. T. Hemolysis of human erythrocytes by transient electric field. Proc Natl Acad Sci U S A. 1977 May;74(5):1923–1927. doi: 10.1073/pnas.74.5.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. Calcium-dependence of catecholamine release from bovine adrenal medullary cells after exposure to intense electric fields. J Membr Biol. 1982;68(2):107–140. doi: 10.1007/BF01872259. [DOI] [PubMed] [Google Scholar]
- López J. R., Alamo L., Caputo C., DiPolo R., Vergara S. Determination of ionic calcium in frog skeletal muscle fibers. Biophys J. 1983 Jul;43(1):1–4. doi: 10.1016/S0006-3495(83)84316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhof P. G., Masui Y. Ca and Mg control of cytostatic factors from Rana pipiens oocytes which cause metaphase and cleavage arrest. Dev Biol. 1977 Dec;61(2):214–229. doi: 10.1016/0012-1606(77)90293-7. [DOI] [PubMed] [Google Scholar]
- Murphy E., Coll K., Rich T. L., Williamson J. R. Hormonal effects on calcium homeostasis in isolated hepatocytes. J Biol Chem. 1980 Jul 25;255(14):6600–6608. [PubMed] [Google Scholar]
- Newport J. W., Kirschner M. W. Regulation of the cell cycle during early Xenopus development. Cell. 1984 Jul;37(3):731–742. doi: 10.1016/0092-8674(84)90409-4. [DOI] [PubMed] [Google Scholar]
- Ochs D. L., Korenbrot J. I., Williams J. A. Intracellular free calcium concentrations in isolated pancreatic acini; effects of secretagogues. Biochem Biophys Res Commun. 1983 Nov 30;117(1):122–128. doi: 10.1016/0006-291x(83)91549-8. [DOI] [PubMed] [Google Scholar]
- Paweletz N. Membranes in the mitotic apparatus. Cell Biol Int Rep. 1981 Apr;5(4):323–336. doi: 10.1016/0309-1651(81)90001-1. [DOI] [PubMed] [Google Scholar]
- Petzelt C. Biochemistry of the mitotic spindle. Int Rev Cytol. 1979;60:53–92. doi: 10.1016/s0074-7696(08)61259-0. [DOI] [PubMed] [Google Scholar]
- Petzelt C., Wülfroth P. Cell cycle specific variations in transport capacity of an isolated Ca2+-transport system. Cell Biol Int Rep. 1984 Oct;8(10):823–840. doi: 10.1016/0309-1651(84)90066-3. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Tsien R. Y., Warner A. E. Free calcium in Xenopus embryos measured with ion-selective microelectrodes. Nature. 1980 Feb 14;283(5748):658–660. doi: 10.1038/283658a0. [DOI] [PubMed] [Google Scholar]
- Schatten G., Schatten H., Simerly C. Detection of sequestered calcium during mitosis in mammalian cell cultures and in mitotic apparatus isolated from sea urchin zygotes. Cell Biol Int Rep. 1982 Aug;6(8):717–724. doi: 10.1016/0309-1651(82)90163-1. [DOI] [PubMed] [Google Scholar]
- Shen S. S., Steinhardt R. A. Direct measurement of intracellular pH during metabolic derepression of the sea urchin egg. Nature. 1978 Mar 16;272(5650):253–254. doi: 10.1038/272253a0. [DOI] [PubMed] [Google Scholar]
- Silver R. B., Cole R. D., Cande W. Z. Isolation of mitotic apparatus containing vesicles with calcium sequestration activity. Cell. 1980 Feb;19(2):505–516. doi: 10.1016/0092-8674(80)90525-5. [DOI] [PubMed] [Google Scholar]
- Steinhardt R. A., Lundin L., Mazia D. Bioelectric responses of the echinoderm egg to fertilization. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2426–2430. doi: 10.1073/pnas.68.10.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolniak S. M., Hepler P. K., Jackson W. T. Detection of the membrane-calcium distribution during mitosis in Haemanthus endosperm with chlorotetracycline. J Cell Biol. 1980 Oct;87(1):23–32. doi: 10.1083/jcb.87.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolniak S. M., Hepler P. K., Jackson W. T. Ionic changes in the mitotic apparatus at the metaphase/anaphase transition. J Cell Biol. 1983 Mar;96(3):598–605. doi: 10.1083/jcb.96.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann U., Pilwat G., Riemann F. Dielectric breakdown of cell membranes. Biophys J. 1974 Nov;14(11):881–899. doi: 10.1016/S0006-3495(74)85956-4. [DOI] [PMC free article] [PubMed] [Google Scholar]



