Abstract
The beta isoform of Neuregulin-1 (NRG-1β), along with its receptors (ErbB2-4), is required for cardiac development. NRG-1β, as well as the ErbB2 and ErbB4 receptors are also essential for maintenance of adult heart function. These observations have led to its evaluation as a therapeutic for heart failure. Animal studies and ongoing clinical trials have demonstrated beneficial effects of two forms of recombinant NRG-1β on cardiac function. In addition to the possible role for recombinant NRG-1βs as heart failure therapies, endogenous NRG-1β/ErbB signaling appears to play a role in restoring cardiac function after injury. The potential mechanisms by which NRG-1β may act as both a therapy and mediator of reverse remodeling remain incompletely understood. In addition to direct effects on cardiac myocytes NRG-1β acts on the vasculature, interstitium, cardiac fibroblasts, and hematopoietic and immune cells which collectively may contribute to NRG-1β’s role in maintaining cardiac structure and function as well as mediating reverse remodeling.
Keywords: Neuregulin, GGF2, ErbB2, ErbB3, ErbB4, cardiac remodeling
Introduction
Neuregulins (NRGs) belong to the epidermal growth factor (EGF) superfamily, and are encoded by four genes (NRG-1, -2, -3, and -4) (1, 2). NRG-1 can be secreted or activated in a juxtracrine or paracrine manner through proteolytic cleavage by membrane associated proteinases (3, 4). There are multiple splicing events that result in expression of many distinct isoforms of NRG-1 that likely regulate its expression and activation. The β isoform of NRG-1 is required for cardiac development and is the best studied of the NRGs in the context of cardiovascular biology and pathophysiology. Intracellular signaling is initiated when NRG binds to one of two tyrosine kinase receptors (ErbB3 or ErbB4), which induces dimer formation with each other (ErbB3/4 heterodimer, ErbB4/4 homodimer) or with ErbB2 (ErbB3/2, ErbB4/2) that couple to intracellular signaling cascades. ErbB2, which possesses an active kinase region but lacks the NRG ligand binding domain, when overexpressed is capable of constitutive activity that mediates its role as an oncogene in adenocarcinoma.
The role of NRG-1β in cardiac development has been well established, and there is a growing appreciation of the importance of the NRG-1/ErbB signaling system in the adult heart. Our understanding of NRG-1’s role in both cardiovascular biology and the pathophysiology of adult heart disease is quickly evolving. Recent studies have identified both cell-specific and context-dependent effects of NRG-1. Clinical trials proceed evaluating two isoforms of NRG-1β as a possible therapy for cardiovascular disease. We will review that progress, and advance the hypothesis that endogenous NRG-1β is a mediator of reverse remodeling.
Neuregulin/ErbB signaling as mediator of cardiovascular adaptation to stress
NRG-1β and ErbB receptors are widely expressed in the postnatal cardiovascular system (Figure 1). A number of studies collectively demonstrate the role of NRG-1/ErbB signaling in maintaining normal physiology of the adult cardiovascular system which have been reviewed in detail elsewhere (1, 5, 6). Multiple studies have independently demonstrated a role for NRG-1 in regulating the adult heart adaptation to physiological and pathological stress. NRG-1 evokes cellular and systemic effects that support an adaptive role for NRG-1/ErbB signaling under a variety of conditions (Figure 1). For instance, NRG-1β enhances cardiomyocyte cell survival (7), cardiac differentiation of embryonic stem cells (8), cell migration (9, 10), angiogenesis (11), cytoskeletal assembly (9), excitation-coupling (12), neuromuscular junction formation (13) and possibly cardiac interstitial matrix structure (14, 15). Depending on cell type and context, NRG-1 activates one or more signaling cascades, including mitogen-activated protein kinase (MAPK), PI3K/AKT, p70/S6K and Src/FAK pathways (7, 16–18).
Figure 1. Role of neuregulin-1/ErbB signaling in the adult cardiovascular system.
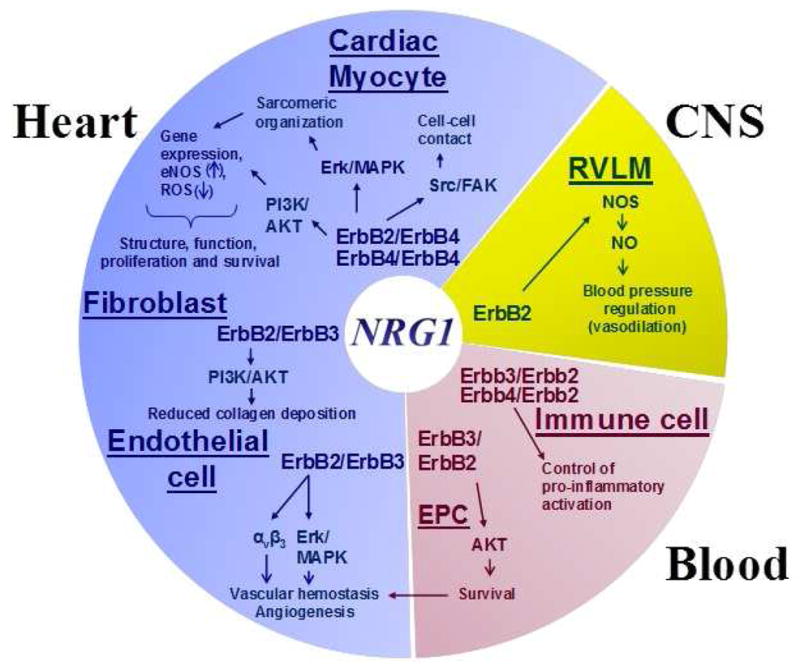
Involvement of NRG/ErbB signaling in the adult cardiovascular system includes multiple tissues, including the heart, the blood and central nervous system (CNS), and cell types, such as cardiomyocytes, endothelial cells, fibroblasts, and circulating monocytes. See text for details. Abbreviations: AKT, protein kinase B; CNS, central nervous system; EPC, endothelial progenitor cells; ERK, extracellular signal-regulated kinases1/2; FAK, focal adhesion kinase; NO, nitric oxide; NOS, nitric oxide synthase; NRG1, neuregulin-1; PI3K, phosphoinositide-3-kinase; RVLM, rostral ventrolateral medulla; Src, proto-oncogene tyrosine-protein kinase.
ErbB2 and ErbB4 receptors mediate the effects of NRG-1β in cardiac myocytes, and each appears to play distinct roles in coupling to intracellular signaling and cellular events (19). For example, ErbB2 appears to be required for activation of focal adhesion formation, one downstream pathway that is implicated in the maintenance of electrical and mechanical coupling in adult rat ventricular myocytes by NRG-1β (20). As ErbB2 has no ligand binding ability, its involvement in NRG-1β signaling is reliant upon heterodimerization with ErbB4 in cardiac myocytes. In contrast, NRG-1β activation of ErbB4 couples to PI3K/AKT with protection of cardiomyocytes against apoptosis, as well as mediating metabolic events (18). The intracellular mediators of NRG-1β/ErbB signaling in cardiomyocytes also include extracellular-regulated kinase (ERK1/2) (17), nitric oxide synthase (21–23) and cardiac myosin light chain kinase (cMLCK) (24). NRG-1β/ErbB signaling promotes cardiomyocyte survival and proliferation and supports normal cellular functions (7, 19, 20). NRG-1β activation of ErbB4/4 homodimers will activate PI3K/AKT, which appears to feed back on activation of ERK1/2 and regulation of cellular hypertrophy (25).
Cardiac endothelial cells isolated from rodent heart express multiple isoforms of NRG-1α and β, most of which are inactive transmembrane proteins (26). In response to stress, NRG-1β is proteolytically released by cardiac microvascular endothelial cells activating ErbB receptors in neighboring cells (21). Expression of NRG and its receptors fluctuate in response to injury, physiological and pathological stress, and metabolic status. NRG-1/ErbB signaling is strongly activated in tissue under a variety of stressed conditions, including cardiac ischemia/reperfusion (18), exercise (27), cerebral ischemia and reperfusion (28–30), and hindlimb ischemia (31). Inhibition of ErbB2 activity in the heart or NRG-1 expression in endothelial cells severely impairs cardiac contractile recovery after ischemic injury (32, 33). NRG-1 “pre-conditioning” is both cardioprotective (34) and neuroprotective (28) from subsequent injury. NRG/ErbB expression levels are dynamic and incompletely understood at present. For example, ErbB2 and ErbB4 mRNA and protein levels are increased in response to ventricular pressure overload during compensatory cardiac hypertrophy in mouse models, but are reduced after transition to heart failure (35). Expression of NRG-1, as well as ErbB2/ErbB4, is likewise reduced in rats with diabetic cardiomyopathy (36). Conversely, ventricular NRG-1 is increased in patients with advanced heart failure, while ErbB2 and ErbB4 expression and activity are reduced, in accordance with what has been observed in animal models (37). Metabolic stress associated with ATP depletion shuts off NRG responsiveness via degradation of ErbB2, which might add yet another layer of complexity to the NRG puzzle (38).
