Abstract
Vitamin D metabolites have been extensively studied as cancer chemopreventive agents. Gc-globulin (GC) isotypes, based on rs7041 and rs4588 diplotypes, have varying affinities for 1α,25-dihydroxyvitamin D (1,25(OH)2D) and 25-hydroxyvitamin D (25(OH)D), which may affect circulating metabolite concentration as well as delivery at the cellular level. We evaluated associations between GC isotype and circulating vitamin D metabolite concentrations in 403 Ursodeoxycholic Acid (UDCA) clinical trial participants. Metabolite uptake was evaluated in human colon cancer (HCT-116) cells treated with ethanol vehicle, 1,25(OH)2D, or 25(OH)D, and with plasma from individuals with known GC isotype. Mammalian-2-hybrid and vitamin D-responsive element-based luciferase assays were employed to measure VDR pathway activation as a marker for metabolite uptake. Regression analysis demonstrated significantly lower serum 25(OH)D concentration for clinical trial participants with 1F_2, 1S_2, or 2_2 isotypes (p<0.01) compared to 1S_1S. Consistent with these in vivo observations, cellular data revealed that 25(OH)D uptake varied less by GC isotype only at the higher concentration tested (p=0.05), while 1,25(OH)2D uptake differed markedly by GC isotype across concentration and assay (p<0.01). The 1F_1S and 1F_2 isotypes produced the greatest reporter gene induction with 1,25(OH)2D treatment and, while activation varied less with 25(OH)D, the 2_2 isotype demonstrated increased induction at the lower concentration. These results suggest that vitamin D metabolite concentration and delivery to colon cells may vary not only by GC isotype, but also that certain isotypes may more effectively deliver 1,25(OH)2D versus 25(OH)D. Overall, these results may help identify populations at risk for cancer and potential recipients of targeted chemoprevention.
Keywords: Gc-globulin, vitamin D, 25(OH)D, colon cancer, megalin
Introduction
Studies demonstrate that individuals with lower circulating vitamin D metabolite concentrations are at increased risk of several diseases, including cancer (1–4). The pro-hormone is endogenously synthesized in skin exposed to UVB or ingested through the diet and/or supplement intake (5–8). Gc-globulin (GC), also known as the vitamin D binding protein, is the primary transport protein for vitamin D metabolites in circulation (9, 10). GC, a serum α2-globulin made of 458 amino acids (51.2 kDa) and part of the albumin superfamily of binding proteins, is primarily synthesized by parenchymal cells in the liver (8, 11). The protein transports 80–90% of vitamin D metabolites and has the greatest affinity for 25(OH)D (10). However, it is unknown how genetic variation in GC affecting the 1,25(OH)2D or 25(OH)D concentration available in circulation relates to uptake at the cellular level.
There are two commonly studied GC polymorphisms (rs4588 and rs7041) and, as shown in Table 1, the amino acid changes at positions 416 and 420 give rise to the 3 common phenotypic alleles of 1F, 1S, and 2 (12, 13). There are six combinations of these phenotypic alleles, defined by diplotypes of rs4588 and rs7041; which represent the GC protein isotypes including 1F_1F, 1F_1S, 1F_2, 1S_1S, 1S_2, and 2_2. These GC isotypes demonstrate differences in affinity for vitamin D and vary dramatically in frequency by race-ethnicity (10, 14). White individuals have a lower frequency of the 1F compared to 1S (10, 11, 15); whereas the 2 allele is the least common in all populations, but has a higher frequency in white populations (10). There is also evidence that both GC genotype and GC isotype, as defined by diplotypes, are associated with variation in circulating vitamin D metabolite concentrations.
Table 1.
Changes in nucleotides and amino acids by GC isotype
| Phenotypic Alslele | Diplotype from rs7041/rs4588 | Amino Acid (position 416/420) |
|---|---|---|
| 1F_1F | TT/CC | Asp/Thr |
| 1S_1S | GG/CC | Glu/Thr |
| 2_2 | TT/AA | Asp/Lys |
Circulating GC concentration is approximately 1000-fold higher than that of vitamin D metabolites with only an estimated 0.2–0.6% of vitamin D metabolites unbound in the serum (10, 11). GC binding is believed to protect metabolites from catabolism and excretion, which increases the half-life of molecules (10, 16). Our previous work demonstrated strong associations between circulating 25(OH)D and the GC gene overall as well as seven individual polymorphisms including rs7041, rs222035, rs842999, rs1155563, rs12512631, rs16846876, rs1746825 (17). Additional epidemiologic studies demonstrate that 25(OH)D levels vary by GC and that higher concentrations are observed for those with 1F_1F or 1S_1S versus 2_2 isotypes (12, 18). Furthermore, studies have identified consistent associations between circulating 25(OH)D levels and colorectal neoplasia risk (2, 19–21). Therefore, variation in the affinity of GC for vitamin D metabolites alters circulating concentrations as well as potentially concentrations that reach the cellular level, independently of circulating concentrations of the binding protein.
We hypothesize that GC isotypes may affect not only circulating vitamin D metabolite concentrations, but also delivery at the cellular level. Chun et al. demonstrated that availability of 25(OH)D in cells differed by GC isotype, as measured by 24-hydroxylase expression in monocytes (22). However, this relationship had not previously been tested with 1,25(OH)2D treatment or in colon cells. The current study expanded upon previous work to evaluate associations between circulating vitamin D metabolite concentration and GC isotypes at the population level, as well as to establish a novel experimental screening system to determine if GC isotype influenced vitamin D metabolite uptake in colorectal carcinoma cells, with measurable biological endpoints relevant to tumorigenesis. The overall goal of this translational research is to identify factors that may influence colorectal neoplasia risk in order to identify individuals at risk for cancer or potential recipients for targeted chemoprevention.
Materials and Methods
Epidemiologic Analysis
Study Population
The epidemiologic analysis included participants from the ursodeoxycholic acid (UDCA) clinical trial conducted at the Arizona Cancer Center, which has been previously described (23–25). Briefly, the UDCA trial was a phase III randomized, double-blind, placebo-controlled trial conducted to test the effect of UDCA on recurrence of colorectal neoplasia (23). The study recruited Arizona residents between 40 to 80 years of age with a history of removal of one or more colorectal adenomas (> 3 mm in diameter) during a colonoscopy prior to study enrollment (23). There were 1192 participants in the overall sample with complete genotype data; however, the sample was further restricted (N=403) to individuals who reported white race with complete vitamin D metabolite and genotype measurements (23, 26). Restriction was necessary because there were not enough individuals of varied race/ethnicity to account for population stratification. The University of Arizona Human Subjects Protection Program approved the UDCA trial and informed consent was obtained for all subjects prior to enrollment.
Genotyping and Vitamin D Metabolite Measurement
Genotyping of participants has been described previously and two GC polymorphisms (rs7041 and rs4588) were chosen a priori and included as part of the original Illumina Golden Gate platform (Illumina®, San Diego, CA) (27, 28). Circulating vitamin D metabolite concentrations were measured at the Bruce Hollis Lab (University of South Carolina) (29, 30). This laboratory utilized multiple QA/QC measures, as described previously, with demonstrated coefficient of variations less than 7.0% for 25(OH)D and 11.0% for 1,25(OH)2D (31, 32).
Statistical Analysis
Linear regression models were utilized to evaluate associations between GC isotype and circulating 25(OH)D concentration. Common factors related to circulating 25(OH)D concentration including age, BMI, and gender were assessed for confounding and none were statistically significant. Each GC group was compared to the 1S_1S isotype as a standard. Due to a low count of individuals with the 1F_1F isotype (N = 9), as expected in a white population, this group was combined with the 1F_1S group. Biologically, these groups differ only by the rs7041 polymorphism, and combining these groups increased power without significantly altering the results.
Cell-based Assays
Cell Culture, Transfection, and Dosing
HCT-116 colorectal carcinoma cells were purchased from the American Tissue Culture Collection (ATCC; Manassas, Virginia) and grown at 37°C in a humidified 5% CO2 incubator. Cells were seeded in 24-well plates at a density of 100,000 cells/well and grown overnight in Dulbecco’s modified eagle medium (DMEM) supplemented with 5% fetal bovine serum (FBS), 100 μg/ml penicillin, and 100 μg/ml streptomycin. The cells were transfected using Express-In Transfection Reagent supplied by Thermo Scientific (Waltham, MA) and plasmids appropriate to the vitamin D receptor-retinoid X receptor (VDR-RXR) Mammalian-two-hybrid (M2H) or vitamin D responsive element (VDRE) biological assay system, which have been previously described (33). Briefly, the M2H plasmids include human VDR cloned into pCMV-AD (prey) and human RXRα cloned into pCMV-BD (bait) along with the luciferase and renilla reporter genes. The M2H system includes five tandem copies of the BD DNA binding site in the pFR-Luc reporter plasmid, compared to two tandem DNA binding sites in the natural VDRE-based system, and thus the M2H is more sensitive to activation with 1,25(OH)2D. Luciferase is expressed following 1,25(OH)2D-activation of the VDR-RXR heterodimer and subsequent transcription of the luciferase reporter gene. The renilla gene is included for measurement and control of transfection efficiency. Each well received 2 μl Express-In, along with 25 ng pCMV-BD-hRXRα (bait) and 25 ng pCMV-AD-hVDR (prey), plus 200 ng pFR-Luc (reporter gene) and 20 ng pRL-NULL (Renilla reniformis) for the M2H system. The VDRE-based assay included transfection of cells with 20 ng pRL-NULL (Renilla reniformis), 50 ng pSG5-hVDR (vitamin D receptor) and 250 ng PER6-Luc (VDRE from the human CYP3A4 gene promoter region linked to luciferase).
Cells were incubated for 24 hours at 37°C and 5% CO2 then dosed with ethanol vehicle or different concentrations of 1,25(OH)2D or 25(OH)D plus 2.5% plasma from individuals with known GC isotype. Plasma samples from six UDCA trial participants, each representing one GC isotype group, were utilized to provide GC for uniform addition to cell culture experiments. We tested the ethanol vehicle plus participant plasma alone with no vitamin D metabolites added, and observed minimal activation of the luciferase systems compared to cells treated with 25(OH)D or 1,25(OH)2D (Figure 1A and B). From this, we concluded that differences in the circulating vitamin D metabolite concentrations or GC concentration in the plasma did not significantly affect activation of the luciferase systems. Furthermore, utilization of 2.5% plasma in each well was also tested compared to 5% plasma, which is traditionally used in cell culture experiments, and no appreciable difference in cell growth or uptake of vitamin D metabolites was detected (data not shown). The lower percent plasma was chosen in order to conserve the limited plasma available from selected participants. Finally, we took into consideration that circulating GC concentration is approximately 1000-fold higher than vitamin D metabolites in vivo. Therefore, using a lower concentration of plasma was still expected to provide sufficient GC concentration in the experimental model for the majority of vitamin D metabolites to be bound to GC, similarly to in vivo, and was not expected to affect delivery at the cellular level.
Fig. 1.
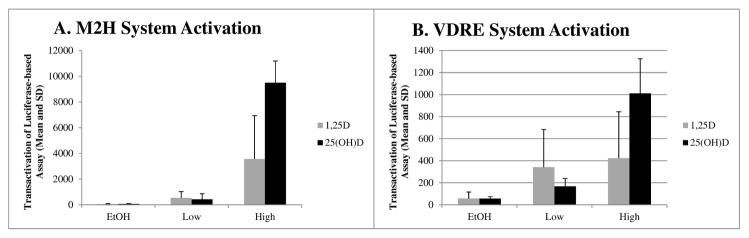
Evaluation of dose-response in M2H versus VDRE-based luciferase reporter systems by vitamin D metabolite and treatment group. Both panels display the mean and standard deviation of VDR-mediated transactivation in HCT-116 cells, as a function of the luciferase/renilla ratio (in RLUs) multiplied by 10,000. EtOH served as the vehicle control in both system and minimal activation of the both assays was observed following EtOH treatment. Cells transfected with components of A) the M2H system, demonstrate increasing dose response with two concentrations of 1,25(OH)2D (1×10−8 M and 5×10−10 M) and 25(OH)D (5×10−7 M and 5×10−8 M). Cells treated with 25(OH)D demonstrated increased activation at the highest concentrations tested in both assays.
