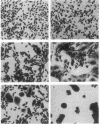
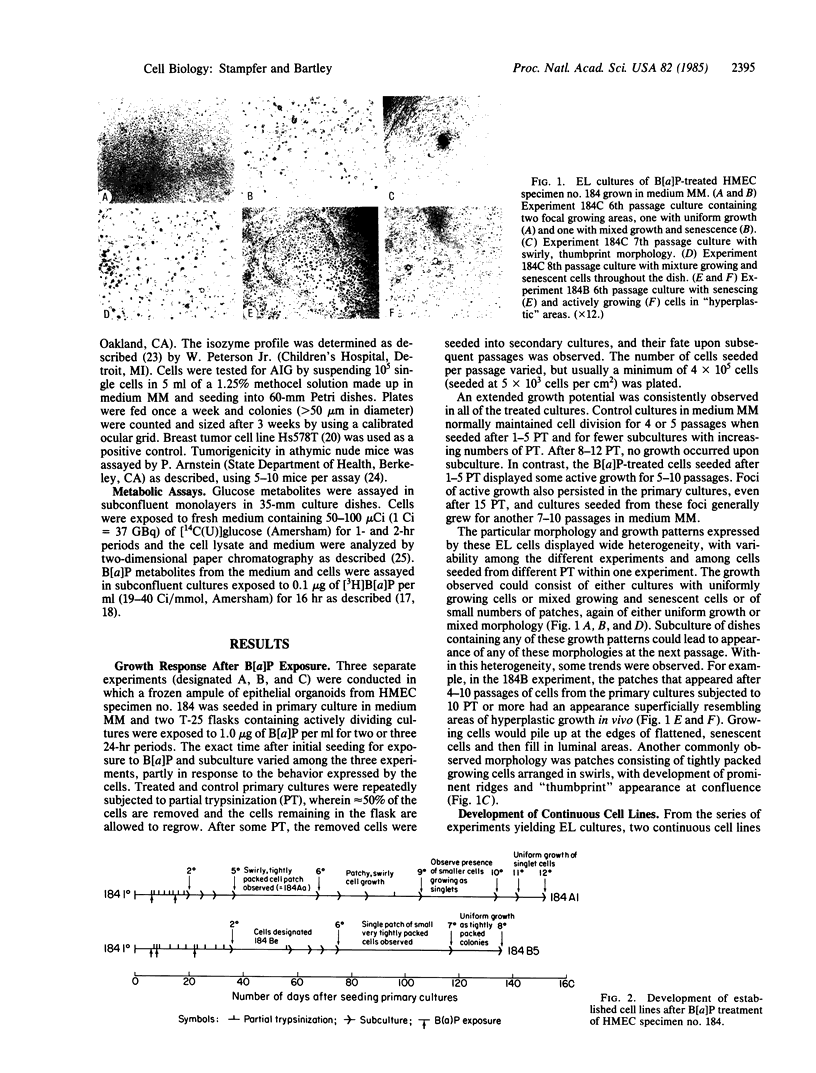
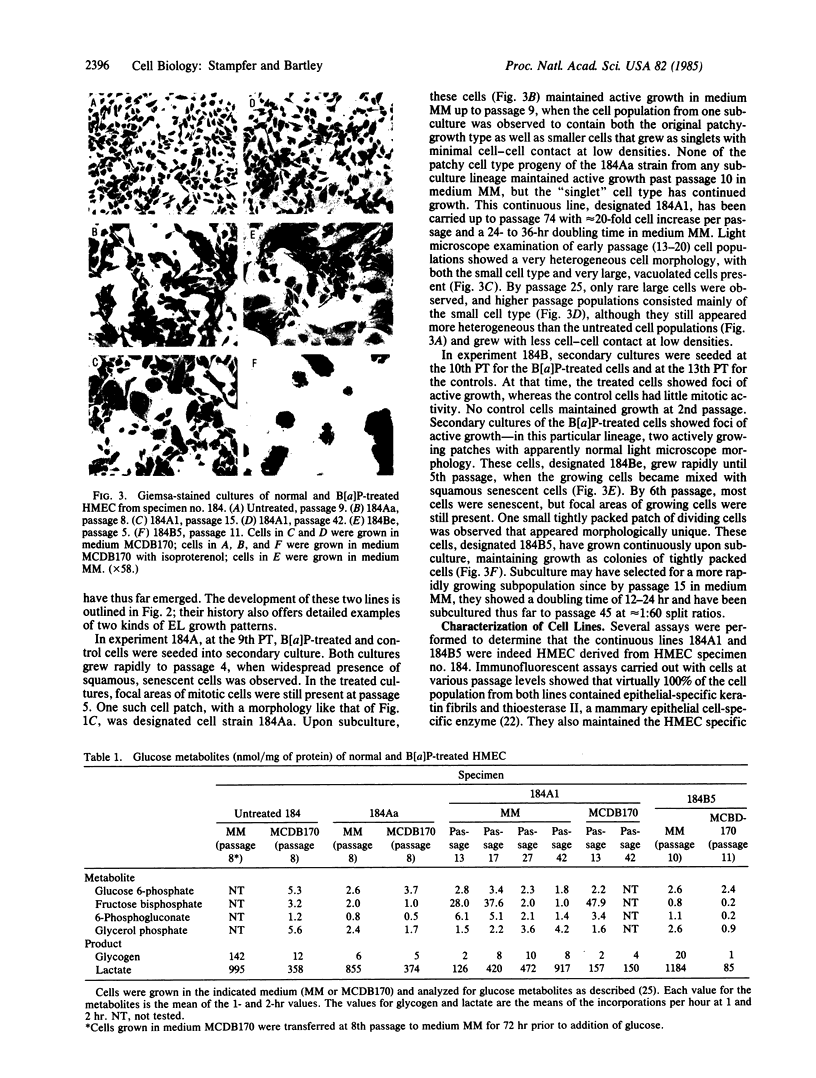
Abstract
Rapidly growing primary cultures of normal human mammary epithelial cells (HMEC) were exposed to 1 microgram of benzo[a]pyrene (B[a]P) per ml for two or three 24-hr periods. The B[a]P-treated populations consistently contained cells displaying a longer period of active growth in culture compared to the untreated control cells. Widespread heterogeneity in morphology and growth patterns was evidenced in these "extended life" (EL) cultures, with multiple sequential changes in these parameters occurring during the course of their life in culture. Two apparently immortal continuous cell lines have thus far emerged from these EL cultures. These lines have been characterized to be of human mammary epithelial origin and derived from the originally treated HMEC specimen. The continuous lines do not appear to be malignantly transformed as they do not cause tumor formation in nude mice and show little or no anchorage-independent growth. Nonetheless, they have acquired several properties characteristic of tumor-derived HMEC, which distinguish them from their normal progenitors. These cell lines, as well as the EL strains, may provide useful substrates for studies to determine what agents can induce further transforming events. Additionally, analysis of the multiple steps occurring in the El cultures, as well as in the emergence of the continuous cell lines, could potentially elucidate the processes occurring during human epithelial cell carcinogenesis and escape from senescence.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arklie J., Taylor-Papadimitrious J., Bodmer W., Egan M., Millis R. Differentiation antigens expressed by epithelial cells in the lactating breast are also detectable in breast cancers. Int J Cancer. 1981 Jul 15;28(1):23–29. doi: 10.1002/ijc.2910280105. [DOI] [PubMed] [Google Scholar]
- Banks-Schlegel S. P., Howley P. M. Differentiation of human epidermal cells transformed by SV40. J Cell Biol. 1983 Feb;96(2):330–337. doi: 10.1083/jcb.96.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley J., Bartholomew J. C., Stampfer M. R. Metabolism of benzo(a)pyrene by human epithelial and fibroblastic cells: metabolite patterns and DNA adduct formation. J Cell Biochem. 1982;18(2):135–148. doi: 10.1002/jcb.1982.240180202. [DOI] [PubMed] [Google Scholar]