The importance of NRG-1/ErbB signaling in the maintenance of adult human heart health is demonstrated by the cardiovascular side effects associated with the anti-cancer agent trastuzumab (39). Approximately 25–30% of breast tumors over-express ErbB2 (also called HER-2), which in this context functions as an oncogene that increases tumor aggressiveness and worsens prognosis (40). A humanized monoclonal antibody that targets ErbB2 (i.e., trastuzumab) was developed as an adjuvant chemotherapeutic agent and tested in combination with standard chemotherapy in 470 women with metastatic breast cancer, compared to 504 patients who received standard therapy alone (doxorubicin, epirubicin, cyclophosphamide, or paclitaxel). Addition of trastuzumab to standard chemotherapeutic agents lessened tumor progression, decreased early mortality, and increased survival (41). However, adjuvant trastuzumab treatment resulted in adverse cardiac effects, including a high incidence of LV systolic dysfunction, particularly those who received concurrent anthracyclines (41, 42). Similar results were obtained in two follow-up trials (43, 44), which led to the implementation of new treatment guidelines, including routine assessment of cardiac function (e.g., LVEF) during and after trastuzumab treatment (44–47). The mechanisms for this effect remain incompletely understood, but support the overall concept that intact myocardial ErbB2 signaling plays a role in maintenance of the human heart.
Neuregulin as a marker of cardiovascular health
Motivated by findings that exercise and injury of skeletal and cardiac muscle activate proteolytic release of NRG-1β in rodents, we and others have found that NRG-1β is detectable in the circulation of humans where it relates to some degree to cardiovascular health (Table 1). While in healthy subjects levels correlate with cardiopulmonary fitness (48), in a number of cardiovascular disease cohorts levels relate to cardiac stress. In a large cohort of systolic heart failure patients, including 293 individuals with ischemic cardiomyopathy and 606 persons with non-ischemic heart failure, circulating levels of NRG-1β were significantly higher in patients with ischemic cardiomyopathy, and higher levels were associated with an increased risk of death or cardiac transplantation (49). When the patients were stratified by disease severity using the New York Heart Association functional classification system, NRG-1β levels were also found to correlate with disease severity (i.e. higher NRG-1β levels in in class II and IV, compared to class I or II) (49). A subsequent study likewise found higher levels of NRG-1β in both plasma and serum of patients with coronary artery disease (CAD) who exhibited stress-induced ischemia (50). However, circulating levels of NRG-1β in stable CAD patients were found to be inversely correlated with angiographic severity of disease (50). In patients with unstable angina pectoris, on the other hand, serum NRG-1β levels were recently shown to be increased compared to age-matched controls (51). Serum NRG-1β levels in these same patients were positively correlated with vascular endothelial growth factor (VEGF) and angiopoietin-1 (Ang-1) levels (51), which hints at potential signaling cross-talk between NRG and VEGF/Ang-1 pathways. One caveat with each of these studies is that current assays measure the pool of all NRG-1β present in the sample. Perhaps as further refinements in the assay are made we might distinguish distinct tissue sources of NRGs, and thereby develop a refined understanding of the relationship between levels and disease states.
Table 1.
Animal and Human Studies of NRG-1 as a Therapy and/or Biomarker for Heart Disease
| Year | Model/Disease | Treatment | Outcome | Ref |
|---|---|---|---|---|
| Animal Studies as Therapy | ||||
| 2006 | Ischemic/post-MI (rats) | IV daily for 5 or 10 days, 1 week or 2 mo after ligation | Improved cardiac performance. Increased capillary density in fibrotic peri-infarct area | (59) |
| Viral myocarditis (mice) | IV daily for 5 days | Improved echocardiographic parameters and survival, reduced lymphocyte infiltration | ||
| Chronic rapid pacing (dogs) | IV daily for 5 days, 3 weeks after start of pacing | Improved LVEDP and LVESP, improved cardiac contractility and relaxation | ||
| Doxorubicin-induced (mice) | IV daily for 5 days, 4 weeks after doxorubicin | Prevented cardiac dysfunction, improved survival, reduced cardiomyocyte necrosis | ||
| 2009 | Ischemic/post-MI (mice) | IP daily for 12 weeks, 1 week after ligation | Induction of cardiomyocyte proliferation, improved cardiac structure and function | (87) |
| 2009 | Doxorubicin-induced (mice) | SCP for 3–5 days after doxorubicin | Improved cardiac function and survival, attenuated troponin degradation | (88) |
| 2009 | Post-MI (mice) | IP daily (details) | Improved cardiac function, reduced scar | (87) |
| 2010 | Post-MI (rats) | IV daily for 7 days, 8 weeks after ligation | Up-regulated cardiac myosin light chain kinase, improved heart function | (24) |
| 2012 | Post-MI (rats) | IV daily for 10 days, 4 weeks after ligation | Improved LV remodeling and cardiac function, reduced mitochondrial dysfunction, myocyte apoptosis, and oxidative stress | (89) |
| 2010 | Ischemic/post-MI (rats) | IV for 20 min before I/R | Improved LV structure, decreased ischemia and apoptosis | (34) |
| 2013 | Post-MI (rats) | IV, every 2 days, 8 weeks | Improved LV contractile function, corrected altered metabolic gene expression | (90) |
| 2011 | Post-MI (swine) | 0.67 mg/kg or 2 mg/kg IV, twice a week from 7 to 35 days post-MI | Improved LV contractile function, mitochondrial morphology, ECM structure, and intercalated disc integrity | (14) |
| 2011 | Diabetic CM (rats) | 10 μg/kg IV every 2 days for 2 weeks | Improved LV function, decreased apoptosis and fibrosis | (60) |
| 2012 | Post-MI (rats) | rhNRG-carrying lentivirus injected into infarcted area | Increased angiogenesis, decreased apoptosis | (91) |
| Human Studies as Therapy | ||||
| 2010 | CHF, Phase II, multicenter | EGF-domain of NRG-1β, 10 hr IV infusions for 10 days | Improved and sustained LVEF%, decreased LVEDV/LVESV at 30 and 90 days (n = 44) | (63) |
| 2011 | Stable CHF, single center, prospective | EGF-domain of NRG-1β, 12 hr for 10 days | Acute increase in CO, Improved LVEF, decrease of serum NA and ALD (n = 15) | (64) |
| Human Biomarker Studies | ||||
| 2009 | Cardio-respiratory fitness | NA | Serum NRG-1beta is correlated with maximum O2 intake (n = 9 normal men) | (48) |
| 2009 | Systolic heart failure | Serum NRG-1β correlates with disease severity, risk of death, transplant (n = 899) | (49) | |
| 2011 | Coronary artery disease | Circulating NRG-1β inversely correlated with angiographic severity of CAD (n = 60) | (50) | |
| Stress-induced ischemia | Circulating NRG-1β levels are higher in patients with stress-induced ischemia (n = 23) compared to without (n = 37) | |||
| 2013 | Breast cancer | Circulating NRG-1β correlates with adverse cardiac effects (n = 78) | (92) | |
| 2013 | Unstable angina pectoris | Serum NRG-1β levels higher in patients with UAP (n = 57) versus controls (n = 36) | (51) | |
| 2013 | Harvard Cohort SCD | Missense variant of NRG-1 gene is associated with sudden cardiac death (SCD: n = 340; Controls: n = 342) | (58) | |
Interestingly, NRG-1 may also provide a link between psychiatric and cardiovascular diseases. Given the critical role of NRG-1 in central nervous system development, it is perhaps not surprising that it has been identified in genome-wide association studies as a schizophrenia susceptibility gene (52–55). Schizophrenia patients have a three-fold higher risk for sudden cardiac death (SCD) than the general population (56, 57), prompting an investigation into whether schizophrenia-related variants of NRG1 are associated with increased SCD susceptibility (58). Patients from the ongoing prospective Oregon Sudden Unexpected Death Study who presented with ventricular fibrillation (n = 340) and control subjects (n = 342) were genotyped for 17 single nucleotide polymorphisms, one of which is located in the NRG1 gene. Out of the 17 genes, only the SNP located in the NRG1 gene was significantly associated with SCD. The authors validated their findings in 1,853 individuals from the Harvard Cohort SCD study and suggested that this non-synonymous (methionine → threonine) SNP in the NRG1 gene might be the first comorbid SNP that links schizophrenia and SCD. Based on these findings it appears that the NRG-1/ErbB signaling network is a complex, multi-functional system that is not bound by the lines separating different clinical pathologies.