Experiments were conducted to determine the best concentrations of 1,25(OH)2D and 25(OH)D to be employed for the current work. We began by testing concentrations utilized in previous experiments (data not shown) (34), and chose final concentrations that demonstrated a consistent dose response in the M2H system (Figure 1A), as well as activation of the VDRE system (Figure 1B), with 2.5% serum. For both M2H and VDRE assays with 1,25(OH)2D, 1×10−8 M and 5×10−10 M were included (final concentration). In order to account for variation in the uptake of 25(OH)D, higher concentrations of 5×10−7 M and 5×10−8M were tested. HCT-116 cells have demonstrated activity of CYP27B1, which is required for intracellular conversion of 25(OH)D to 1,25(OH)2D (34). Plates were then again incubated for 24 hours, cells lysed, and luciferase assays (Promega; Madison, WI) were performed to measure luciferase activity as an index of uptake of vitamin D metabolites.
Statistical Analysis
For cellular experiments, luciferase expression was normalized to renilla levels by dividing luciferase by renilla values then multiplying by 10,000. The mean and standard deviation of at least replicate experiments were calculated (N = 7 for M2H and N = 4 for VDRE), with triplicate samples in each treatment group within each independent experiment. As described above, the 1S_1S isotype was used as the standard and percent 1S_1S expression was calculated. ANOVA regression was performed to test for an overall pattern of statistically significant differences between the GC isotypes then, if the overall p-value was less than 0.05, Tukey’s adjustment for pairwise comparisons was utilized to identify which individual GC isotypes were significantly different from 1S_1S. Data management and analyses were performed using SAS 9.3 (Cary, NC) and STATA (College Station, TX).
Results
Baseline characteristics: UDCA study participants
The baseline characteristics of the UDCA clinical trial participants (N=1192), as well as the subset used for the current analysis (N=403), are presented in Table 2 and have been previously described in detail (17, 26). The subset of UDCA trial participants had a mean age of 66.1 ± 8.5 years and 65.5% were male. Mean BMI was 28.4 ± 4.8 kg/m2 while total calcium intake was within range of the recommended daily allowance (1034.5 ± 516.8 g/day), and reported supplement use was high (77.9%). This subset of participants with vitamin D metabolite measures did not differ with respect to any of the selected characteristics from the UDCA study as a whole.
Table 2.
Baseline characteristics of study population
| Characteristics | UDCA | Subset |
|---|---|---|
|
| ||
| N = 1192 | N = 4031 | |
| Mean age, y ± SD | 66.2 ± 8.5 | 66.1 ± 8.5 |
| Sex, Male, n (%) | 804 (67.5) | 294 (65.5) |
| Race, White, n (%) | 1108 (94.5) | 403 (100.0) |
| Mean BMI, kg/m2 ± SD | 28.2 ± 4.8 | 28.4 ± 4.8 |
| Aspirin use, n (%) | 331 (27.8) | 120 (29.8) |
| Ever smoker, n (%) | 802 (69.3) | 271 (68.3) |
| Current smoker, n (%) | 140 (11.7) | 52 (12.9) |
| Total Fat, g/day ± SD | 62.2 ± 31.7 | 61.1 ± 30.3 |
| Energy, kcal/day ± SD | 1987.9 ± 815.6 | 1998.1 ± 812.6 |
| Calcium intake, g/day ± SD | 1008.5 ± 494.4 | 1034.5 ± 516.8 |
| 25(OH)D concentration, ng/ml ± SD | N/A | 26.6 ± 9.2 |
| 1,25(OH)2D concentration, pg/ml ± SD | N/A | 35.3 ± 9.2 |
| Supplemental vitamin D use, Yes, n (%) | 856 (71.8) | 314 (77.9) |
Missing for BMI, N = 10; ever smoker, N = 6.
Epidemiologic analysis: Associations between GC isotype and circulating vitamin D metabolites
The results for associations between circulating 25(OH)D concentration and GC isotype are presented in Table 3. Our analysis demonstrated statistically significantly lower circulating 25(OH)D with the isotypes including the 2 phenotypic allele compared to 1S_1S (p-trend < 0.001). Circulating 25(OH)D concentrations decreased with additional copies of the 2 allele, with the 2_2 isotype demonstrating a mean concentration 6.8 ng/ml (95% CI −10.23 – −3.39) less than the 1S_1S isotype reference group, which had an observed mean of 28.4 ng/ml (95% CI 26.8 – 30.0). The 1S_2 and 1F_2 isotypes also demonstrated statistically significantly (p< 0.02) lower circulating 25(OH)D concentrations compared to the 1S_1S group (−3.03 ng/ml and −3.76 ng/ml, respectively). The combined 1F_1F and 1F_1S groups did not demonstrate significantly different 25(OH)D levels from the reference group (p=0.82). There was no statistically significant difference in circulating 1,25(OH)2D concentrations by GC isotype (data not shown). These differences in the UDCA study participants were also evaluated in cellular experiments.
Table 3.
Associations between GC isotype and circulating 25(OH)D concentrations.
| Frequency N (%)1 |
Difference in 25(OH)D (ng/ml) from 1S_1S Isotype Mean (95% CI) |
p-value2 | |
|---|---|---|---|
| Gc Isotype | |||
| 1S_1S | 120 (29.8) | REF3 | REF |
| 1F_1F/1F_1S | 82 (20.4) | 0.30 (−2.23, 2.82) | 0.82 |
| 1S_2 | 125 (31.0) | −3.03 (−5.29, −0.78) | 0.01 |
| 1F_2 | 42 (10.4) | −3.76 (−6.92, −0.60) | 0.02 |
| 2_2 | 34 (8.4) | −6.81 (−10.23, −3.39) | <0.01 |
| p-trend | 0.001 | ||
N=403. One participant was excluded because the diplotype was not represented in the common categories, as previously described by Abbas et al. (12).
Compared to 1S_1S using linear regression among white participants.
The reference group mean is 28.42, 95% CI (26.8, 30.0)
Cellular-based assays: GC isotype and uptake of vitamin D metabolites
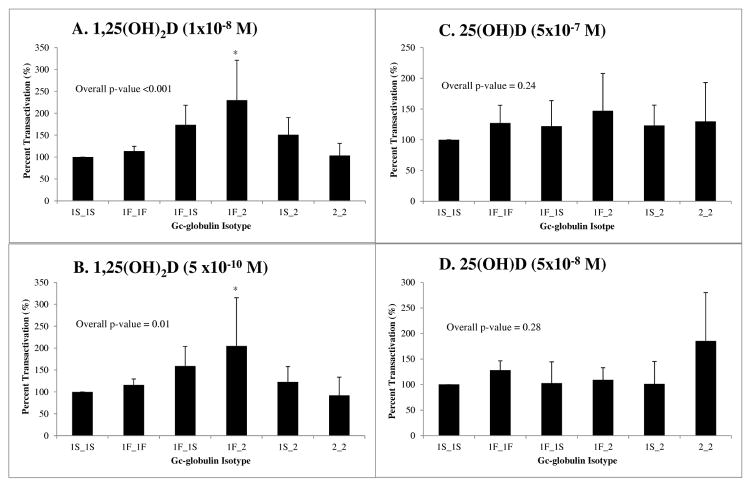
The in vitro experiments using HCT-116 cells demonstrated statistically significant differences in 1,25(OH)2D and 25(OH)D uptake by GC isotype, as illustrated in Figures 2 and 3. The percent transactivation of the luciferase reporter gene in HCT-116 cells by GC isotype, using 1S_1S set to 100 as a reference, was significantly different overall using ANOVA analysis in the M2H system (Figure 2), at both concentrations of 1,25(OH)2D tested (p<0.01). At 1×10−8 M of 1,25(OH)2D (Fig. 2A), the 1F_1S, 1F_2, and 1S_2 isotypes demonstrated increased activation relative to 1S_1S. However, at 5×10−10 M 1,25(OH)2D (Fig. 2B), overall induction of luciferase decreased compared to 1S_1S, but the majority of isotypes followed a similar pattern as the higher concentration. At both concentrations, the reporter gene induction with the 1F_2 isotype was statistically significantly greater than the 1S_1S isotype (p<0.05) using Tukey’s method for pairwise comparisons. We were unable to detect a statistically significant difference in activation of the M2H luciferase system with 25(OH)D treatment. However, all isotypes demonstrated higher activation compared to 1S_1S at 5×10−7 M 25(OH)D (Fig. 2C), while only the 1F_1F and 2_2 isotypes activated luciferase to a greater extent than 1S_1S at the lower concentration of 5×10−8 M 25(OH)D (Fig. 2D).
Fig. 2.
Assessment of 1,25(OH)2D and 25(OH)D uptake in colon cancer cells employing a M2H luciferase reporter system. Each panel depicts the percent transactivation (luciferase/renilla RLUs) of the luciferase system compared to 1S_1S isotype as a control (set to 100%) in HCT-116 cells using the M2H system. Seven replicate experiments with 3 wells per group were conducted for the M2H system. Cells dosed with either A) 1×10−8 M or B) 5 x10−10 M 1,25(OH)2D demonstrate significantly different activation of the M2H system (Overall ANOVA p<0.01). The 1F_1S and 1F_2 isotypes led to a statistically significant increased activation compared to 1S_1S in this system (Tukey’s pairwise *p<0.05). Cells treated with either C) 5×10−7 M or D) 5×10−8 M 25(OH)D did not reveal statistically significant variation in activation of the luciferase system (Overall ANOVA p=0.24 and 0.28, respectively), though the 2_2 isotype demonstrates higher activation at the lower concentration of 25(OH)D (panel D).
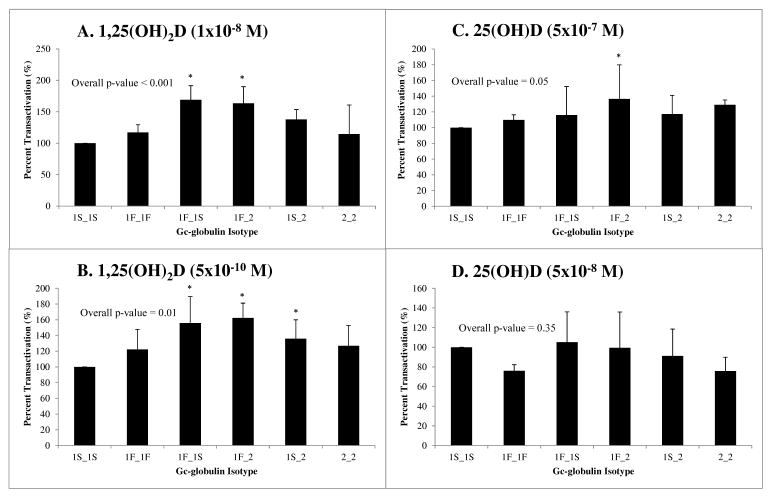
Fig. 3.
Assessment of 1,25(OH)2D and 25(OH)D uptake in colon cancer cells employing a VDRE-based luciferase reporter system. Each panel depicts the percent transactivation (luciferase/renilla RLUs) of the luciferase system using the 1S_1S isotype as a control (set to 100%) in HCT-116 cells using the VDRE system. Four replicate experiments with 3 wells per group were conducted for the M2H system. Cells dosed with either A) 1×10−8 M or B) 5 x10−10 M 1,25(OH)2D demonstrate significantly different induction of luciferase via VDRE-directed transcription (Overall ANOVA p<0.001 and 0.01, respectively). The 1F_1S, 1F_2, and 1S_2 isotypes exhibited statistically significantly increased activation above 1S_1S in this system (Tukey’s pairwise *p<0.05). Cells treated with either C) 5×10−7 M or D) 5×10−8 M 25(OH)D revealed varied results in activation of the luciferase system. Treatment with 5×10−7 M 25(OH)D led to a marginally significant overall variation in system activation (Overall ANOVA p = 0.05), with the greatest change demonstrated by increased activation with 1F_2 compared to 1S_1S treatment (Tukey’s pairwise *p<0.05).
We also tested uptake of vitamin D metabolites using a VDRE-based luciferase system as shown in Figure 3. In this VDRE-based system, the percent transactivation of the luciferase reporter gene was significantly different (Overall ANOVA p<0.001 and p<0.01, respectively) with the two concentrations of 1,25(OH)2D tested (Fig. 3A and B). Again, the 1F_1S and 1F_2 isotypes demonstrated increased activation at the highest concentration of 5×10−9 M 1,25(OH)2D, while activation for these isotypes as well as 1S_2 was significantly increased at 5×10−10 M compared to the 1S_1S isotype (Tukey’s p<0.05) (Fig. 3A and B). Uptake of 25(OH)D was also marginally statistically significantly different when tested in the VDRE system at 5×10−7 M 25(OH)D (Overall ANOVA p=0.05), though not at the lower concentration, with the 1F_2 isotype demonstrating the greatest difference in activation compared to 1S_1S (Tukey’s p<0.05) ((Fig. 3C and D). For all experiments, the EtOH vehicle plus serum only treatment group did not significantly activate the luciferase reporter gene system, indicating that variation in vitamin D metabolite concentration between participant samples did not affect the results. These results provide evidence that delivery and uptake of vitamin D metabolites may vary at the cellular level by GC isotype.
Discussion
Scientific evidence regarding a relationship between vitamin D and cancer is building, but there are still many unanswered questions related to factors affecting vitamin D status and delivery at the cellular level. This study provides evidence that functional variation in GC affects circulating 25(OH)D concentration as well as delivery of vitamin D metabolites at the cellular level to colon cells. The epidemiologic analysis identified associations between GC isotypes and clinically relevant differences in circulating 25(OH)D concentration. Furthermore, at the cellular level in human colonocytes, the experiments demonstrated statistically significant differences in activation of two VDR-based luciferase systems, across multiple concentrations of 1,25(OH)2D and a single concentration of 25(OH)D, by GC isotype. Overall, this evidence provides a translational basis for further studies of the vitamin D endocrine system and cancer prevention in diverse and at-risk populations.
The results of the present analyses support the hypothesis that GC isotype, as defined by diplotypes of two commonly studied polymorphisms, are associated with circulating 25(OH)D concentration at the population level. We found that overall, the 1F_2, 1S_2 and 2_2 isotypes demonstrate statistically significantly lower circulating 25(OH)D compared to the 1S_1S, 1F_1S, and 1F_1F isotypes. Previous studies primarily evaluated associations by genotype or allele. Our previous work (17) as well as analyses by Ahn et al. (35) identified GC polymorphisms significantly associated with serum 25(OH)D concentrations, including rs7041 and rs4588. Sinotte et al. also found that, in premenopausal women, 25(OH)D levels significantly declined with additional copies of the rare genotypes defining 1F/1S and 2 (36). However, more recent studies have demonstrated that the 2_2 isotype is associated with decreased circulating concentrations of vitamin D metabolites (12, 37). It has been proposed that the 1F_1F isotype, which has a greater affinity for vitamin D metabolites, may lead to more efficient transport and protection from catabolism (10, 14). However, there may be factors not accounted for in these population level studies as there is also evidence, as described below, that the reported differences in circulating vitamin D metabolites by GC isotype may translate to tissue-specific and/or cellular level effects.
There are two models related to vitamin D metabolite delivery at the cellular level. The “free-hormone hypothesis” states that unbound metabolites act upon target tissues, while the GC-bound metabolites act as stores in the body (8, 38). A second, complementary theory identifies the additional proteins of megalin and cubilin that act to take up the GC-bound metabolites into cells (39, 40). With either mechanism, variations in affinity of GC isotypes for vitamin D metabolites could alter the intracellular concentration and ability to activate the transcriptional activity of VDR. At the cellular level, 1,25(OH)2D has been demonstrated to reduce cancer-promoting phenotypes in a variety of cell types (7, 8, 18, 41, 42). However, only one previous study to date has attempted to quantify the effect of factors that influence intracellular metabolite concentration and hence the potential anti-cancer activity of vitamin D. Using induction of 24-hydroxylase (CYP24A1) as a surrogate for intracellular 1,25(OH)2D concentration, Chun et al. demonstrated that the 1S_2 and 2_2 isotypes led to the greatest induction of the enzyme when monocytes were treated with 25(OH)D (22). We only detected a marginally significant difference by GC genotype with 5×10−7 M 25(OH)D in the VDRE-based assay; however, the 2_2 isotype did demonstrate increased activity in the M2H assay at the lower concentration tested. Furthermore, with 1,25(OH)2D treatment, similar patterns of activation across GC isotypes and assay were observed. In contrast, only 25(OH)D treatment was tested by Chun et al. using real-time PCR in monocytes, cells which they also demonstrated do not utilize megalin-mediated endocytosis (22). Our previous work has demonstrated that HCT-116 cells do utilize megalin for uptake of vitamin D metabolites (submitted for publication), which could explain the differences between the results of these studies and requires further exploration. The results of the present study may indicate that additional factors play a role in vitamin D metabolite uptake in colon cells, including the potential role for megalin-mediated endocytosis of GC-bound vitamin D metabolites, which may have implications for colon cancer prevention.
The strongest evidence from epidemiologic studies of a relationship between low vitamin D status and cancer is for increased risk of colorectal neoplasia (21, 43, 44). Low circulating 25(OH)D concentration has been associated with adenoma incidence and recurrence (20, 26), as well as increased colon cancer incidence (4, 45, 46). However, though the associations are consistent, there are observations in some populations that indicate a potential role for unaccounted factors. For example, historically, age-adjusted rates of colorectal neoplasia are lower among Hispanic populations compared to non-Hispanic, while circulating 25(OH)D concentrations are lower (47). There is also an observed paradox for African American populations with low circulating 25(OH)D level, yet reduced risk of health outcomes such as fractures (48). The GC-megalin interaction could also provide a potential mechanism for this relationship as bone is one tissue in which megalin-mediated endocytosis has been demonstrated (49, 50). This further supports the hypothesis that the increased affinity of 1F_1F isotype for vitamin D metabolites, though rare in white populations, may have evolved to protect from catabolism and deliver limited stores of vitamin D metabolites more efficiently to cells. Interestingly, the 2_2 isotype demonstrated relatively higher activation at the lower concentration of 25(OH)D compared to 1,25(OH)2D. This could mean that in white individuals with the 2_2 isotype, who demonstrate lower circulating 25(OH)D concentrations, the GC is able to deliver and release the metabolite more efficiently at the cellular level, whereupon it is metabolized to 1,25(OH)2D. This hypothesis was supported by the results of the current work in colon cancer cells.
The results of the current work identified differences in activation of a VDR-based luciferase system by GC isotype and vitamin D metabolite concentration in colon cancer cells. At the highest concentration of 1,25(OH)2D tested, the 1F_1S and 1F_2 isotypes demonstrated increased activation, while the 1F_1F or 2_2 isotypes did not activate the system as well. Arnaud et al. demonstrated that the 1F_1F isotype had the highest affinity for both 25(OH)D and 1,25(OH)2D compared to 1S_1S and 2_2 (14). This may indicate that at low circulating vitamin D metabolite concentrations, 1F_1F binds more tightly in order to help maintain a store of these lipophilic molecules, but at the cellular interface additional factors likely play a role in delivery within certain tissues. For example, since 1F_1F has the highest affinity constant, and thus lowest dissociation constant, it may be the interaction of GC with proteins such as megalin and cubilin that actively mediate uptake in place of diffusion alone when metabolite concentrations are low. This hypothesis supports further testing of GC isotypes to more clearly determine their role in cancer risk in both the circulation and once they reach the tissue and cellular levels.
There were strengths as well as limitations of the current work. For the epidemiologic analysis, the samples size was sufficient to detect the large difference in 25(OH)D concentration by GC isotype. However, the sample was not large enough to appropriately account for population stratification by race/ethnicity. Furthermore, the 1F_1F isotype is more common in African American populations and the next step will be to examine associations between GC isotype, circulating vitamin D metabolites concentrations, and risk for colorectal neoplasia in large, diverse populations. For the colon cell-based experiments, the strengths included testing the associations in two different, but complementary, versions of the luciferase assay system as well as across multiple doses of 25(OH)D and 1,25(OH)2D. However, future studies should incorporate testing of purified GC with interactions between megalin and cubilin as well as compare the mechanism of vitamin D uptake in normal colon versus colon cancer cells. Furthermore, variation is common in the luciferase assay and testing the ranking of GC isotypes using additional techniques, such as qPCR, would be beneficial. Nonetheless, these novel results provide quantitative evidence for the effect of variation in GC on the vitamin D endocrine system in the colon; and further analysis and experimentation is warranted.
We have presented evidence for the effect of Gc-globulin isotype on vitamin D status at the population-level as well as a potential mechanism for variation in vitamin D metabolite uptake in colon cancer cells. This translational research supports the hypothesis that circulating 25(OH)D concentration alone is not the only factor of importance in evaluating risk for disease, including cancer. Factors that affect delivery of vitamin D metabolites at the tissue-level, including GC-globulin, and potentially tissue-specific variation in that mechanism may alter the risk of neoplasia in that tissue. We thus suggest that studying GC isotype, in addition to circulating vitamin D metabolite concentrations, may identify heterogeneity in associations between circulating vitamin D metabolite concentration and colorectal neoplasia risk, specifically. Identifying genetic variation that modifies colorectal neoplasia risk could ultimately help identify individuals at risk for cancer or potential recipients for targeted chemoprevention.
Acknowledgments
Financial Support: This work was supported by the R25T training fellowship (R25CA078447) awarded to E. Hibler as well as a grant from the National Cancer Institute (R01CA140285) awarded to E. Jacobs and P. Jurutka
Footnotes
Competing interests: No authors have any financial conflicts of interest to disclose.
References
- 1.Davis CD. Vitamin D and cancer: current dilemmas and future research needs. Am J Clin Nutr. 2008;88:565S–9S. doi: 10.1093/ajcn/88.2.565S. [DOI] [PubMed] [Google Scholar]
- 2.Gandini S, Boniol M, Haukka J, Byrnes G, Cox B, Sneyd MJ, et al. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. 2011;128:1414–24. doi: 10.1002/ijc.25439. [DOI] [PubMed] [Google Scholar]
- 3.Garland CF, Gorham ED, Mohr SB, Garland FC. Vitamin D for cancer prevention: global perspective. Ann Epidemiol. 2009;19:468–83. doi: 10.1016/j.annepidem.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 4.Giovannucci E. Vitamin D and Cancer Incidence in the Harvard Cohorts. Ann Epidemiol. 2008 doi: 10.1016/j.annepidem.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 5.IOM. Dietary Reference Intake for Calcium and Vitamin D. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 6.Adams JS, Hewison M. Update in Vitamin D. J Clin Endocrinol Metab. 2010;95:471–8. doi: 10.1210/jc.2009-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dixon KM, Mason RS. Vitamin D. Int J Biochem Cell Biol. 2009;41:982–5. doi: 10.1016/j.biocel.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 8.Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008;88:491S–9S. doi: 10.1093/ajcn/88.2.491S. [DOI] [PubMed] [Google Scholar]
- 9.Willnow TE, Nykjaer A. Cellular uptake of steroid carrier proteins--mechanisms and implications. Mol Cell Endocrinol. 2010;316:93–102. doi: 10.1016/j.mce.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 10.Speeckaert M, Huang G, Delanghe JR, Taes YE. Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clinica chimica acta; international journal of clinical chemistry. 2006;372:33–42. doi: 10.1016/j.cca.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Gomme PT, Bertolini J. Therapeutic potential of vitamin D-binding protein. Trends Biotechnol. 2004;22:340–5. doi: 10.1016/j.tibtech.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Abbas S, Linseisen J, Slanger T, Kropp S, Mutschelknauss EJ, Flesch-Janys D, et al. The Gc2 allele of the vitamin D binding protein is associated with a decreased postmenopausal breast cancer risk, independent of the vitamin D status. Cancer Epidemiol Biomarkers Prev. 2008;17:1339–43. doi: 10.1158/1055-9965.EPI-08-0162. [DOI] [PubMed] [Google Scholar]
- 13.Christiansen M, Jorgensen CS, Laursen I, Hirschberg D, Hojrup P, Houen G. Protein chemical characterization of Gc globulin (vitamin D-binding protein) isoforms; Gc-1f, Gc-1s and Gc-2. Biochim Biophys Acta. 2007;1774:481–92. doi: 10.1016/j.bbapap.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Arnaud J, Constans J. Affinity differences for vitamin D metabolites associated with the genetic isoforms of the human serum carrier protein (DBP) Hum Genet. 1993;92:183–8. doi: 10.1007/BF00219689. [DOI] [PubMed] [Google Scholar]
- 15.Fu L, Yun F, Oczak M, Wong BY, Vieth R, Cole DE. Common genetic variants of the vitamin D binding protein (DBP) predict differences in response of serum 25-hydroxyvitamin D [25(OH)D] to vitamin D supplementation. Clin Biochem. 2009 doi: 10.1016/j.clinbiochem.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Haddad J. The Vitamin D Binding Protein and Its Clinical Significance. In: Holick MF, editor. Vitamin D: Physiology, Molecular Biology, and Clinical Applications. Totowa, NJ: Humana Press, Inc; 1999. pp. 101–7. [Google Scholar]
- 17.Hibler EA, Hu C, Jurutka PW, Martinez ME, Jacobs ET. Polymorphic variation in the GC and CASR genes and associations with vitamin D metabolite concentration and metachronous colorectal neoplasia. Cancer Epidemiol Biomarkers Prev. 2012;21:368–75. doi: 10.1158/1055-9965.EPI-11-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289:F8–28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 19.Harriss DJ, Atkinson G, Batterham A, George K, Cable NT, Reilly T, et al. Lifestyle factors and colorectal cancer risk (2): a systematic review and meta-analysis of associations with leisure-time physical activity. Colorectal Dis. 2009;11:689–701. doi: 10.1111/j.1463-1318.2009.01767.x. [DOI] [PubMed] [Google Scholar]
- 20.Hartman TJ, Albert PS, Snyder K, Slattery ML, Caan B, Paskett E, et al. The association of calcium and vitamin D with risk of colorectal adenomas. J Nutr. 2005;135:252–9. doi: 10.1093/jn/135.2.252. [DOI] [PubMed] [Google Scholar]
- 21.Lee JE, Li H, Chan AT, Hollis BW, Lee IM, Stampfer MJ, et al. Circulating Levels of Vitamin D and Colon and Rectal Cancer: The Physicians’ Health Study and a Meta-analysis of Prospective Studies. Cancer Prev Res (Phila) 2011;4:735–43. doi: 10.1158/1940-6207.CAPR-10-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chun RF, Lauridsen AL, Suon L, Zella LA, Pike JW, Modlin RL, et al. Vitamin D-binding protein directs monocyte responses to 25-hydroxy- and 1,25-dihydroxyvitamin D. J Clin Endocrinol Metab. 2010;95:3368–76. doi: 10.1210/jc.2010-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alberts DS, Martinez ME, Hess LM, Einspahr JG, Green SB, Bhattacharyya AK, et al. Phase III trial of ursodeoxycholic acid to prevent colorectal adenoma recurrence. J Natl Cancer Inst. 2005;97:846–53. doi: 10.1093/jnci/dji144. [DOI] [PubMed] [Google Scholar]
- 24.Alberts DS, Martinez ME, Roe DJ, Guillen-Rodriguez JM, Marshall JR, van Leeuwen JB, et al. Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas. Phoenix Colon Cancer Prevention Physicians’ Network. The New England journal of medicine. 2000;342:1156–62. doi: 10.1056/NEJM200004203421602. [DOI] [PubMed] [Google Scholar]
- 25.Martinez ME, Reid ME, Guillen-Rodriguez J, Marshall JR, Sampliner R, Aickin M, et al. Design and baseline characteristics of study participants in the Wheat Bran Fiber trial. Cancer Epidemiol Biomarkers Prev. 1998;7:813–6. [PubMed] [Google Scholar]
- 26.Jacobs ET, Alberts DS, Benuzillo J, Hollis BW, Thompson PA, Martinez ME. Serum 25(OH)D levels, dietary intake of vitamin D, and colorectal adenoma recurrence. J Steroid Biochem Mol Biol. 2007;103:752–6. doi: 10.1016/j.jsbmb.2006.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egan JB, Thompson PA, Ashbeck EL, Conti DV, Duggan D, Hibler E, et al. Genetic polymorphisms in vitamin D receptor VDR/RXRA influence the likelihood of colon adenoma recurrence. Cancer research. 2010;70:1496–504. doi: 10.1158/0008-5472.CAN-09-3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levine AJ, Figueiredo JC, Won L, Poynter JN, Conti D, Duggan DJ, et al. Genetic Variability in the MTHFR gene and colorectal cancer risk in the Colorectal Cancer Family Registry. Cancer Epidemiol Biomarkers Prev. 2009;19:89–100. doi: 10.1158/1055-9965.EPI-09-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin Chem. 1993;39:529–33. [PubMed] [Google Scholar]
- 30.Hollis BW. Assay of circulating 1,25-dihydroxyvitamin D involving a novel single-cartridge extraction and purification procedure. Clin Chem. 1986;32:2060–3. [PubMed] [Google Scholar]
- 31.Jacobs E, Martinez ME, Buckmeier J, Lance P, May M, Jurutka P. Circulating fibroblast growth factor-23 is associated with increased risk for metachronous colorectal adenoma. J Carcinog. 2011;10:3. doi: 10.4103/1477-3163.76723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobs ET, Alberts DS, Foote JA, Green SB, Hollis BW, Yu Z, et al. Vitamin D insufficiency in southern Arizona. Am J Clin Nutr. 2008;87:608–13. doi: 10.1093/ajcn/87.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartik L, Whitfield GK, Kaczmarska M, Lowmiller CL, Moffet EW, Furmick JK, et al. Curcumin: a novel nutritionally derived ligand of the vitamin D receptor with implications for colon cancer chemoprevention. J Nutr Biochem. 2010;21:1153–61. doi: 10.1016/j.jnutbio.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobs ET, Van Pelt C, Forster RE, Zaidi W, Hibler EA, Galligan MA, et al. CYP24A1 and CYP27B1 Polymorphisms Modulate Vitamin D Metabolism in Colon Cancer Cells. Cancer research. 2013;73:2563–73. doi: 10.1158/0008-5472.CAN-12-4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahn J, Albanes D, Berndt SI, Peters U, Chatterjee N, Freedman ND, et al. Vitamin D-related genes, serum vitamin D concentrations and prostate cancer risk. Carcinogenesis. 2009;30:769–76. doi: 10.1093/carcin/bgp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sinotte M, Diorio C, Berube S, Pollak M, Brisson J. Genetic polymorphisms of the vitamin D binding protein and plasma concentrations of 25-hydroxyvitamin D in premenopausal women. Am J Clin Nutr. 2009;89:634–40. doi: 10.3945/ajcn.2008.26445. [DOI] [PubMed] [Google Scholar]
- 37.Lauridsen AL, Vestergaard P, Hermann AP, Brot C, Heickendorff L, Mosekilde L, et al. Plasma concentrations of 25-hydroxy-vitamin D and 1,25-dihydroxy-vitamin D are related to the phenotype of Gc (vitamin D-binding protein): a cross-sectional study on 595 early postmenopausal women. Calcif Tissue Int. 2005;77:15–22. doi: 10.1007/s00223-004-0227-5. [DOI] [PubMed] [Google Scholar]
- 38.Zerwekh JE. Blood biomarkers of vitamin D status. Am J Clin Nutr. 2008;87:1087S–91S. doi: 10.1093/ajcn/87.4.1087S. [DOI] [PubMed] [Google Scholar]
- 39.Nykjaer A, Fyfe JC, Kozyraki R, Leheste JR, Jacobsen C, Nielsen MS, et al. Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D(3) Proc Natl Acad Sci U S A. 2001;98:13895–900. doi: 10.1073/pnas.241516998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rowling MJ, Kemmis CM, Taffany DA, Welsh J. Megalin-mediated endocytosis of vitamin D binding protein correlates with 25-hydroxycholecalciferol actions in human mammary cells. J Nutr. 2006;136:2754–9. doi: 10.1093/jn/136.11.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adorini L, Penna G, Amuchastegui S, Cossetti C, Aquilano F, Mariani R, et al. Inhibition of prostate growth and inflammation by the vitamin D receptor agonist BXL-628 (elocalcitol) J Steroid Biochem Mol Biol. 2007;103:689–93. doi: 10.1016/j.jsbmb.2006.12.065. [DOI] [PubMed] [Google Scholar]
- 42.Palmer HG, Gonzalez-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, et al. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol. 2001;154:369–87. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baron JA, Beach M, Mandel JS, van Stolk RU, Haile RW, Sandler RS, et al. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. The New England journal of medicine. 1999;340:101–7. doi: 10.1056/NEJM199901143400204. [DOI] [PubMed] [Google Scholar]
- 44.Wei MY, Garland CF, Gorham ED, Mohr SB, Giovannucci E. Vitamin D and prevention of colorectal adenoma: a meta-analysis. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17:2958–69. doi: 10.1158/1055-9965.EPI-08-0402. [DOI] [PubMed] [Google Scholar]
- 45.Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–9. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 46.Ng K, Wolpin BM, Meyerhardt JA, Wu K, Chan AT, Hollis BW, et al. Prospective study of predictors of vitamin D status and survival in patients with colorectal cancer. Br J Cancer. 2009;101:916–23. doi: 10.1038/sj.bjc.6605262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng XE, Lipka S, Li T, Shahzad G, Levine E, Vlacancich R, et al. The relationship of vitamin D status, smoking, and colorectal adenoma: A retrospective study in an ethnically diverse community. The Journal of steroid biochemistry and molecular biology. 2012 doi: 10.1016/j.jsbmb.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 48.Aloia JF. African Americans, 25-hydroxyvitamin D, and osteoporosis: a paradox. Am J Clin Nutr. 2008;88:545S–50S. doi: 10.1093/ajcn/88.2.545S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Atkins GJ, Anderson PH, Findlay DM, Welldon KJ, Vincent C, Zannettino AC, et al. Metabolism of vitamin D3 in human osteoblasts: evidence for autocrine and paracrine activities of 1 alpha,25-dihydroxyvitamin D3. Bone. 2007;40:1517–28. doi: 10.1016/j.bone.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 50.Wang C, Hu YM, He JW, Gu JM, Zhang H, Hu WW, et al. Association between low density lipoprotein receptor-related protein 2 gene polymorphisms and bone mineral density variation in Chinese population. PloS one. 2011;6:e28874. doi: 10.1371/journal.pone.0028874. [DOI] [PMC free article] [PubMed] [Google Scholar]