- Borek C. X-ray induced in vitro neoplastic transformation of human diploid cells. Nature. 1980 Feb 21;283(5749):776–778. doi: 10.1038/283776a0. [DOI] [PubMed] [Google Scholar]
- Ceriani R. L., Thompson K., Peterson J. A., Abraham S. Surface differentiation antigens of human mammary epithelial cells carried on the human milk fat globule. Proc Natl Acad Sci U S A. 1977 Feb;74(2):582–586. doi: 10.1073/pnas.74.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. E., Keen J., Lane E. B., Taylor-Papadimitriou J. Establishment and characterization of SV40-transformed human breast epithelial cell lines. Cancer Res. 1982 May;42(5):2040–2053. [PubMed] [Google Scholar]
- Colburn N. H., Bruegge W. F., Bates J. R., Gray R. H., Rossen J. D., Kelsey W. H., Shimada T. Correlation of anchorage-independent growth with tumorigenicity of chemically transformed mouse epidermal cells. Cancer Res. 1978 Mar;38(3):624–634. [PubMed] [Google Scholar]
- Dorman B. H., Siegfried J. M., Kaufman D. G. Alterations of human endometrial stromal cells produced by N-methyl-N'-nitro-N-nitrosoguanidine. Cancer Res. 1983 Jul;43(7):3348–3357. [PubMed] [Google Scholar]
- Emerman J. T., Bartley J. C., Bissell M. J. Glucose metabolite patterns as markers of functional differentiation in freshly isolated and cultured mouse mammary epithelial cells. Exp Cell Res. 1981 Jul;134(1):241–250. doi: 10.1016/0014-4827(81)90481-x. [DOI] [PubMed] [Google Scholar]
- Greiner J. W., DiPaolo J. A., Evans C. H. Carcinogen-induced phenotypic alterations in mammary epithelial cells accompanying the development of neoplastic transformation. Cancer Res. 1983 Jan;43(1):273–278. [PubMed] [Google Scholar]
- Hackett A. J., Smith H. S., Springer E. L., Owens R. B., Nelson-Rees W. A., Riggs J. L., Gardner M. B. Two syngeneic cell lines from human breast tissue: the aneuploid mammary epithelial (Hs578T) and the diploid myoepithelial (Hs578Bst) cell lines. J Natl Cancer Inst. 1977 Jun;58(6):1795–1806. doi: 10.1093/jnci/58.6.1795. [DOI] [PubMed] [Google Scholar]
- Halton D. M., Peterson W. D., Jr, Hukku B. Cell culture quality control by rapid isoenzymatic characterization. In Vitro. 1983 Jan;19(1):16–24. doi: 10.1007/BF02617989. [DOI] [PubMed] [Google Scholar]
- Hammond S. L., Ham R. G., Stampfer M. R. Serum-free growth of human mammary epithelial cells: rapid clonal growth in defined medium and extended serial passage with pituitary extract. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5435–5439. doi: 10.1073/pnas.81.17.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaighn M. E., Narayan K. S., Ohnuki Y., Jones L. W., Lechner J. F. Differential properties among clones of simian virus 40-transformed human epithelial cells. Carcinogenesis. 1980 Aug;1(8):635–645. doi: 10.1093/carcin/1.8.635. [DOI] [PubMed] [Google Scholar]
- Knowles M. A., Franks L. M. Stages in neoplastic transformation of adult epithelial cells by 7,12-dimethylbenz(a)anthracene in vitro. Cancer Res. 1977 Nov;37(11):3917–3924. [PubMed] [Google Scholar]
- Kulesz-Martin M. F., Koehler B., Hennings H., Yuspa S. H. Quantitative assay for carcinogen altered differentiation in mouse epidermal cells. Carcinogenesis. 1980;1(12):995–1006. doi: 10.1093/carcin/1.12.995. [DOI] [PubMed] [Google Scholar]
- Milo G. E., Jr, DiPaolo J. A. Neoplastic transformation of human diploid cells in vitro after chemical carcinogen treatment. Nature. 1978 Sep 14;275(5676):130–132. doi: 10.1038/275130a0. [DOI] [PubMed] [Google Scholar]
- Milo G. E., Noyes I., Donahoe J., Weisbrode S. Neoplastic transformation of human epithelial cells in vitro after exposure to chemical carcinogens. Cancer Res. 1981 Dec;41(12 Pt 1):5096–5102. [PubMed] [Google Scholar]
- Owens R. B., Smith H. S., Nelson-Rees W. A., Springer E. L. Epithelial cell cultures from normal and cancerous human tissues. J Natl Cancer Inst. 1976 Apr;56(4):843–849. doi: 10.1093/jnci/56.4.843. [DOI] [PubMed] [Google Scholar]
- Parsa I., Marsh W. H., Sutton A. L. An in vitro model of human pancreas carcinogenesis: effects of nitroso compounds. Cancer. 1981 Mar 15;47(6 Suppl):1543–1551. doi: 10.1002/1097-0142(19810315)47:6+<1543::aid-cncr2820471417>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Rhim J. S., Trimmer R., Arnstein P., Huebner R. J. Neoplastic transformation of chimpanzee cells induced by adenovirus type 12--simian virus 40 hybrid virus. Proc Natl Acad Sci U S A. 1981 Jan;78(1):313–317. doi: 10.1073/pnas.78.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinskas K. C., Kateley S. A., Tower J. E., Maher V. M., McCormick J. J. Induction of anchorage-independent growth in human fibroblasts by propane sultone. Cancer Res. 1981 May;41(5):1620–1627. [PubMed] [Google Scholar]
- Sina J. F., Bradley M. O., O'Brien T. G. Neoplastic transformation of Syrian hamster epidermal cells in vitro. Cancer Res. 1982 Oct;42(10):4116–4123. [PubMed] [Google Scholar]
- Smith S., Pasco D., Pawlak J., Thompson B. J., Stampfer M., Nandi S. Thioesterase II, a new marker enzyme for human cells of breast epithelial origin. J Natl Cancer Inst. 1984 Aug;73(2):323–329. doi: 10.1093/jnci/73.2.323. [DOI] [PubMed] [Google Scholar]
- Stampfer M. R., Bartholomew J. C., Smith H. S., Bartley J. C. Metabolism of benzo[a]pyrene by human mammary epithelial cells: toxicity and DNA adduct formation. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6251–6255. doi: 10.1073/pnas.78.10.6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfer M. R. Cholera toxin stimulation of human mammary epithelial cells in culture. In Vitro. 1982 Jun;18(6):531–537. doi: 10.1007/BF02810076. [DOI] [PubMed] [Google Scholar]
- Stampfer M. R., Vlodavsky I., Smith H. S., Ford R., Becker F. F., Riggs J. Fibronectin production by human mammary cells. J Natl Cancer Inst. 1981 Aug;67(2):253–261. [PubMed] [Google Scholar]
- Stampfer M., Hallowes R. C., Hackett A. J. Growth of normal human mammary cells in culture. In Vitro. 1980 May;16(5):415–425. doi: 10.1007/BF02618365. [DOI] [PubMed] [Google Scholar]
- Steinberg M. L., Defendi V. Altered pattern of growth and differentiation in human keratinocytes infected by simian virus 40. Proc Natl Acad Sci U S A. 1979 Feb;76(2):801–805. doi: 10.1073/pnas.76.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomassen D. G., Gray T. E., Mass M. J., Barrett J. C. High frequency of carcinogen-induced early, preneoplastic changes in rat tracheal epithelial cells in culture. Cancer Res. 1983 Dec;43(12 Pt 1):5956–5963. [PubMed] [Google Scholar]
- Walen K. H. Chromosome analyses and anchorage-independent growth of SV40-induced morphologically transformed epithelial cells from amniotic fluids. In Vitro. 1981 Jun;17(6):531–539. doi: 10.1007/BF02633514. [DOI] [PubMed] [Google Scholar]