Neuregulin-1β as a Heart Failure Therapy
Recombinant NRG-1β has been evaluated as a potential therapy in many animal models of heart injury, including myocardial infarction, ischemia/reperfusion injury, diabetic cardiomyopathy, myocarditis, and chronic rapid pacing (Table 1). Intravenous administration of recombinant NRG-1β in rats after LAD (left anterior descending artery) ligation resulted in reduced ventricular pressure and increased capillary density in fibrotic lesions at the periphery of the infarct (59). Improvement in cardiac function was still observed in treated rats, albeit less markedly, even when rhNRG-1 was not administered until 2 months after LAD ligation (59). These findings could be interpreted as NRG-1β –stimulated reverse remodeling, as opposed to merely preventing compensatory changes in cardiac dimensions and function.
More direct evidence for NRG-1β’s role in remodeling is its apparent anti-fibrotic effects in several animal models of heart failure. In rats with diabetic cardiomyopathy NRG-1 attenuates myocardial interstitial fibrosis (60). We have also observed reduced fibrosis in a swine model of cardiomyopathy after treatment with recombinant glial growth factor 2 (GGF2) (14), which is a pseudonym for NRG-1β3, a longer NRG-1 isoform (Type II) that contains a Kringle domain in addition to the immunoglobin-like and EGF-like domains which characterize NRG-1 Type I isoforms (61). Treatment of primary murine fibroblasts with recombinant Type I NRG-1β caused a reduction in the pro-fibrotic myofibroblast phenotype, along with down-regulation in TGFβ-induced fibrotic transcripts and proteins (62).
Recent progress has been made in the effort to develop NRG-based therapies using what has thus far been learned from patient prospective studies and animal models. Two Phase II human trials have reported that daily infusions of recombinant human NRG-1 (rhNRG-1) was safe and well-tolerated in patients with stable chronic heart failure (63, 64). The first published trial was a randomized Phase II, double-blind multicenter study including 44 subjects with chronic heart failure (63). Participants were given daily infusions of rhNRG-1 or a placebo for 10 days, in addition to standard therapy (63). At day 30, patients who received rhNRG-1 exhibited significantly increased left ventricular ejection fraction (LVEF) (63). Most interesting, patients who received the 10-day rhNRG-1 infusion therapy showed reduced end-systolic and end-diastolic volumes at day 30 that continued to day 90 (63). This was the first human study to demonstrate a role for NRG-1 in reverse remodeling. Another clinical trial, published in 2011, demonstrated improved and sustained hemodynamics in a cohort of 15 patients with chronic heart failure who received daily infusions of rhNRG-1 for 10 days (64). Larger ongoing trials include a Phase II interventional study aimed at determining the efficacy and safety of rh-NRG-1 in 120 patients with chronic heart failure, a Phase III trial to evaluate the efficacy of subcutaneous administration of rhNRG-1 in 120 patients with chronic systolic heart failure, and a Phase III trial to measure the effects of rhNRG-1 treatment on the death rate in 1,600 chronic heart failure patients. Study details provided at http://www.clinicaltrials.gov (NCT01251406, NCT1214096, and NCT01541202).
A Phase I trial to evaluate the safety of the GGF2 version of NRG-1β in 40 patients with left ventricular dysfunction and symptomatic heart failure was recently completed (NCT01258387). Administration of a single dose was generally well tolerated, and trial participants who received GGF2 showed a consistent and dose-responsive increase in ejection fraction at 28 days that was sustained up to 90 days (65, 66). Preliminary data suggest that a single dose of GGF2 has similar efficacy to the EGF-domain only fragment of NRG-1β (rhNRG-1) given over multiple days. Perhaps this is due to the presence of an additional domain (i.e., the Kringle domain) in GGF2 that is not present in rhNRG-1. More detailed mechanistic studies are warranted to address the relative potency of rhNRG-1 and GGF2.
Despite the mounting evidence that NRG exerts beneficial effects in the setting of heart disease, some caution is warranted as these studies proceed. One issue is the possibility that specific disease states may change myocardial response to exogenous NRG. An example of this was the demonstration that diabetes abrogates compensatory NRG/ErbB receptor responses to MI in rats (67). In neonatal cardiomyocytes, the fatty acid palmitate not only reverses the protective effects of NRG-1β, it causes a drastic increase in apoptosis (68). These negative effects are alleviated in the presence of the mono-unsaturated fatty acid oleate. If applicable to humans, these data have important implications for dietary interactions in patients who receive NRG treatment. There is also concern that NRG treatment might exert “off-target” effects by nature of its mitogenic properties as a growth factor, and the well-established role of ErbB2 as an oncogene in a variety of malignancies. To address this issue, a bivalent version of NRG was engineered to preferentially target ErbB4 homodimer activation, thereby preventing doxorubicin-induced cardiomyocyte death without activating or potentiating cancer cell signaling (69–71). Further studies are clearly warranted to determine whether this or other modified ligands, or alternative delivery methods are needed to increase the therapeutic window for NRGs.
Mechanisms of NRG’s effects on the adult cardiovascular system - beyond regulation of cardiac myocyte biology
Our knowledge about the role of NRG-1 and its receptors in the cardiovascular system is in rapid evolution, with a great deal more to learn. The variety of NRG-1 mediated biological effects in different cell types implies that NRG-1/ErbB signaling is required for the adaptive response of the cardiovascular system to changes in cardiac demand (Table 2). As discussed above, the role of NRG/ErbB signaling in the regulation of cardiac myocyte biology is well established. More recent it has become clear that intact NRG-1/ErbB signaling is a prerequisite for normal cardiac adaptation to the physiological stress of pregnancy (72). Adaptation to exercise has yet to be established, although the positive correlation between aerobic capacity and serum NRG-1β suggests that may be the case (48). These data suggest the intriguing hypothesis that NRG activation in skeletal muscle may act through release in the circulation to regulate cardiac function at a distance. A similar interesting story of NRG having effects at a distance is the role of NRG-1/ErbB signaling in the central nervous system rostral ventrolateral medulla, a region of the brainstem crucial for blood pressure control (73).
Table 2.
Cellular effects of NRG-1/ErbB signaling in the heart
| Cell | Cellular phenotype | Role of NRG-1/ErbB in phenotype | Ref |
|---|---|---|---|
| Myocytes | Hypertrophy, cell lengthening | Mice with conditional ErbB2 deletion or ErbB4 inactivation develop severe DCM, along with chamber dilation, wall thinning and decreased contractility | (93–95) |
| Altered excitation–contraction coupling | NRG-1β attenuates doxorubicin-induced excitation- coupling changes | (12) | |
| Altered energy metabolism | NRG-1β reduces oxidative stress, attenuates mitochondrial dysfunction in rats with HF, induces glucose uptake and protein synthesis | (12, 26, 84, 89) | |
| Altered myofibrillar content and function | NRG-1β enhances sarcomere organization, reduces anthracycline-induced disarray, myofibrillar degradation | (12, 16, 17, 59) | |
| Apoptosis/Necrosis | NRG-1β prevents apoptosis in rats with diabetic CM or doxorubicin-induced CM, in rhesus monkeys with rapid- pacing HF, and cardiomyocytes in vitro | (5, 7, 18, 19, 59, 96–99) | |
| Conduction System | Electrical remodeling | NRG-1β promotes conversion of contractile cardiomyocytes into Purkinje fibers, increases percentage of pacemaker cells in mixed populations of cardiomyocytes | (100–102) |
| Vasculature | Endothelial dysfunction | NRG-1β/ErbB signaling inhibition in rostral ventrolateral medulla causes hypertension, uncoupled eNOS prevents NRG-1β-induced cardioprotection, promotes proliferation/angiogenesis in vascular ECs | (11, 23, 73, 103) |
| Intimal thickening, smooth muscle hyperplasia | NRG-1β attenuates neointimal formation following vascular injury and inhibits vascular smooth muscle cell proliferation, a NRG-1β single nucleotide polymorphism moderates job strain-associated atherosclerosis | (10, 104) | |
| Rarefaction of capillaries | NRG-1β stimulates neovasculogenesis, arteriogenesis and angiogenesis | (11, 31, 74, 105) | |
| Interstitium | Induction of matrix metalloproteases | NRG-1β inhibits MMP-9 activation in brain of ischemia/reperfusion rats | (83) |
| Increased collagen synthesis, fibrosis | NRG-1β decreases collagen synthesis and fibrosis in diabetic rats with CM, dramatic LV fibrosis observed in conjunction with impaired NRG-1β/ErbB expression in diabetic rats | (36, 60) | |
| Fibroblasts | Myofibroblast conversion/activation, fibrosis | Improved ECM structure and fewer ventricular myofibroblasts in NRG-1β-treated post-MI swine, reduced expression of myofibroblast markers, collagens, and pro- fibrotic matricellular proteins in NRG-1β-treated swine and isolated rodent cardiac fibroblasts | (14) |
| Immune cells | Accumulation of leukocytes | NRG-1β treatment decreases lymphocytic infiltration in viral CM, expression induced in activated monocytes in atherosclerotic plaques, increased leukocyte accumulation in NRG-1-deficient ECs, EC-derived NRG-1β protects against ischemic injury, NRG-1β inhibits development of inflammation in brain of ischemia/reperfusion rats, NRG- 1β prevents IL-1β-induced endothelial permeability, NRG-1β decreases activation of monocytic cells, IL-6, TNF-α and IL-8 increased in lymphoblastoid cells from patients with mutation that may affect NRG-1β bioavailability | (28, 32, 59, 83, 106–108) |
A schematic of known NRG-1/ErbB signaling and its general effects in the central nervous system, blood, and heart is shown in Figure 1. In addition to cardiac myocytes in the heart, both endothelial cells and fibroblasts are responsive to NRG-1β and these may be involved in its effects on cardiovascular disease. NRG induces angiogenesis in vascular endothelial cells in several tissue beds (31). NRG-1β also regulates endothelial progenitor cells (EPCs) via ErbB2/ErbB3 receptors to support survival of EPCs (74). The possibility that NRG-1β has direct effects on cardiac fibrosis is rapidly evolving. A recent study of hypertrophic skin fibroblasts suggests that NRG induces scarring in these cells (75), but in cardiac fibroblasts, NRG appears to be anti-fibrotic (14, 62). In post-MI swine, infusion of the GGF2 isoform of NRG-1β reduces fibrosis remote from the site of infarct (14). When compared to untreated control animals, left ventricular tissues of GGF2-treated swine have fewer αSMA positive myofibroblasts, based on immunohistochemistry, and the cardiac matrix is visually more organized when tissue is macerated and viewed by scanning electron microscopy (14). We are finding that primary rat, mouse, and human cardiac fibroblasts respond to NRG-1β with antifibrotic signaling.
Chronic heart failure (CHF) is characterized by immune activation to a degree that is correlated to CHF severity and disease progression (76–79). There is some evidence suggesting that NRG-1/ErbB signaling plays a role in the regulation of immune cell activation. Protein expression of ErbB2, ErbB3 and ErbB4 receptors have been found in human circulating blood monocytes (28, 80). Moreover, during differentiation of human monocytes into macrophages, NRG, ErbB3 and ErbB4 expression levels are up-regulated, and NRG-1 prevents transition of macrophages into foam cells (81, 82). Based on this observation, along with multiple other studies (12, 21, 22, 26, 32, 60, 73, 83–85), NRG has emerged as a possible mitigator of atherosclerosis and restenosis (86). However, only a few investigations have been conducted to directly address this hypothesis. For example, chronic infusion of NRG-1β in apolipoprotein-E deficient mice prevented macrophage infiltration and atherosclerotic lesions (82). NRG-1β has also been shown to attenuate carotid artery neointimal formation in balloon-injured rats (10) and diminish ischemic damages in several rat models (24, 29, 59, 83). Thus, NRG may have effects on immune system function that provide additional benefit in the setting of heart disease.
Conclusions
NRG/ErbB signaling has emerged as an important determinant of cardiovascular function in the adult, and promising clinical data suggest that recombinant NRGs may become a new class of cardiovascular therapies that directly promote reverse remodeling in systolic heart failure. A better understanding of NRG-1’s role in this context, including an in-depth knowledge of effects on the many cells that regulate this process, is paramount to the development of therapeutics that fully harness the beneficial effects of this pathway. Further exploration of how endogenous NRG/ErbB signaling is regulated may provide new insights into how we might prevent cardiac injury, preserve cardiac function, and promote recovery of myocardial function under various clinical conditions. NRG’s role as a regulator of progenitor cell populations and as an adjunct in regenerative therapies also warrants further investigation. The ongoing program examining the clinical translation of recombinant NRGs promises to further illuminate the role and potential of multipotent growth factor.
Acknowledgments
This work was supported by the National Institutes of Health (P20 HL101425, U01 HL100398). Douglas B. Sawyer is an inventor on a patent assigned to Brigham and Women’s Hospital for the use of neuregulin for the treatment of heart failure, and on a patent assigned to the University of Pennsylvania and Vanderbilt University Medical Center for the use of neuregulin as a prognostic indicator in heart disease.
Footnotes
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
Cristi L. Galindo declares that she has no conflict of interest.
Sergey Ryzhov declares that he has no conflict of interest.
Douglas B. Sawyer has received financial support through a grant from Acorda Therapeutics for the study of neuregulin as a therapeutic for heart failure; has received a consulting fee/honorarium from Acorda Therapeutics;
References
- 1.Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- 2.Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res. 2003;284:14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- 3.Fleck D, Garratt AN, Haass C, Willem M. BACE1 dependent neuregulin processing: review. Curr Alzheimer Res. 2012;9:178–183. doi: 10.2174/156720512799361637. [DOI] [PubMed] [Google Scholar]
- 4.Fleck D, van Bebber F, Colombo A, Galante C, Schwenk BM, Rabe L, Hampel H, Novak B, Kremmer E, Tahirovic S, et al. Dual cleavage of neuregulin 1 type III by BACE1 and ADAM17 liberates its EGF-like domain and allows paracrine signaling. J Neurosci. 2013;33:7856–7869. doi: 10.1523/JNEUROSCI.3372-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendes-Ferreira P, De Keulenaer GW, Leite-Moreira AF, Bras-Silva C. Therapeutic potential of neuregulin-1 in cardiovascular disease. Drug Discov Today. 2013;18:836–842. doi: 10.1016/j.drudis.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Odiete O, Hill MF, Sawyer DB. Neuregulin in cardiovascular development and disease. Circ Res. 2012;111:1376–1385. doi: 10.1161/CIRCRESAHA.112.267286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukazawa R, Miller TA, Kuramochi Y, Frantz S, Kim YD, Marchionni MA, Kelly RA, Sawyer DB. Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt. J Mol Cell Cardiol. 2003;35:1473–1479. doi: 10.1016/j.yjmcc.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Chen M, Bi LL, Wang ZQ, Zhao F, Gan XD, Wang YG. Time-dependent regulation of neuregulin-1beta/ErbB/ERK pathways in cardiac differentiation of mouse embryonic stem cells. Mol Cell Biochem. 2013;380:67–72. doi: 10.1007/s11010-013-1658-y. [DOI] [PubMed] [Google Scholar]
- 9.Adam L, Vadlamudi R, Kondapaka SB, Chernoff J, Mendelsohn J, Kumar R. Heregulin regulates cytoskeletal reorganization and cell migration through the p21-activated kinase-1 via phosphatidylinositol-3 kinase. J Biol Chem. 1998;273:28238–28246. doi: 10.1074/jbc.273.43.28238. [DOI] [PubMed] [Google Scholar]
- 10.Clement CM, Thomas LK, Mou Y, Croslan DR, Gibbons GH, Ford BD. Neuregulin-1 attenuates neointimal formation following vascular injury and inhibits the proliferation of vascular smooth muscle cells. J Vasc Res. 2007;44:303–312. doi: 10.1159/000101776. [DOI] [PubMed] [Google Scholar]
- 11.Russell KS, Stern DF, Polverini PJ, Bender JR. Neuregulin activation of ErbB receptors in vascular endothelium leads to angiogenesis. Am J Physiol. 1999;277:H2205–2211. doi: 10.1152/ajpheart.1999.277.6.H2205. [DOI] [PubMed] [Google Scholar]
- 12.Timolati F, Ott D, Pentassuglia L, Giraud MN, Perriard JC, Suter TM, Zuppinger C. Neuregulin-1 beta attenuates doxorubicin-induced alterations of excitation-contraction coupling and reduces oxidative stress in adult rat cardiomyocytes. J Mol Cell Cardiol. 2006;41:845–854. doi: 10.1016/j.yjmcc.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Fukazawa T, Matsumoto M, Imura T, Khalesi E, Kajiume T, Kawahara Y, Tanimoto K, Yuge L. Electrical stimulation accelerates neuromuscular junction formation through ADAM19/neuregulin/ErbB signaling in vitro. Neurosci Lett. 2013;545:29–34. doi: 10.1016/j.neulet.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Kasasbeh E, Murphy A, Ahmad FA, Tu E, Williams P, Nunnally A, Adcock J, Caggiano AO, Parry TJ, Ganguly A, et al. Neuregulin-1β Improves Cardiac Remodeling After Myocardial Infarction In Swine. Circulation. 2011;124:Abstract 15531. [Google Scholar]
- 15.Pentassuglia L, Sawyer DB. ErbB/integrin signaling interactions in regulation of myocardial cell-cell and cell-matrix interactions. Biochim Biophys Acta. 2013;1833:909–916. doi: 10.1016/j.bbamcr.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sawyer DB, Zuppinger C, Miller TA, Eppenberger HM, Suter TM. Modulation of anthracycline-induced myofibrillar disarray in rat ventricular myocytes by neuregulin-1beta and anti-erbB2: potential mechanism for trastuzumab-induced cardiotoxicity. Circulation. 2002;105:1551–1554. doi: 10.1161/01.cir.0000013839.41224.1c. [DOI] [PubMed] [Google Scholar]
- 17.Baliga RR, Pimental DR, Zhao YY, Simmons WW, Marchionni MA, Sawyer DB, Kelly RA. NRG-1-induced cardiomyocyte hypertrophy. Role of PI-3-kinase, p70(S6K), and MEK-MAPK-RSK. Am J Physiol. 1999;277:H2026–2037. doi: 10.1152/ajpheart.1999.277.5.H2026. [DOI] [PubMed] [Google Scholar]
- 18.Kuramochi Y, Cote GM, Guo X, Lebrasseur NK, Cui L, Liao R, Sawyer DB. Cardiac endothelial cells regulate reactive oxygen species-induced cardiomyocyte apoptosis through neuregulin-1beta/erbB4 signaling. J Biol Chem. 2004;279:51141–51147. doi: 10.1074/jbc.M408662200. [DOI] [PubMed] [Google Scholar]
- 19.Zhao YY, Sawyer DB, Baliga RR, Opel DJ, Han X, Marchionni MA, Kelly RA. Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem. 1998;273:10261–10269. doi: 10.1074/jbc.273.17.10261. [DOI] [PubMed] [Google Scholar]
- 20.Kuramochi Y, Guo X, Sawyer DB. Neuregulin activates erbB2-dependent src/FAK signaling and cytoskeletal remodeling in isolated adult rat cardiac myocytes. J Mol Cell Cardiol. 2006;41:228–235. doi: 10.1016/j.yjmcc.2006.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemmens K, Fransen P, Sys SU, Brutsaert DL, De Keulenaer GW. Neuregulin-1 induces a negative inotropic effect in cardiac muscle: role of nitric oxide synthase. Circulation. 2004;109:324–326. doi: 10.1161/01.CIR.0000114521.88547.5E. [DOI] [PubMed] [Google Scholar]
- 22.Brero A, Ramella R, Fitou A, Dati C, Alloatti G, Gallo MP, Levi R. Neuregulin-1beta1 rapidly modulates nitric oxide synthesis and calcium handling in rat cardiomyocytes. Cardiovasc Res. 2010;88:443–452. doi: 10.1093/cvr/cvq238. [DOI] [PubMed] [Google Scholar]
- 23**.Ebner B, Lange SA, Eckert T, Wischniowski C, Ebner A, Braun-Dullaeus RC, Weinbrenner C, Wunderlich C, Simonis G, Strasser RH. Uncoupled eNOS annihilates neuregulin-1beta-induced cardioprotection: a novel mechanism in pharmacological postconditioning in myocardial infarction. Mol Cell Biochem. 2013;373:115–123. doi: 10.1007/s11010-012-1480-y. This study made the interesting observation that NRG administered during ischemia and prior to reperfusion increases infarct size. They go on to show that this effect is due to enhancing ROS formation by activating uncoupled nitric oxide synthase (NOS). Either removing eNOS, or improving coupling through co-administration of L-arginine as a substrate for eNOS converted NRG to a cardioprotective lingand, likely through GSK3β inactivation. [DOI] [PubMed] [Google Scholar]
- 24.Gu X, Liu X, Xu D, Li X, Yan M, Qi Y, Yan W, Wang W, Pan J, Xu Y, et al. Cardiac functional improvement in rats with myocardial infarction by up-regulating cardiac myosin light chain kinase with neuregulin. Cardiovasc Res. 2010;88:334–343. doi: 10.1093/cvr/cvq223. [DOI] [PubMed] [Google Scholar]
- 25.Baliga RR, Pimental DR, Zhao YY, Simmons WW, Marchionni MA, Sawyer DB, Kelly RA. NRG-1-induced cardiomyocyte hypertrophy. Role of PI-3-kinase, p70(S6K), and MEK-MAPK-RSK. Am J Physiol Heart Circ Physiol. 1999;277:H2026–H2037. doi: 10.1152/ajpheart.1999.277.5.H2026. [DOI] [PubMed] [Google Scholar]
- 26.Cote GM, Miller TA, Lebrasseur NK, Kuramochi Y, Sawyer DB. Neuregulin-1alpha and beta isoform expression in cardiac microvascular endothelial cells and function in cardiac myocytes in vitro. Exp Cell Res. 2005;311:135–146. doi: 10.1016/j.yexcr.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 27.Lebrasseur NK, Cote GM, Miller TA, Fielding RA, Sawyer DB. Regulation of neuregulin/ErbB signaling by contractile activity in skeletal muscle. Am J Physiol Cell Physiol. 2003;284:C1149–1155. doi: 10.1152/ajpcell.00487.2002. [DOI] [PubMed] [Google Scholar]
- 28.Xu Z, Ford GD, Croslan DR, Jiang J, Gates A, Allen R, Ford BD. Neuroprotection by neuregulin-1 following focal stroke is associated with the attenuation of ischemia-induced pro-inflammatory and stress gene expression. Neurobiol Dis. 2005;19:461–470. doi: 10.1016/j.nbd.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 29.Shyu WC, Lin SZ, Chiang MF, Yang HI, Thajeb P, Li H. Neuregulin-1 reduces ischemia-induced brain damage in rats. Neurobiol Aging. 2004;25:935–944. doi: 10.1016/j.neurobiolaging.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 30.Xu Z, Jiang J, Ford G, Ford BD. Neuregulin-1 is neuroprotective and attenuates inflammatory responses induced by ischemic stroke. Biochem Biophys Res Commun. 2004;322:440–446. doi: 10.1016/j.bbrc.2004.07.149. [DOI] [PubMed] [Google Scholar]
- 31*.Hedhli N, Dobrucki LW, Kalinowski A, Zhuang ZW, Wu X, Russell RR, 3rd, Sinusas AJ, Russell KS. Endothelial-derived neuregulin is an important mediator of ischaemia-induced angiogenesis and arteriogenesis. Cardiovasc Res. 2012;93:516–524. doi: 10.1093/cvr/cvr352. These investigators defined a role for endothelial derived NRG as an important mediator of ischemia-induced angiogenesis in peripheral vascular disease. These findings have clear therapeutic implications that have yet to be explored. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32**.Hedhli N, Huang Q, Kalinowski A, Palmeri M, Hu X, Russell RR, Russell KS. Endothelium-derived neuregulin protects the heart against ischemic injury. Circulation. 2011;123:2254–2262. doi: 10.1161/CIRCULATIONAHA.110.991125. These investigators used endothelial specific ablation of NRG to demonstrate a role for endogenous cardiac NRG in the protection of the heart from ischemic injury. The elegant work sets the stage to explore how endogenous and exogenous recombinant neuregulin might be used to enhance recovery from myocardial infarction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pentassuglia L, Graf M, Lane H, Kuramochi Y, Cote G, Timolati F, Sawyer DB, Zuppinger C, Suter TM. Inhibition of ErbB2 by receptor tyrosine kinase inhibitors causes myofibrillar structural damage without cell death in adult rat cardiomyocytes. Exp Cell Res. 2009;315:1302–1312. doi: 10.1016/j.yexcr.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang SJ, Wu XS, Han ZH, Zhang XX, Wang CM, Li XY, Lu LQ, Zhang JL. Neuregulin-1 preconditioning protects the heart against ischemia/reperfusion injury through a PI3K/Akt-dependent mechanism. Chin Med J (Engl) 2010;123:3597–3604. [PubMed] [Google Scholar]
- 35.Rohrbach S, Yan X, Weinberg EO, Hasan F, Bartunek J, Marchionni MA, Lorell BH. Neuregulin in cardiac hypertrophy in rats with aortic stenosis. Differential expression of erbB2 and erbB4 receptors. Circulation. 1999;100:407–412. doi: 10.1161/01.cir.100.4.407. [DOI] [PubMed] [Google Scholar]
- 36.Gui C, Zhu L, Hu M, Lei L, Long Q. Neuregulin-1/ErbB signaling is impaired in the rat model of diabetic cardiomyopathy. Cardiovasc Pathol. 2012;21:414–420. doi: 10.1016/j.carpath.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 37.Rohrbach S, Niemann B, Silber RE, Holtz J. Neuregulin receptors erbB2 and erbB4 in failing human myocardium -- depressed expression and attenuated activation. Basic Res Cardiol. 2005;100:240–249. doi: 10.1007/s00395-005-0514-4. [DOI] [PubMed] [Google Scholar]
- 38.Peng X, Guo X, Borkan SC, Bharti A, Kuramochi Y, Calderwood S, Sawyer DB. Heat shock protein 90 stabilization of ErbB2 expression is disrupted by ATP depletion in myocytes. J Biol Chem. 2005;280:13148–13152. doi: 10.1074/jbc.M410838200. [DOI] [PubMed] [Google Scholar]
- 39.Geisberg C, Pentassuglia L, Sawyer DB. Cardiac side effects of anticancer treatments: new mechanistic insights. Curr Heart Fail Rep. 2012;9:211–218. doi: 10.1007/s11897-012-0098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seshadri R, Matthews C, Dobrovic A, Horsfall DJ. The significance of oncogene amplification in primary breast cancer. Int J Cancer. 1989;43:270–272. doi: 10.1002/ijc.2910430218. [DOI] [PubMed] [Google Scholar]
- 41.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 42.Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, Murphy M, Stewart SJ, Keefe D. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–1221. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 43.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 44.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 45.Suter TM, Procter M, van Veldhuisen DJ, Muscholl M, Bergh J, Carlomagno C, Perren T, Passalacqua R, Bighin C, Klijn JG, et al. Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol. 2007;25:3859–3865. doi: 10.1200/JCO.2006.09.1611. [DOI] [PubMed] [Google Scholar]
- 46.Jones AL, Barlow M, Barrett-Lee PJ, Canney PA, Gilmour IM, Robb SD, Plummer CJ, Wardley AM, Verrill MW. Management of cardiac health in trastuzumab-treated patients with breast cancer: updated United Kingdom National Cancer Research Institute recommendations for monitoring. Br J Cancer. 2009;100:684–692. doi: 10.1038/sj.bjc.6604909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davis M, Witteles RM. Cardiac testing to manage cardiovascular risk in cancer patients. Semin Oncol. 2013;40:147–155. doi: 10.1053/j.seminoncol.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 48.Moondra V, Sarma S, Buxton T, Safa R, Cote G, Storer T, Lebrasseur NK, Sawyer DB. Serum Neuregulin-1beta as a Biomarker of Cardiovascular Fitness. Open Biomark J. 2009;2:1–5. doi: 10.2174/1875318300902010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ky B, Kimmel SE, Safa RN, Putt ME, Sweitzer NK, Fang JC, Sawyer DB, Cappola TP. Neuregulin-1 beta is associated with disease severity and adverse outcomes in chronic heart failure. Circulation. 2009;120:310–317. doi: 10.1161/CIRCULATIONAHA.109.856310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geisberg CA, Wang G, Safa RN, Smith HM, Anderson B, Peng XY, Veerkamp B, Zhao DX, Blakemore D, Yu C, et al. Circulating neuregulin-1beta levels vary according to the angiographic severity of coronary artery disease and ischemia. Coron Artery Dis. 2011;22:577–582. doi: 10.1097/MCA.0b013e32834d3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51**.Zeng Z, Gui C, Nong Q, Du F, Zhu L. Serum neuregulin-1beta levels are positively correlated with VEGF and Angiopoietin-1 levels in patients with diabetes and unstable angina pectoris. Int J Cardiol. 2013 doi: 10.1016/j.ijcard.2013.04.088. Based upon animal studies one might predict that NRG is released by ischemia, even before myocardial injury. These authors measured serum NRG in patients with unstable angina and provide evidence to support this idea. The potential of NRG as a biomarker of ischemia is further supported by Ref 50. [DOI] [PubMed] [Google Scholar]
- 52.Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stefansson H, Sarginson J, Kong A, Yates P, Steinthorsdottir V, Gudfinnsson E, Gunnarsdottir S, Walker N, Petursson H, Crombie C, et al. Association of neuregulin 1 with schizophrenia confirmed in a Scottish population. Am J Hum Genet. 2003;72:83–87. doi: 10.1086/345442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nicodemus KK, Luna A, Vakkalanka R, Goldberg T, Egan M, Straub RE, Weinberger DR. Further evidence for association between ErbB4 and schizophrenia and influence on cognitive intermediate phenotypes in healthy controls. Mol Psychiatry. 2006;11:1062–1065. doi: 10.1038/sj.mp.4001878. [DOI] [PubMed] [Google Scholar]
- 55.Norton N, Moskvina V, Morris DW, Bray NJ, Zammit S, Williams NM, Williams HJ, Preece AC, Dwyer S, Wilkinson JC, et al. Evidence that interaction between neuregulin 1 and its receptor erbB4 increases susceptibility to schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:96–101. doi: 10.1002/ajmg.b.30236. [DOI] [PubMed] [Google Scholar]
- 56.Ruschena D, Mullen PE, Burgess P, Cordner SM, Barry-Walsh J, Drummer OH, Palmer S, Browne C, Wallace C. Sudden death in psychiatric patients. Br J Psychiatry. 1998;172:331–336. doi: 10.1192/bjp.172.4.331. [DOI] [PubMed] [Google Scholar]
- 57.Manu P, Kane JM, Correll CU. Sudden deaths in psychiatric patients. J Clin Psychiatry. 2011;72:936–941. doi: 10.4088/JCP.10m06244gry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huertas-Vazquez A, Teodorescu C, Reinier K, Uy-Evanado A, Chugh H, Jerger K, Ayala J, Gunson K, Jui J, Newton-Cheh C, et al. A common missense variant in the neuregulin 1 gene is associated with both schizophrenia and sudden cardiac death. Heart Rhythm. 2013;10:994–998. doi: 10.1016/j.hrthm.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu X, Gu X, Li Z, Li X, Li H, Chang J, Chen P, Jin J, Xi B, Chen D, et al. Neuregulin-1/erbB-activation improves cardiac function and survival in models of ischemic, dilated, and viral cardiomyopathy. J Am Coll Cardiol. 2006;48:1438–1447. doi: 10.1016/j.jacc.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 60.Li B, Zheng Z, Wei Y, Wang M, Peng J, Kang T, Huang X, Xiao J, Li Y, Li Z. Therapeutic effects of neuregulin-1 in diabetic cardiomyopathy rats. Cardiovasc Diabetol. 2011;10:69. doi: 10.1186/1475-2840-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carraway KL, 3rd, Burden SJ. Neuregulins and their receptors. Curr Opin Neurobiol. 1995;5:606–612. doi: 10.1016/0959-4388(95)80065-4. [DOI] [PubMed] [Google Scholar]
- 62.Galindo C, Truc-Tran L, Ryzhov S, Feoktistov I, Sawyer DB. Neuregulin-1β Inhibits TGFβ-induced Cardiofibroblast to Myofibroblast Transition. Cardiovascular Research. 2013;113:Abstract 302. [Google Scholar]
- 63.Gao R, Zhang J, Cheng L, Wu X, Dong W, Yang X, Li T, Liu X, Xu Y, Li X, et al. A Phase II, randomized, double-blind, multicenter, based on standard therapy, placebo-controlled study of the efficacy and safety of recombinant human neuregulin-1 in patients with chronic heart failure. J Am Coll Cardiol. 2010;55:1907–1914. doi: 10.1016/j.jacc.2009.12.044. [DOI] [PubMed] [Google Scholar]
- 64**.Jabbour A, Hayward CS, Keogh AM, Kotlyar E, McCrohon JA, England JF, Amor R, Liu X, Li XY, Zhou MD, et al. Parenteral administration of recombinant human neuregulin-1 to patients with stable chronic heart failure produces favourable acute and chronic haemodynamic responses. Eur J Heart Fail. 2011;13:83–92. doi: 10.1093/eurjhf/hfq152. In this clinical trial the investigators define the hemodynamic response to recombinant NRG, showing its inodilator properties. Interestingly the treatment was associated with improved myocardial function chronically that was most pronounced in the intermediate dose group. [DOI] [PubMed] [Google Scholar]
- 65.Brittain E, Muldowney J, Geisberg C, Caggiano A, Eisen A, Anderson S, Sawyer D, Mendes L, Lenihan D. Evaluation of cardiac function in symptomatic heart failure patients in a single infusion, Phase 1, dose escalation study of glial growth factor 2. J Am Coll Cardiol. 2013:61. [Google Scholar]
- 66.Lenihan DJ, Anderson S, Geisberg C, Caggiano A, Eisen A, Brittain E, Muldowney JAS, III, Mendes L, Sawyer D. SAFETY AND TOLERABILITY OF GLIAL GROWTH FACTOR 2 IN PATIENTS WITH CHRONIC HEART FAILURE: A PHASE I SINGLE DOSE ESCALATION STUDY. J Am Coll Cardiol. 2013:61. [Google Scholar]
- 67.Odiete O, Konik EA, Sawyer DB, Hill MF. Type 1 diabetes mellitus abrogates compensatory augmentation of myocardial neuregulin-1beta/ErbB in response to myocardial infarction resulting in worsening heart failure. Cardiovasc Diabetol. 2013;12:52. doi: 10.1186/1475-2840-12-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miller TA, Icli B, Cote GM, Lebrasseur NK, Borkan SC, Pimentel DR, Peng X, Sawyer DB. Palmitate alters neuregulin signaling and biology in cardiac myocytes. Biochem Biophys Res Commun. 2009;379:32–37. doi: 10.1016/j.bbrc.2008.11.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69**.Jay SM, Murthy AC, Hawkins JF, Wortzel JR, Steinhauser ML, Alvarez LM, Gannon J, Macrae CA, Griffith LG, Lee RT. An engineered bivalent neuregulin protects against Doxorubicin-induced cardiotoxicity with reduced proneoplastic potential. Circulation. 2013;128:152–161. doi: 10.1161/CIRCULATIONAHA.113.002203. These investigators engineered NRG to favor ErbB4 or ErbB3 homodimer formation, with the goal to remove ErbB2 activation during NRG stimulation. They provide intriguing evidence that such an approach can protect the heart and limit tumor growth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Force T, Wang Y. Mechanism-based engineering against anthracycline cardiotoxicity. Circulation. 2013;128:98–100. doi: 10.1161/CIRCULATIONAHA.113.003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jay SM, Kurtagic E, Alvarez LM, de Picciotto S, Sanchez E, Hawkins JF, Prince RN, Guerrero Y, Treasure CL, Lee RT, et al. Engineered bivalent ligands to bias ErbB receptor-mediated signaling and phenotypes. J Biol Chem. 2011;286:27729–27740. doi: 10.1074/jbc.M111.221093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lemmens K, Doggen K, De Keulenaer GW. Activation of the neuregulin/ErbB system during physiological ventricular remodeling in pregnancy. Am J Physiol Heart Circ Physiol. 2011;300:H931–942. doi: 10.1152/ajpheart.00385.2010. [DOI] [PubMed] [Google Scholar]
- 73.Matsukawa R, Hirooka Y, Ito K, Sunagawa K. Inhibition of neuregulin-1/ErbB signaling in the rostral ventrolateral medulla leads to hypertension through reduced nitric oxide synthesis. Am J Hypertens. 2013;26:51–57. doi: 10.1093/ajh/hps005. [DOI] [PubMed] [Google Scholar]
- 74*.Safa RN, Peng XY, Pentassuglia L, Lim CC, Lamparter M, Silverstein C, Walker J, Chen B, Geisberg C, Hatzopoulos AK, et al. Neuregulin-1beta regulation of embryonic endothelial progenitor cell survival. Am J Physiol Heart Circ Physiol. 2011;300:H1311–1319. doi: 10.1152/ajpheart.01104.2009. This work demonstrates that a subpopulation of monocytes in the circulation that have been associated with cardiac repair respond to NRG with enhanced survival. Whether this set of cells are activated by NRG during cardiac injury/stress, and mediate cardiac repair remains an open question. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim JS, Choi IG, Lee BC, Park JB, Kim JH, Jeong JH, Seo CH. Neuregulin induces CTGF expression in hypertrophic scarring fibroblasts. Mol Cell Biochem. 2012;365:181–189. doi: 10.1007/s11010-012-1258-2. [DOI] [PubMed] [Google Scholar]
- 76.Rauchhaus M, Doehner W, Francis DP, Davos C, Kemp M, Liebenthal C, Niebauer J, Hooper J, Volk HD, Coats AJ, et al. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation. 2000;102:3060–3067. doi: 10.1161/01.cir.102.25.3060. [DOI] [PubMed] [Google Scholar]
- 77.Barisione C, Garibaldi S, Ghigliotti G, Fabbi P, Altieri P, Casale MC, Spallarossa P, Bertero G, Balbi M, Corsiglia L, et al. CD14CD16 monocyte subset levels in heart failure patients. Dis Markers. 2010;28:115–124. doi: 10.3233/DMA-2010-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dixon DL, Griggs KM, Bersten AD, De Pasquale CG. Systemic inflammation and cell activation reflects morbidity in chronic heart failure. Cytokine. 2011;56:593–599. doi: 10.1016/j.cyto.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 79.Mann DL. The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circ Res. 2011;108:1133–1145. doi: 10.1161/CIRCRESAHA.110.226936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leone F, Perissinotto E, Cavalloni G, Fonsato V, Bruno S, Surrenti N, Hong D, Capaldi A, Geuna M, Piacibello W, et al. Expression of the c-ErbB-2/HER2 proto-oncogene in normal hematopoietic cells. J Leukoc Biol. 2003;74:593–601. doi: 10.1189/jlb.0203068. [DOI] [PubMed] [Google Scholar]
- 81.Mograbi B, Rochet N, Imbert V, Bourget I, Bocciardi R, Emiliozzi C, Rossi B. Human monocytes express amphiregulin and heregulin growth factors upon activation. Eur Cytokine Netw. 1997;8:73–81. [PubMed] [Google Scholar]
- 82.Xu G, Watanabe T, Iso Y, Koba S, Sakai T, Nagashima M, Arita S, Hongo S, Ota H, Kobayashi Y, et al. Preventive effects of heregulin-beta1 on macrophage foam cell formation and atherosclerosis. Circ Res. 2009;105:500–510. doi: 10.1161/CIRCRESAHA.109.193870. [DOI] [PubMed] [Google Scholar]
- 83.Li Q, Zhang R, Ge YL, Mei YW, Guo YL. Effects of neuregulin on expression of MMP-9 and NSE in brain of ischemia/reperfusion rat. J Mol Neurosci. 2009;38:207–215. doi: 10.1007/s12031-008-9150-y. [DOI] [PubMed] [Google Scholar]
- 84.Guma A, Martinez-Redondo V, Lopez-Soldado I, Canto C, Zorzano A. Emerging role of neuregulin as a modulator of muscle metabolism. Am J Physiol Endocrinol Metab. 2010;298:E742–750. doi: 10.1152/ajpendo.00541.2009. [DOI] [PubMed] [Google Scholar]
- 85.Okoshi K, Nakayama M, Yan X, Okoshi MP, Schuldt AJ, Marchionni MA, Lorell BH. Neuregulins regulate cardiac parasympathetic activity: muscarinic modulation of beta-adrenergic activity in myocytes from mice with neuregulin-1 gene deletion. Circulation. 2004;110:713–717. doi: 10.1161/01.CIR.0000138109.32748.80. [DOI] [PubMed] [Google Scholar]
- 86.Watanabe T, Sato K, Itoh F, Iso Y. Pathogenic involvement of heregulin-beta(1) in anti-atherogenesis. Regul Pept. 2012;175:11–14. doi: 10.1016/j.regpep.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 87.Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 88.Bian Y, Sun M, Silver M, Ho KK, Marchionni MA, Caggiano AO, Stone JR, Amende I, Hampton TG, Morgan JP, et al. Neuregulin-1 attenuated doxorubicin-induced decrease in cardiac troponins. Am J Physiol Heart Circ Physiol. 2009;297:H1974–1983. doi: 10.1152/ajpheart.01010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guo YF, Zhang XX, Liu Y, Duan HY, Jie BZ, Wu XS. Neuregulin-1 attenuates mitochondrial dysfunction in a rat model of heart failure. Chin Med J (Engl) 2012;125:807–814. [PubMed] [Google Scholar]
- 90*.Hill MF, Patel AV, Murphy A, Smith HM, Galindo CL, Pentassuglia L, Peng X, Lenneman CG, Odiete O, Friedman DB, et al. Intravenous glial growth factor 2 (GGF2) isoform of neuregulin-1beta improves left ventricular function, gene and protein expression in rats after myocardial infarction. PLoS One. 2013;8:e55741. doi: 10.1371/journal.pone.0055741. In this study the effects of recombinant NRG were examined in a rat model of cardiac remodeling after myocardial infarction, and was found to improve cardiac function in concert with suppressing/reversing a number of the protein and gene expression changes that occur in chronic heart failure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xiao J, Li B, Zheng Z, Wang M, Peng J, Li Y, Li Z. Therapeutic effects of neuregulin-1 gene transduction in rats with myocardial infarction. Coron Artery Dis. 2012;23:460–468. doi: 10.1097/MCA.0b013e32835877da. [DOI] [PubMed] [Google Scholar]
- 92.Geisberg CA, Abdallah WM, da Silva M, Silverstein C, Smith HM, Abramson V, Mayer I, Means-Powell J, Freehardt D, White B, et al. Circulating neuregulin during the transition from stage A to stage B/C heart failure in a breast cancer cohort. J Card Fail. 2013;19:10–15. doi: 10.1016/j.cardfail.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ozcelik C, Erdmann B, Pilz B, Wettschureck N, Britsch S, Hubner N, Chien KR, Birchmeier C, Garratt AN. Conditional mutation of the ErbB2 (HER2) receptor in cardiomyocytes leads to dilated cardiomyopathy. Proc Natl Acad Sci U S A. 2002;99:8880–8885. doi: 10.1073/pnas.122249299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garcia-Rivello H, Taranda J, Said M, Cabeza-Meckert P, Vila-Petroff M, Scaglione J, Ghio S, Chen J, Lai C, Laguens RP, et al. Dilated cardiomyopathy in Erb-b4-deficient ventricular muscle. Am J Physiol Heart Circ Physiol. 2005;289:H1153–1160. doi: 10.1152/ajpheart.00048.2005. [DOI] [PubMed] [Google Scholar]
- 95.Crone SA, Zhao YY, Fan L, Gu Y, Minamisawa S, Liu Y, Peterson KL, Chen J, Kahn R, Condorelli G, et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8:459–465. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 96.Jie B, Zhang X, Wu X, Xin Y, Liu Y, Guo Y. Neuregulin-1 suppresses cardiomyocyte apoptosis by activating PI3K/Akt and inhibiting mitochondrial permeability transition pore. Mol Cell Biochem. 2012;370:35–43. doi: 10.1007/s11010-012-1395-7. [DOI] [PubMed] [Google Scholar]
- 97.Li J, Qiang O, Wang L. Effects of neuregulin on cardiac myocyte apoptosis and PI-3K signal transduction pathway in rapid pacing-induced heart failure in rhesus monkeys. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2007;32:408–412. [PubMed] [Google Scholar]
- 98.Rohrbach S, Muller-Werdan U, Werdan K, Koch S, Gellerich NF, Holtz J. Apoptosis-modulating interaction of the neuregulin/erbB pathway with anthracyclines in regulating Bcl-xS and Bcl-xL in cardiomyocytes. J Mol Cell Cardiol. 2005;38:485–493. doi: 10.1016/j.yjmcc.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 99.Pentassuglia L, Timolati F, Seifriz F, Abudukadier K, Suter TM, Zuppinger C. Inhibition of ErbB2/neuregulin signaling augments paclitaxel-induced cardiotoxicity in adult ventricular myocytes. Exp Cell Res. 2007;313:1588–1601. doi: 10.1016/j.yexcr.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 100.Rentschler S, Zander J, Meyers K, France D, Levine R, Porter G, Rivkees SA, Morley GE, Fishman GI. Neuregulin-1 promotes formation of the murine cardiac conduction system. Proc Natl Acad Sci U S A. 2002;99:10464–10469. doi: 10.1073/pnas.162301699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ruhparwar A, Er F, Martin U, Radke K, Gruh I, Niehaus M, Karck M, Haverich A, Hoppe UC. Enrichment of cardiac pacemaker-like cells: neuregulin-1 and cyclic AMP increase I(f)-current density and connexin 40 mRNA levels in fetal cardiomyocytes. Med Biol Eng Comput. 2007;45:221–227. doi: 10.1007/s11517-007-0164-3. [DOI] [PubMed] [Google Scholar]
- 102.Ruhparwar A, Haverich A. Prospects for biological cardiac pacemaker systems. Pacing Clin Electrophysiol. 2003;26:2069–2071. doi: 10.1046/j.1460-9592.2003.00322.x. [DOI] [PubMed] [Google Scholar]
- 103.Kalinowski A, Plowes NJ, Huang Q, Berdejo-Izquierdo C, Russell RR, Russell KS. Metalloproteinase-dependent cleavage of neuregulin and autocrine stimulation of vascular endothelial cells. FASEB J. 2010;24:2567–2575. doi: 10.1096/fj.08-129072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hintsanen M, Elovainio M, Puttonen S, Kivimaki M, Raitakari OT, Lehtimaki T, Rontu R, Juonala M, Kahonen M, Viikari J, et al. Neuregulin-1 genotype moderates the association between job strain and early atherosclerosis in young men. Ann Behav Med. 2007;33:148–155. doi: 10.1007/BF02879896. [DOI] [PubMed] [Google Scholar]
- 105.Lok J, Sardi SP, Guo S, Besancon E, Ha DM, Rosell A, Kim WJ, Corfas G, Lo EH. Neuregulin-1 signaling in brain endothelial cells. J Cereb Blood Flow Metab. 2009;29:39–43. doi: 10.1038/jcbfm.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Panutsopulos D, Arvanitis DL, Tsatsanis C, Papalambros E, Sigala F, Spandidos DA. Expression of heregulin in human coronary atherosclerotic lesions. J Vasc Res. 2005;42:463–474. doi: 10.1159/000088100. [DOI] [PubMed] [Google Scholar]
- 107.Lok J, Zhao S, Leung W, Seo JH, Navaratna D, Wang X, Whalen MJ, Lo EH. Neuregulin-1 effects on endothelial and blood-brain-barrier permeability after experimental injury. Transl Stroke Res. 2012;3:S119–S124. doi: 10.1007/s12975-012-0157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marballi K, Quinones MP, Jimenez F, Escamilla MA, Raventos H, Soto-Bernardini MC, Ahuja SS, Walss-Bass C. In vivo and in vitro genetic evidence of involvement of neuregulin 1 in immune system dysregulation. J Mol Med (Berl) 2010;88:1133–1141. doi: 10.1007/s00109-010-0653-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


