Abstract
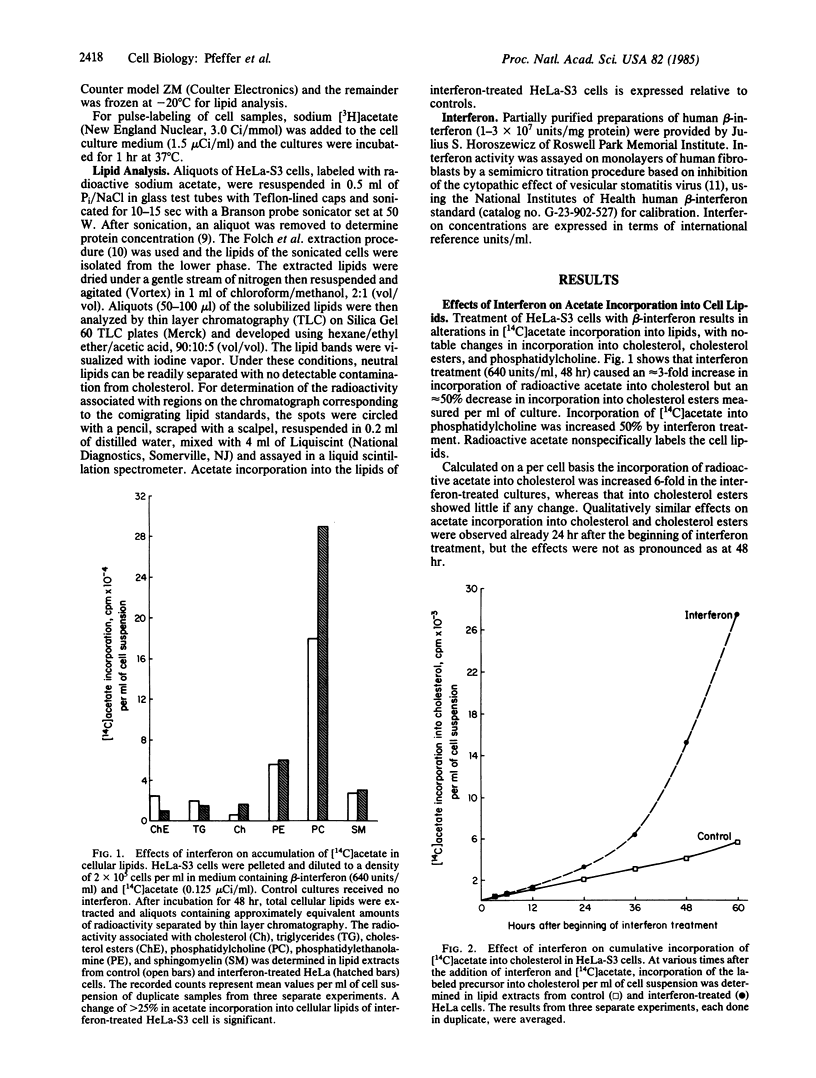
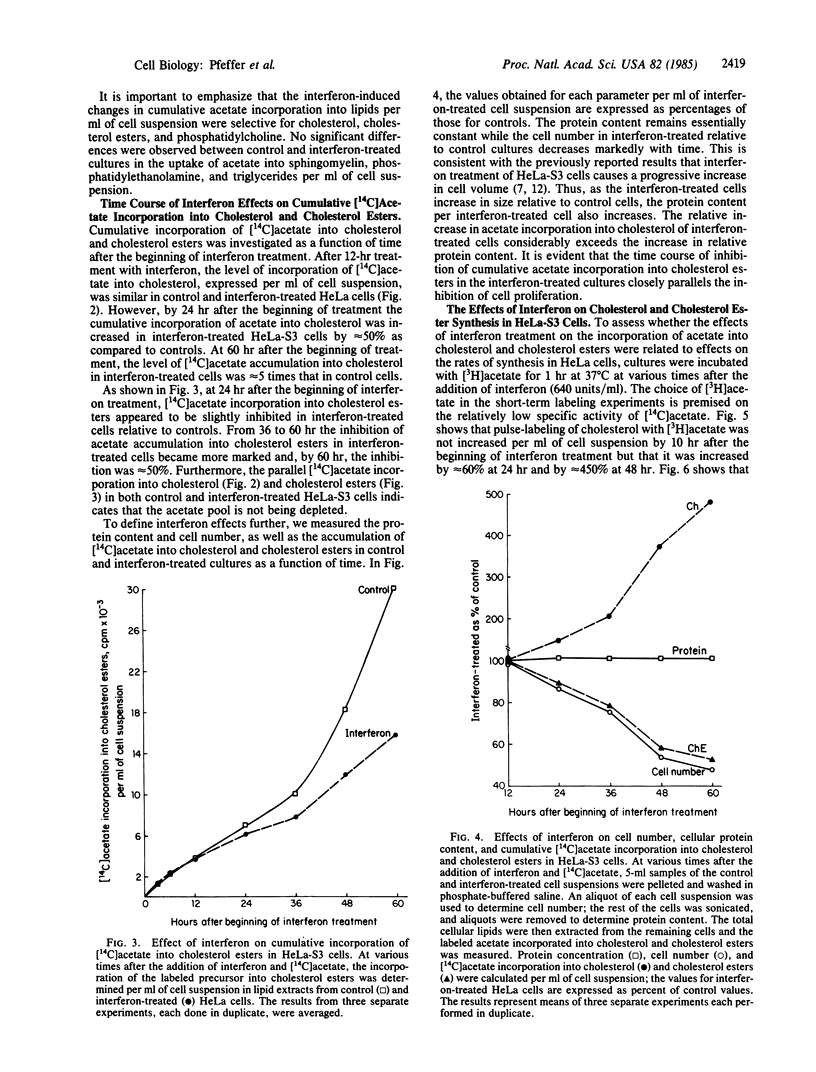
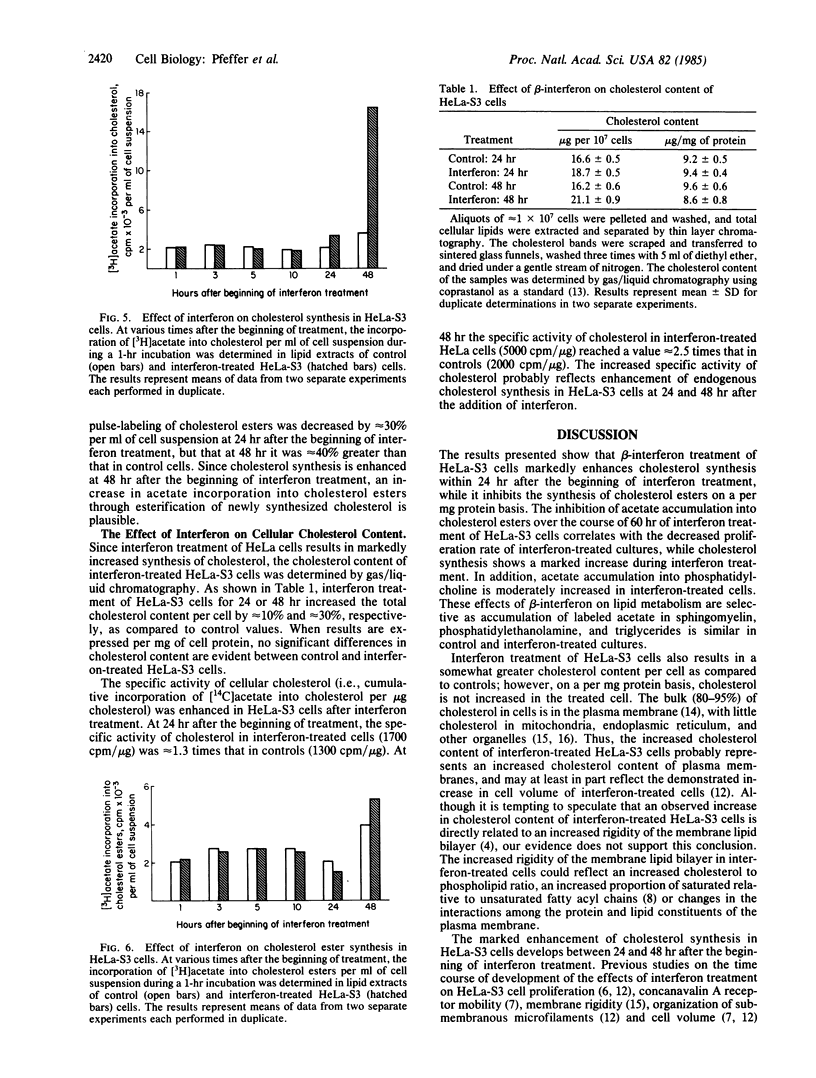
Treatment of human HeLa-S3 cells (an epidermoid carcinoma line) with human beta-interferon (640 units/ml) selectively alters lipid metabolism by increasing cholesterol synthesis per mg of cell protein as measured by 1-hr pulse-labeling of cells with [3H]acetate. Cholesterol synthesis in interferon-treated cells is increased approximately equal to 60% at 24 hr after the beginning of treatment and approximately equal to 450% at 48 hr. Continuous labeling of interferon-treated cells with [14C]acetate shows increased accumulation of label in cholesterol when normalized per mg of cell protein, as well as an increase in the specific activity of cholesterol in the treated cells. In contrast, interferon treatment decreases the accumulation of [14C]acetate into cholesterol esters. The [14C]acetate labeling of sphingomyelin, phosphatidylethanolamine, and triglycerides shows no change compared to untreated controls. The labeling of phosphatidylcholine was moderately increased in treated cells. The interferon-induced changes in lipid metabolism are a part of a coordinated response of cells to interferon treatment, characterized by reduced cell proliferation and cell motility and an increase in cell size and mass. The increased cholesterol synthesis is consistent with a model in which beta-interferon treatment of HeLa cells inhibits the endocytosis of cholesterol-containing low density lipoprotein, which results in an increase in cholesterol synthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G., Brown M. S., Goldstein J. L. Role of the coated endocytic vesicle in the uptake of receptor-bound low density lipoprotein in human fibroblasts. Cell. 1977 Mar;10(3):351–364. doi: 10.1016/0092-8674(77)90022-8. [DOI] [PubMed] [Google Scholar]
- Bhathena S. J., Avigan J., Schreiner M. E. Effect of insulin on sterol and fatty acid synthesis and hydroxymethylglutaryl CoA reductase activity in mammalian cells grown in culture. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2174–2178. doi: 10.1073/pnas.71.6.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmann H. B., Hagopian A., Eylar E. H. Cellular membranes: the isolation and characterization of the plasma and smooth membranes of HeLa cells. Arch Biochem Biophys. 1968 Oct;128(1):51–69. doi: 10.1016/0003-9861(68)90008-8. [DOI] [PubMed] [Google Scholar]
- Chandrabose K., Cuatrecasas P., Pottathil R. Changes in fatty acyl chains of phospholipids induced by interferon in mouse sarcoma S-180 cells. Biochem Biophys Res Commun. 1981 Feb 12;98(3):661–668. doi: 10.1016/0006-291x(81)91165-7. [DOI] [PubMed] [Google Scholar]
- Colbeau A., Nachbaur J., Vignais P. M. Enzymic characterization and lipid composition of rat liver subcellular membranes. Biochim Biophys Acta. 1971 Dec 3;249(2):462–492. doi: 10.1016/0005-2736(71)90123-4. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Goldstein J. L., Basu S. K., Brunschede G. Y., Brown M. S. Release of low density lipoprotein from its cell surface receptor by sulfated glycosaminoglycans. Cell. 1976 Jan;7(1):85–95. doi: 10.1016/0092-8674(76)90258-0. [DOI] [PubMed] [Google Scholar]
- Havell E. A., Vilcek J. Production of high-titered interferon in cultures of human diploid cells. Antimicrob Agents Chemother. 1972 Dec;2(6):476–484. doi: 10.1128/aac.2.6.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lange Y., Ramos B. V. Analysis of the distribution of cholesterol in the intact cell. J Biol Chem. 1983 Dec 25;258(24):15130–15134. [PubMed] [Google Scholar]
- MIETTINEN T. A., AHRENS E. H., Jr, GRUNDY S. M. QUANTITATIVE ISOLATION AND GAS--LIQUID CHROMATOGRAPHIC ANALYSIS OF TOTAL DIETARY AND FECAL NEUTRAL STEROIDS. J Lipid Res. 1965 Jul;6:411–424. [PubMed] [Google Scholar]
- Pfeffer L. M., Landsberger F. R., Tamm I. Beta-interferon-induced time-dependent changes in the plasma membrane lipid bilayer of cultured cells. J Interferon Res. 1981;1(4):613–620. doi: 10.1089/jir.1981.1.613. [DOI] [PubMed] [Google Scholar]
- Pfeffer L. M., Murphy J. S., Tamm I. Interferon effects on the growth and division of human fibroblasts. Exp Cell Res. 1979 Jun;121(1):111–120. doi: 10.1016/0014-4827(79)90450-6. [DOI] [PubMed] [Google Scholar]
- Pfeffer L. M., Tamm I. Comparison of the effects of alpha and beta interferons on the proliferation and volume of human tumor cells (HeLa-S3, Daudi, P3HR-1). J Interferon Res. 1983;3(4):395–408. doi: 10.1089/jir.1983.3.395. [DOI] [PubMed] [Google Scholar]
- Pfeffer L. M., Tamm I. Effects of beta interferon on concanavalin A binding and size of HeLa cells. J Interferon Res. 1982;2(3):431–440. doi: 10.1089/jir.1982.2.431. [DOI] [PubMed] [Google Scholar]
- Pfeffer L. M., Wang E., Tamm I. Interferon inhibits the redistribution of cell surface components. J Exp Med. 1980 Aug 1;152(2):469–474. doi: 10.1084/jem.152.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum M. F., Huttler C. R., Strauss J. F., 3rd Control of sterol metabolism in cultured rat granulosa cells. Endocrinology. 1981 Nov;109(5):1518–1527. doi: 10.1210/endo-109-5-1518. [DOI] [PubMed] [Google Scholar]
- Schuler L. A., Scavo L., Kirsch T. M., Flickinger G. L., Strauss J. F., 3rd Regulation of de novo biosynthesis of cholesterol and progestins, and formation of cholesteryl ester in rat corpus luteum by exogenous sterol. J Biol Chem. 1979 Sep 10;254(17):8662–8668. [PubMed] [Google Scholar]
- Wang E., Michl J., Pfeffer L. M., Silverstein S. C., Tamm I. Interferon suppresses pinocytosis but stimulates phagocytosis in mouse peritoneal macrophages: related changes in cytoskeletal organization. J Cell Biol. 1984 Apr;98(4):1328–1341. doi: 10.1083/jcb.98.4.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E., Pfeffer L. M., Tamm I. Interferon increases the abundance of submembranous microfilaments in HeLa-S3 cells in suspension culture. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6281–6285. doi: 10.1073/pnas.78.10.6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker-Dowling P. A., