Abstract
Objectives
To quantitatively summarize the association of dietary magnesium (Mg) intake with serum C-reactive protein (CRP) levels in the general population.
Methods
Observational and experimental studies through February 2013 were reviewed in PubMed and EMBASE. Additional information was retrieved through Google or hand search of related reference lists. The main outcome is either adjusted geometric mean of CRP or odds ratio (OR) of having serum CRP≥3 mg/L. Meta-regression was used to determine the linear association of dietary Mg intake and adjusted geometric means of CRP levels. A fixed-effects model was used to pool ORs of interest, comparing those in the lowest with those in the highest group of dietary Mg intake.
Results
A dataset derived from seven cross-sectional studies including 32,918 participants was quantitatively assessed. A weighted inverse association between Mg intake and serum CRP levels was observed[β coefficient: −0.0028; 95% CI, −0.0043 to −0.0013; P for trend=0.001] from four cross-sectional studies. The pooled OR (95%CI) of having CRP≥3 mg/L was 1.49(1.18 to 1.89) comparing the lowest to the highest group of Mg intake from three studies with data available. Qualitative assessment among five intervention studies also showed a potential beneficial effect of Mg intake on serum CRP levels.
Conclusion
This meta-analysis and systematic review indicate that dietary Mg intake is significantly and inversely associated with serum CRP levels. The potential beneficial effect of Mg intake on chronic diseases may be, at least in part, explained by inhibiting inflammation.
Keywords: magnesium, inflammation, C-reactive protein, meta-analysis, systematic review
INTRODUCTION
Magnesium (Mg), an essential mineral, is found abundantly in whole grains, leafy green vegetables, legumes and nuts, and hundreds of body physiologies involving over 350 enzymes require it(1). However, according to National Health and Nutrition Examination Survey (NHANES), 1999–2000, about 60% of US population consumed inadequate dietary Mg. Dietary Mg intake has been related to several health outcomes including those related to metabolic and inflammatory processes such as hypertension, metabolic syndrome(2–4), type 2 diabetes(5), cardiovascular diseases(1, 6–8), osteoporosis, and some cancers (e.g. colon, breast)(9). One suggested mechanism for the beneficial effect of Mg intake is that Mg may reduce the levels of C-reactive protein (CRP) - a well-documented indicator of a low grade or chronic inflammation.
Laboratory studies have linked Mg deficiency to acute inflammatory response mediated by calcium, N-Methyl-D-aspartic acid or N-Methyl-D-aspartate (NMDA), interleukin-6(IL-6), and tumor necrosing factor–alpha (TNF-α)(9). So far, findings from epidemiological studies on the association of Mg and CRP levels are not consistent. Several cross-sectional studies reported an inverse association between serum Mg concentrations and CRP levels in children(10),13, women(4), and obese patients(11). Also, in a study of samples from NHANES 1999–2002, a national representative population in the United States, people with total daily Mg intake below the recommended daily allowance(RDA) were 40% more likely to have elevated CRP levels(12). In addition, a cross-sectional study also found an inverse association between dietary Mg intake and serum CRP levels(13). However, another recent cross-sectional study found no association between dietary Mg intake and CRP levels(14). Therefore, we summarized the literature quantitatively to estimate the overall association of Mg and CRP levels by conducting a meta-analysis as well as systematic review.
METHODS
Data Source and Study Selection
We searched the databases including PubMed, EMBASE and Google up to February 2013, along with the references obtained from the identified studies and reviews using the terms ‘magnesium’, ‘Mg’, ‘dietary micronutrient’ combined with ‘C-reactive Protein’, ‘high sensitivity C-reactive protein’, ‘CRP’, ‘hs-CRP’, and ‘biomarkers of inflammation’.
Observational studies, reporting either mean or odds ratio (OR), are included in the quantitative meta-analysis. Other studies which included one cohort study, two cross-sectional studies reporting outcomes (correlation coefficient (r) or ORs) on continuous scale and five intervention studies that reported correlation coefficients, geometric mean, or median level of CRP or changes in CRP levels between baseline and the end of study are included for a systematic review.
Data Extraction
Data were carefully extracted from the original studies independently by two authors (D. D and P. X.), and any disagreements were resolved by consensus. The data that we collected included the first author’s name, year of publication, study name, number of participants, age, percent of male participants, exposure assessment and category, outcome assessment, adjusted covariates, and adjusted average levels of CRP or ORs of having serum CRP ≥ 3 mg/L with 95%CIs for corresponding categories and/or continuous exposure.
Data Synthesis and analysis
The included studies were categorized into two groups: cross-sectional studies with adjusted geometric mean as the outcome, and cross-sectional studies with OR as the outcome. We extracted adjusted geometric means of CRP levels with 95% CIs as well as median Mg levels for each quintiles of Mg intake in cross-sectional studies(4, 13–15). In all the studies, data from fully adjusted models were used.
Data transformed to their natural logarithms (ln) were used to compute the corresponding standard errors (SEs) and inverse variance. A random effects meta-regression(16) was used to assess the overall linear relation between Mg intake and geometric mean levels of CRP with inverse variance as weights for each study. We also extracted ORs with 95% confidence intervals (CIs)in three cross-sectional studies(10, 17, 18). Natural logarithms transformed ORs and 95% CIs were used to compute the corresponding SEs. Fixed-effects models were used to combine ORs of having CRP ≥3 mg/L by comparing participants in the lowest to those in the highest group of Mg intake because there was no significant heterogeneity found among studies. Cochran’s Chi-square test was used to examine heterogeneity among included studies and I2 was computed to determine the degree of inconsistency across studies(19, 20). Publication bias was assessed by Egger’s test(21) and Begg’s test(22). All analyses were conducted using STATA statistical software (version 12.1, STATA Corp., College Station, TX, USA). All statistical tests were two-sided and P-value ≤0.05 was considered statistically significant.
RESULTS
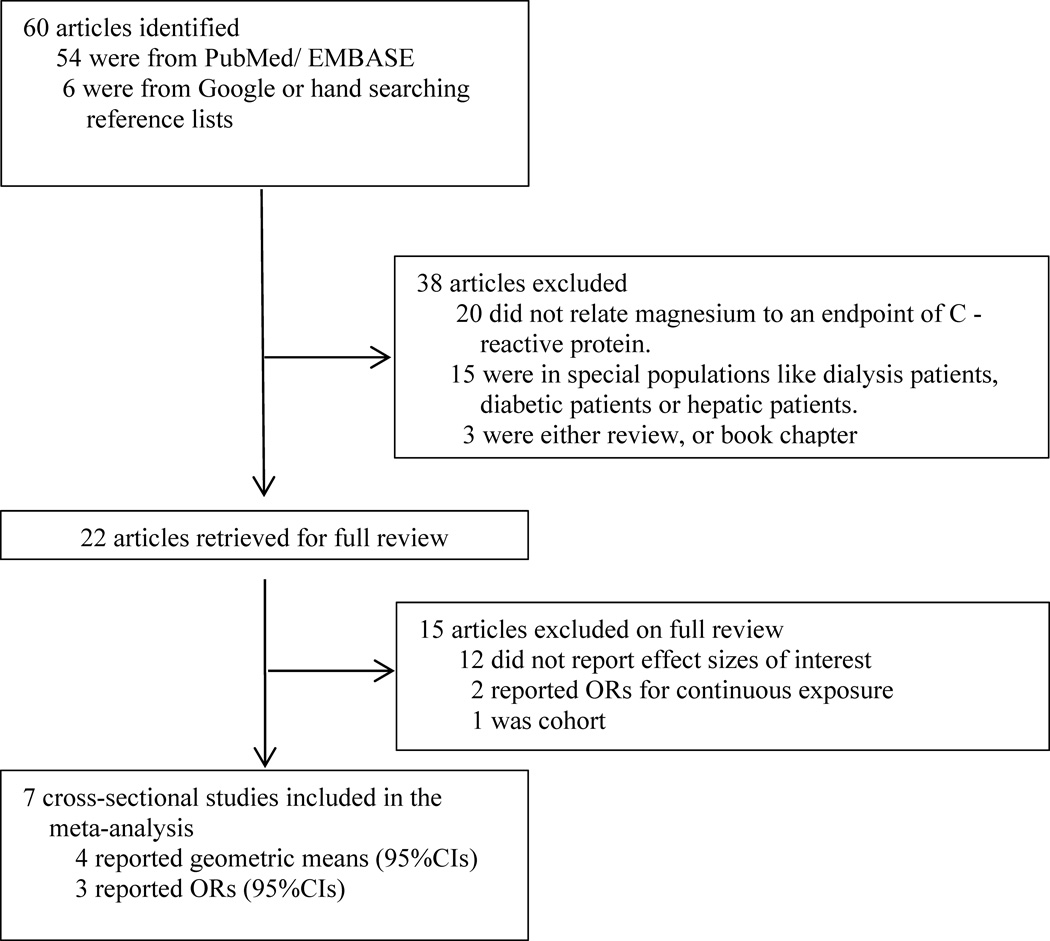
Initially, 60 articles were identified, but 53 articles were excluded because they did not meet the pre-specified inclusion criteria. So, seven cross-sectional analyses were included in the meta-analysis. The study selection process for the meta-analysis was presented in Figure 1. Two more cross-sectional studies and one cohort study were also identified and included in the systematic review because the results could not be pooled with other studies (Table 1). In addition, five intervention studies were identified for systematic review (Table 2).
Figure 1.
Process of study selection for the meta-analyses.
Table 1.
Characteristics of 9 cross-sectional analyses and 1 longitudinal analysis included in the study
| Source | Participants (n) | Males (%) | Age, year |
Exposure assessment |
Exposure Categories |
Outcome assessment |
Adjusted variables |
Reported measures of association |
|---|---|---|---|---|---|---|---|---|
| Cross-sectional analysis | ||||||||
| King D, et al. ,2005, NHANES (1999–2000),USA(18) | 5,021 | 25.4 | ≥17 | Dietary magnesium intake was derived from the 24 hour recall information for each respondent using the NHANES Computer assisted dietary interview (CADI) system | Four groups based on RDA1 <50% RDA; 50–74% RDA; 75–99% RDA; ≥ 100% RDA. |
CRP was analyzed using a high sensitivity assay technique that quantifies CRP by latex-enhanced nephlometry. | BMI, smoking, income level, alcohol consumption, exercise, and medical conditions like congestive heart failure, coronary heart disease, angina, heart attack, diabetes and hypertension. | ORs with 95% CIs |
| Song Y, et al., 2005,WHS, USA(4) | 11,686 | 0 | ≥45 | Dietary Magnesium was assessed by semi quantitative FFQ. | Quintiles (median, mg/day): 252; 293; 324; 359; 422. |
CRP was measured by a validated high-sensitivity assay (Denka Seiken, Nigata, Japan). | Age, BMI, smoking, exercise, alcohol, total calorie intake, multivitamin use, diabetes, hypertension, high cholesterol, and parental history of MI before 60 years of age and dietary intakes of total fat, cholesterol, folate, glycemic loadand fiber. | Geometric Means with 95%CIs |
| Bo S, et al., 2006, Italy(17) | 1,653 | 47.2 | 54.6±5.7 | Dietary magnesium was assessed by semi quantitative FFQ. | Tertiles (Median intake with 95% CIs, mg/day): 241.29(38.6–278); 308.2(269.1–338.6); 397.9(337.5–1052.3). |
Serum CRP was measured with a high-sensitivity CRP latex agglutination method on HITACH 911 Analyzer (Sentinel Ch., Milan, Italy). | Age, sex, BMI, smoking, level of physical activity, alcohol consumption, dietary total energy intake, and percentage of total fat and fiber intake. | ORs with 95%CIs, Medians with 95%CIs, and beta coefficients |
| King D, et al., 2007, NHANES (1999–2002), USA(10) | 5,007 | 51.4 | 6–17 | Secondary data on dietary magnesium intake was from NHANES 1999–2002. | Three groups based on RDA1 <75% RDA; 75–99% RDA; ≥100% RDA. |
High sensitivity CRP was measured as part of the NHANES 1999–2002 physical and laboratory examination. | Age, sex, race, income level, exercise, BMI and dietary intakes of fiber and total energy. | ORs with 95%CIs |
| Song Y, et al., 2007, NHS, USA(15) | 657 | 0 | 56 (43–69) | Dietary magnesium was assessed by semi quantitative FFQ. | Quintiles (median, mg/day): 225; 262; 289; 316; 356 |
Blood CRP was measured with a latex-enhanced turbidimetric assay on a Hitachi 911. | Age, smoking status, physical activity, alcohol consumption, total energyintake, menopausal status, postmenopausal hormone use, and BMI. | Geometric means with 95%CIs, and beta coefficients with SEs |
| Chacko SA, et al., 2010,WHI-OS, USA(13) | 3,713 | 50–79 | 0 | Dietary magnesium, was assessed by semi quantitative FFQ. | Quintiles (median, mg/day): 168.5; 204.4; 233.2; 263.1; 310.2 |
hs-CRP was measured on a chemistry analyzer (Hitachi 911; Roche Diagnostics, Indianapolis, IN) using an immunoturbidimetri-c assay with reagents and calibrators (Denka Seiken, Niigata, Japan). | Age, race, ethnicity, clinical center, time of blood draw, smoking, alcohol, energy expenditure from recreational physical activity /week, total energy intake, BMI, case-control status and dietary intakes of fiber, fruit and vegetable, folate , saturated and trans fat | Geometric means with 95%CIs, beta coefficients with SEs |
| de Oleivera Otto M, et al., 2011,MESA, USA(14) | 5,181 | 45–84 | 47.6 | Dietary magnesium measured by MESA FFQ. | Quintiles (mg/day): ≤206; 207–237; 238–262; 263–298; ≥299 |
Plasma CRP was measured with a particle enhanced immunephelometric assay by a BNII nephelometer (high-sensitivity CRP; Dade Behring). | Age, sex,, race-ethnicity, total energy intake, field center, education, physical activity, alcohol consumption, smoking, fiber intake and dietary supplement use. | Geometric Means with 95%CIs |
| Guerrero-Romero F, et al., 2002, Mexico(11) | 371 | 23–52 | 27 | Serum magnesium was measured by colorimetric method. | Serum Magnesium quartiles (mg/dl) Lowest, 2nd and 3rd and Highest | CRP was measured by automated microparticle enzyme immunoassay (Imx, Abbot Laboratories, USA). | Age, sex, BMI and Glucose Tolerance status | Correlation coefficient (r), and OR on continuous scale with 95%CIs |
| Rodriguez-Morán M, et al., 2008,Mexico(23) | 488 | 50.8 | 10–13. | Serum magnesium concentrations were measured using a colorimetric method. | Tertiles magnesium (mg/dl) mean (SD): 1.3(0.3); 1.8(0.03); 2.2(0.4). |
High sensitive CRP was measured by automated micro particle enzyme immunoassay (Imx, Abbot Laboratories, Minneapolis, MN, USA). | BMI and body fat percentage. | OR with 95%CI, and mean with SD |
| Longitudinal analysis | ||||||||
| Kim D, et al., 2010, USA(24) | 4,497 | 18–30 | 42.9 | Dietary magnesium intake was assessed by using a validated interview -administered CARDIA Diet History Questionnaire. | Quintiles of magnesium intake (mg/day): 178.68; 275.18; 351.25; 455.74; 669.30. |
High sensitive CRP was measured at at years 7,15 and 20 with a nephelometry-based high throughput assay. | Age, sex, ethnicity, study center, education, smoking status, alcohol consumption, physical activity, family history of diabetes, BMI, systolic Blood pressure, energy intake, dietary intake of saturated fat, and crude fiber. | Log transformed median , beta coefficients with 95%CIs |
Abbreviations: BMI: body mass index; CARDIA: Coronary Artery Risk Development in Young Adults; CI: confidence interval; CRP: C-reactive protein; MESA: Multi-Ethnic Study of Atherosclerosis; NHANES: National Health and Nutritional Examination Survey; NHS: Nurse Health Study; OR: odds ratio; RDA: Recommended Dietary Allowance;SD: standard deviation; SE: standard error; WHI-OS: Women’s Health Initiative- Observational Study; WHS: Women Health Study.
Magnesium RDA (mg/day) for both males and female: 130for those aged 6–8years old, 240 for those aged 9–13 years old; for males: 410 for those aged 14–18years, for females: 360 for those aged 14–18 years
Table 2.
Randomized controlled trials investigating the effect of magnesium supplementation on CRP in humans.
| Study | Country | Intervention group | Control group | Duration | Outcome Measure | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n/N | Male, % | Age, year | Treatment | n/N | Male, % | Age, year | Treatment | ||||
| Almoznino-Sarafian, et al., 2007(25) | Israel | 17/17 | 52.9 | 71.0±7.8 | Oral magnesium citrate: 300 mg/day | 18/18 | 55.6 | 72.38 | Untreated Controls | 5 weeks | ln(CRP) ± SD; Pearson’s correlation coefficient |
| Chacko, et al., 2010(27) | USA | 13/14 | 71 | 44.4 ±13.0 | Oral magnesium citrate: 500 mg/day | 13/14 | 71 | 44.4 ± 13.0 | Placebo | 4 weeks | Geometric mean (95%CI) |
| Nielsen, et al., 2010(26) | USA | 46/50 | 22 | 51–85 | Oral magnesium citrate: 320 mg/day | 49/50 | 22 | 51–85 | Placebo (Sodium Citrate) | 8 weeks | Change in mean CRP (increase/decrease) |
| Rodriguez-Hernandez, et al., 2010(33) | Mexico | 15/20 | 0 | 30–65 | Oral Solution containing 50ml of 5% Magnesium Chloride (equivalent to 450 mg/day elemental magnesium) | 15/18 | 0 | 30–65 | Control | 4 months | Mean CRP ± SD; OR for reduction in CRP |
| Mosleh, et al., 2012(32) | Iran | 35/37 | 0 | 46.3±4.2 | Oral Magnesium Oxide: 250mg/day | 34/37 | 0 | 46.3±4.2 | Placebo | 8 weeks | Median (IQR) for CRP |
Abbreviations: n in n/N is the number of participants who completed the study in each arm; and N is the number of participants who started the study in each arm; CRP: C-reactive protein; IQR: inter-quartile range; SD: standard deviation.
The final dataset for this meta-analysis is comprised of 32,918 participants from seven cross-sectional studies. Six studies were conducted in USA and one study in Italy (Table 1). Five studies included both men and women, and two studies consisted women only. Five studies used semi-quantitative food frequency questionnaires to collect dietary data, while other two studies used secondary dietary data from NHANES (1999–2000 and 1999–2002).In the original studies, dietary Mg intake was either categorized into three / four groups based on RDA levels(10, 18) or divided into tertiles or quintiles(4, 13–15, 17). Serum CRP was measured by using high sensitivity assay techniques (Table 1).
In the three cross-sectional studies(10, 17, 18) that calculated ORs as the effect sizes, the median Mg intake ranged from 205 to397.9 mg/day. In the four cross-sectional studies that calculated geometric means as the outcomes, median dietary Mg intake ranged from 225 to 422 mg/day. In almost all studies, age, body mass index (BMI,) smoking, physical activity, alcohol intake, and dietary calorie intake were considered as potential confounders.
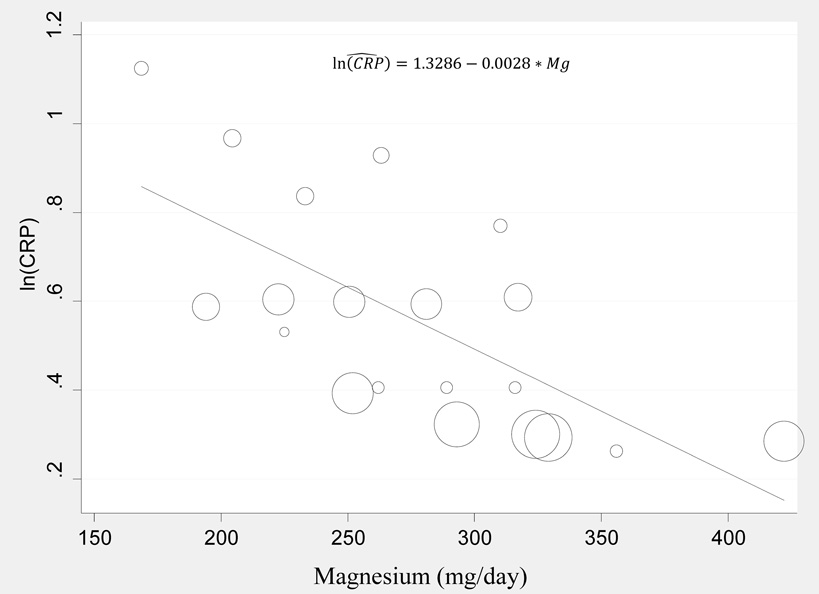
As shown in Figure 2, for the four cross-sectional studies that measured geometric mean CRP, the pooled estimate indicated that Mg intake (mg/day) was inversely associated with serum CRP levels (mg/L), with the meta-regression model:
ln (CRP)=1.3286 – 0.028* Mg, Adjusted R2 = 45.75%, P=0.001
Statistically significant heterogeneity was found among these studies (I2= 94.54%).
Figure 2.
Meta-regression for modeling ln(CRP)levels against dietary magnesium intake from four cross-sectional studies. The dots represent observations from each quintile relating ln(CRP) to dietary magnesium intake. Size of dot is proportional to the inverse of squared standard error of ln(CRP).
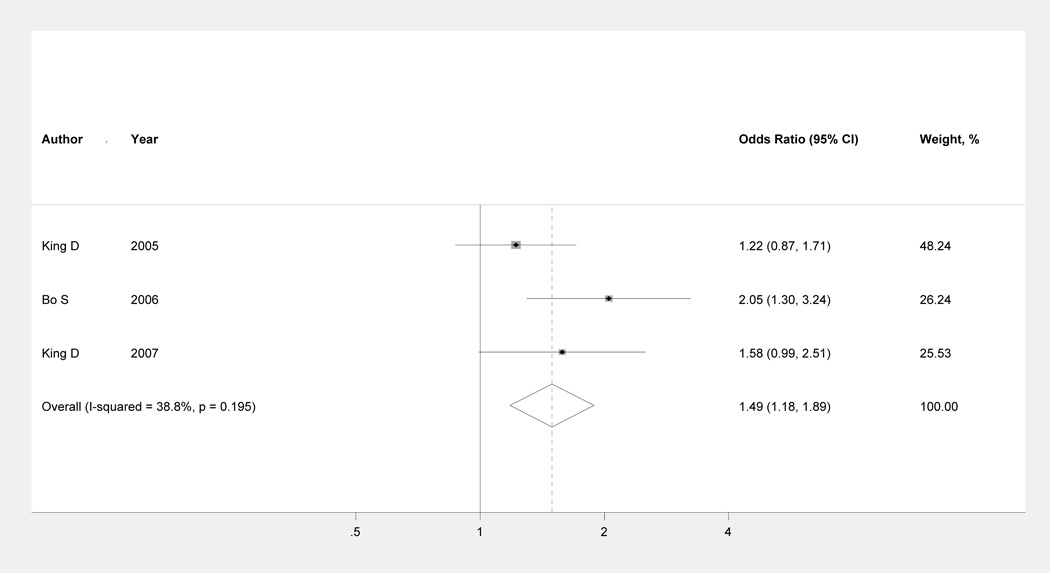
The combined association between adjusted OR of having serum CRP ≥3 mg/L with each unit (mg/day) increment in Mg intake was presented in Figure3. A statistically significant inverse association was observed (pooled OR: 1.49; 95%CI: 1.18 to 1.89). Non-significant heterogeneity among the studies was found (I2 = 38.8%, P =0.195). In addition, no evidence of publication bias existed (Egger’s test: P = 0.308; Begg’s test: P = 0.602).
Two cross-sectional studies (11, 23) and one prospective cohort study(24) were not included in the meta-analyses because the exposure measurement, effect size or the study design was different. The two cross-sectional studies found that serum Mg level was significantly inversely associated with serum CRP levels. In a study(23) conducted in Mexican children aged 10–13 years old, a continuous decrease in mean CRP levels from the lowest tertiles (2.45 mg/L, SD=10.6) to the highest tertile (0.8 mg/L, SD=0.5) of serum Mg level was observed. In a similar study(11) conducted in adults aged from 23–52 years old, it was found that serum Mg level was significantly inversely correlated to serum CRP levels (r=−0.39, P < 0.002). In a prospective cohort study(24) conducted among young adults aged 18 to 30 years, the researchers found a significant inverse association between dietary Mg intake and serum CRP levels, β-coefficient (95% CI) in the highest quintile of Mg intake was −0.160 (−0.262 to −0.058, Ptrend<0.01).
In addition, five Mg intervention studies (Table 2) reported significant inverse association between Mg supplementation and serum CRP levels. Two of the studies were conducted in USA, the rest were conducted in Mexico, Israel, and Iran, respectively. The age of participants ranged from 30 to 85 years. The duration of the studies ranged from 4 weeks to 4 months. One of the intervention studies(25) measured CRP at baseline and the end of study, and reported it in natural logarithmic scale. The ln(CRP) decreased significantly after the intervention. Another study(26) showed CRP levels decreased by 1.6mg/L in the Mg intervention group, while it increased by 1.5 mg/L in the placebo group (P<0.002). Two other studies did not find a significant association between Mg supplementation and CRP levels. Of note, one study(27) found that CRP levels increased after 500 mg elemental Mg supplementation in the form of Mg citrate for 4 weeks, while it decreased in the placebo group. The difference in changes between groups was not significant (P =0.50).
DISCUSSION
Evidence from this meta-analysis and systematic review indicates that dietary Mg intake is inversely associated with serum CRP levels. Our findings are robust since the meta-analysis is based on both continuous and binary outcomes and the results are supportive of each other. In addition, the study participants are comprised of male and female, adults and children with a wide age range, which improve the generalizability of the findings. In addition, the summarization of findings both from observational and intervention studies either in the meta-analyses or in the systematic review makes the available information on the association of Mg and serum CRP levels more aggregated.
Some limitations should also be considered when interpreting the results from this meta-analysis. First, most of the studies had just a single measure of exposure / effect sizes, e.g., mean level of CRP, ORs or correlation coefficients. The different measures of exposure and effect size made it difficult or even impossible to pool the results and estimate the overall association. For example, we had to exclude two studies(11, 23) from the meta-analysis because they reported mean CRP (SD), and/or OR (95%CI) based on a continuous scale of Mg intake. Second, none of the primary studies considered the health impact of high Mg intake (hypermagnesaemia, e.g., serum Mg>1.9 mEq/L(28)). Anyway, no sufficient evidence to date indicates any substantial adverse effect of dietary magnesium overdose, though two case studies reported that Mg intake above 6 mEq/L, a rare phenomenon in a general population, caused parathyroid gland dysfunction, respiratory, cardiac and CNS dysfunction(29, 30). Of note, an estimated 75% of Americans have daily magnesium intakes less than the RDA (31).
In addition, the possibility of residual confounding from primary studies cannot be completely excluded, though various potential confounders including lifestyle and demographic variables were well adjusted in the primary studies.
Five Mg intervention studies(25–27, 32, 33) used different doses of Mg supplementation (50 to 450 mg / day) for relatively short durations(4weeks to 4months). These studies cannot be pooled due to different measures of outcome across studies. Nevertheless, all these intervention studies reported a generally inverse association between Mg supplementation and serum CRP levels, which is consistent with the pooled estimate from observational studies. In addition, a study conducted in patients with cardiac surgery found a moderate inverse correlation between serum Mg concentration and pre-operative CRP levels(34).
Findings from this meta-analysis are biologically plausible. Studies indicate that Mg deficiency may increase CRP production mediated through interlinked chains of the following events. Inadequate dietary Mg intake depletes extracellular Mg ion and consequently causes activation of macrophages and influx of calcium ions into cells (adipocytes, neuronal and peritoneal cells). The increased calcium level in the cells causes enhanced Mg need to block influx of calcium ion, which further leads to increased stimulation of NMDA receptors. The increased stimulation of NMDA results in opening of channels non-selective to cations thus increasing calcium ions in neuronal cells(9). This causes release of neurotromediators (e.g., substance P) and inflammatory cytokines. Major proinflammatory cytokines including IL-6 and tumor necrosis factor TNF-α are released into the bloodstream and act as signaling molecules to promote the release of CRP from the liver as part of the acute phase response, which further prolongs the inflammatory response in the body(1). CRP production by liver is regulated by TNF- α and IL-6(9). A well-documented link between Mg deficiency and acute inflammatory response in animal models characterized by leukocyte and macrophages activation, release of inflammatory cytokines and acute phase proteins, and excessive production of free radicals has also been reported in experimental settings(35, 36). Studies based on infrared spectrometry techniques have also demonstrated substantial alteration in secondary structures of human CRP in presence of Mg ions(37).
In summary, findings from this meta-analysis and systematic review indicate that dietary Mg intake is inversely associated with serum CRP levels. Our results suggest that the potential beneficial effect of Mg intake on the risk of chronic diseases may be, at least in part, explained by inhibiting inflammation. Because inflammation is a risk factor of various chronic diseases, increasing Mg intake is certainly of great public health significance.
Figure 3.
Multivariable adjusted ORs and 95% CIs of having elevated CRP levels (≥3 mg/L) comparing those in the lowest with those in the highest dietary magnesium intake group from three cross-sectional studies. The summary estimate was obtained by using a fixed-effects model. The dots indicate the adjusted ORs. The size of the shade square is proportional to the weight of each study. The horizontal lines represent 95% CIs. The diamond markers indicate the pooled ORs. CI indicates confidence interval.
ACKNOWLEDGEMENT
This study was partially supported by NIH grants (R01HL081572 and R01ES021735).
Footnotes
CONFLICT OF INTEREST STATEMENT
None to declare.
The authors declare no conflict of interest.
REFERENCES
- 1.Liu S, Chacko S. In: Dietary Mg Intake and Biomarkers of Inflammation and Endothelial Dysfunction. Watson RR, Preedy VR, Zibadi S, editors. Humana Press: Magnesium in Human Health and Disease; 2013. pp. 35–50. [Google Scholar]
- 2.He K, Liu K, Daviglus ML, Morris SJ, Loria CM, Van Horn L, et al. Magnesium intake and incidence of metabolic syndrome among young adults. Circulation. 2006;113(13):1675–1682. doi: 10.1161/CIRCULATIONAHA.105.588327. [DOI] [PubMed] [Google Scholar]
- 3.Rayssiguier Y, Gueux E, Nowacki W, Rock E, Mazur A. High fructose consumption combined with low dietary magnesium intake may increase the incidence of the metabolic syndrome by inducing inflammation. Magnesium research : official organ of the International Society for the Development of Research on Magnesium. 2006;19(4):237–243. [PubMed] [Google Scholar]
- 4.Song Y, Ridker PM, Manson JE, Cook NR, Buring JE, Liu S. Magnesium intake, C-reactive protein, and the prevalence of metabolic syndrome in middle-aged and older U.S. women. Diabetes care. 2005;28(6):1438–1444. doi: 10.2337/diacare.28.6.1438. [DOI] [PubMed] [Google Scholar]
- 5.Song Y, Manson JE, Buring JE, Liu S. Dietary magnesium intake in relation to plasma insulin levels and risk of type 2 diabetes in women. Diabetes care. 2004;27(1):59–65. doi: 10.2337/diacare.27.1.59. Epub 2003/12/25. [DOI] [PubMed] [Google Scholar]
- 6.Abbott RD, Ando F, Masaki KH, Tung KH, Rodriguez BL, Petrovitch H, et al. Dietary magnesium intake and the future risk of coronary heart disease (the Honolulu Heart Program) The American journal of cardiology. 2003;92(6):665–669. doi: 10.1016/s0002-9149(03)00819-1. [DOI] [PubMed] [Google Scholar]
- 7.Al-Delaimy WK, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Magnesium intake and risk of coronary heart disease among men. Journal of the American College of Nutrition. 2004;23(1):63–70. doi: 10.1080/07315724.2004.10719344. [DOI] [PubMed] [Google Scholar]
- 8.Stevanovic S, Nikolic M, Stankovic A. Dietary magnesium intake and coronary heart disease risk: a study from Serbia. Medicinski glasnik : official publication of the Medical Association of Zenica-Doboj Canton, Bosnia and Herzegovina. 2011;8(2):203–208. [PubMed] [Google Scholar]
- 9.Nielsen FH. Magnesium, inflammation, and obesity in chronic disease. Nutrition reviews. 2010;68(6):333–340. doi: 10.1111/j.1753-4887.2010.00293.x. Epub 2010/06/12. [DOI] [PubMed] [Google Scholar]
- 10.King DE, Mainous AG, 3rd, Geesey ME, Ellis T. Magnesium intake and serum C-reactive protein levels in children. Magnesium research : official organ of the International Society for the Development of Research on Magnesium. 2007;20(1):32–36. [PubMed] [Google Scholar]
- 11.Guerrero-Romero F, Rodriguez-Moran M. Relationship between serum magnesium levels and C-reactive protein concentration, in non-diabetic, non-hypertensive obese subjects. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2002;26(4):469–474. doi: 10.1038/sj.ijo.0801954. Epub 2002/06/22. [DOI] [PubMed] [Google Scholar]
- 12.King DE, Mainous AG, Geesey ME, Egan BM, Rehman S. Magnesium supplement intake and C-reactive protein levels in adults. Nutrition research (New York, NY) 2006;26(5):193–196. [Google Scholar]
- 13.Chacko SA, Song Y, Nathan L, Tinker L, de Boer IH, Tylavsky F, et al. Relations of dietary magnesium intake to biomarkers of inflammation and endothelial dysfunction in an ethnically diverse cohort of postmenopausal women. Diabetes care. 2010;33(2):304–310. doi: 10.2337/dc09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Oliveira Otto MC, Alonso A, Lee DH, Delclos GL, Jenny NS, Jiang R, et al. Dietary micronutrient intakes are associated with markers of inflammation but not with markers of subclinical atherosclerosis. The Journal of nutrition. 2011;141(8):1508–1515. doi: 10.3945/jn.111.138115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song Y, Li TY, van Dam RM, Manson JE, Hu FB. Magnesium intake and plasma concentrations of markers of systemic inflammation and endothelial dysfunction in women. The American Journal of Clinical Nutrition. 2007;85(4):1068–1074. doi: 10.1093/ajcn/85.4.1068. [DOI] [PubMed] [Google Scholar]
- 16.Higgins RMHaJPT. STATA Journal. 2008;8(Number 4):493–519. [Google Scholar]
- 17.Bo S, Durazzo M, Guidi S, Carello M, Sacerdote C, Silli B, et al. Dietary magnesium and fiber intakes and inflammatory and metabolic indicators in middle-aged subjects from a population-based cohort. Am J Clin Nutr. 2006;84(5):1062–1069. doi: 10.1093/ajcn/84.5.1062. [DOI] [PubMed] [Google Scholar]
- 18.King DE, Mainous AG, 3rd, Geesey ME, Woolson RF. Dietary magnesium and C-reactive protein levels. Journal of the American College of Nutrition. 2005;24(3):166–171. doi: 10.1080/07315724.2005.10719461. [DOI] [PubMed] [Google Scholar]
- 19.Bowden J, Tierney JF, Copas AJ, Burdett S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC medical research methodology. 2011;11:41. doi: 10.1186/1471-2288-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in medicine. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. Epub 1994/12/01. [PubMed] [Google Scholar]
- 23.Rodriguez-Moran M, Guerrero-Romero F. Serum magnesium and C-reactive protein levels. Archives of disease in childhood. 2008;93(8):676–680. doi: 10.1136/adc.2006.109371. Epub 2007/07/21. [DOI] [PubMed] [Google Scholar]
- 24.Kim DJ, Xun P, Liu K, Loria C, Yokota K, Jacobs DR, Jr, et al. Magnesium intake in relation to systemic inflammation, insulin resistance, and the incidence of diabetes. Diabetes care. 2010;33(12):2604–2610. doi: 10.2337/dc10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Almoznino-Sarafian D, Berman S, Mor A, Shteinshnaider M, Gorelik O, Tzur I, et al. Magnesium and C-reactive protein in heart failure: an anti-inflammatory effect of magnesium administration? European journal of nutrition. 2007;46(4):230–237. doi: 10.1007/s00394-007-0655-x. Epub 2007/05/05. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen FH, Johnson LK, Zeng H. Magnesium supplementation improves indicators of low magnesium status and inflammatory stress in adults older than 51 years with poor quality sleep. Magnesium research : official organ of the International Society for the Development of Research on Magnesium. 2010;23(4):158–168. doi: 10.1684/mrh.2010.0220. Epub 2011/01/05. [DOI] [PubMed] [Google Scholar]
- 27.Chacko SA, Sul J, Song Y, Li X, LeBlanc J, You Y, et al. Magnesium supplementation, metabolic and inflammatory markers, and global genomic and proteomic profiling: a randomized, double-blind, controlled, crossover trial in overweight individuals. Am J Clin Nutr. 2011;93(2):463–473. doi: 10.3945/ajcn.110.002949. Epub 2010/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong ET, Rude RK, Singer FR, Shaw ST., Jr A high prevalence of hypomagnesemia and hypermagnesemia in hospitalized patients. American journal of clinical pathology. 1983;79(3):348–352. doi: 10.1093/ajcp/79.3.348. [DOI] [PubMed] [Google Scholar]
- 29.McLaughlin SA, McKinney PE. Antacid-induced hypermagnesemia in a patient with normal renal function and bowel obstruction. The Annals of pharmacotherapy. 1998;32(3):312–315. doi: 10.1345/aph.17284. Epub 1998/04/09. [DOI] [PubMed] [Google Scholar]
- 30.Uchiyama C, Kato T, Tomida K, Suzuki R, Nakata K, Hamanaka M, et al. Fatal hypermagnesemia induced by preoperative colon preparation in an elderly woman: report of a case. Clinical Journal of Gastroenterology. 2013;6(2):105. doi: 10.1007/s12328-012-0353-y. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization. Calcium and magnesium in drinking-water : public health significance. xi. Geneva, Switzerland: World Health Organization; 2009. p. 180. [Google Scholar]
- 32.Moslehi N, Vafa M, Rahimi-Foroushani A, Golestan B. Effects of oral magnesium supplementation on inflammatory markers in middle-aged overweight women. Journal of Research in Medical Sciences. 2012;17(7):607–613. [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez-Hernandez H, Cervantes-Huerta M, Rodriguez-Moran M, Guerrero-Romero F. Oral magnesium supplementation decreases alanine aminotransferase levels in obese women. Magnesium research : official organ of the International Society for the Development of Research on Magnesium. 2010;23(2):90–96. doi: 10.1684/mrh.2010.0204. Epub 2010/05/13. [DOI] [PubMed] [Google Scholar]
- 34.Svagzdiene M, Rupsa T, Sirvinskas E, Baranauskiene D, Abdrachmanovas O. P-74 Relation of magnesium level in serum, erythrocytes, and cardiac tissue and C-reactive protein. Journal of cardiothoracic and vascular anesthesia. 2011;25(3):S64. [Google Scholar]
- 35.Bussiere FI, Tridon A, Zimowska W, Mazur A, Rayssiguier Y. Increase in complement component C3 is an early response to experimental magnesium deficiency in rats. Life sciences. 2003;73(4):499–507. doi: 10.1016/s0024-3205(03)00291-1. Epub 2003/05/22. [DOI] [PubMed] [Google Scholar]
- 36.Mazur A, Maier JA, Rock E, Gueux E, Nowacki W, Rayssiguier Y. Magnesium and the inflammatory response: potential physiopathological implications. Archives of biochemistry and biophysics. 2007;458(1):48–56. doi: 10.1016/j.abb.2006.03.031. Epub 2006/05/23. [DOI] [PubMed] [Google Scholar]
- 37.Dong A, Caughey WS, Du Clos TW. Effects of calcium, magnesium, and phosphorylcholine on secondary structures of human C-reactive protein and serum amyloid P component observed by infrared spectroscopy. The Journal of biological chemistry. 1994;269(9):6424–6430. [PubMed] [Google Scholar]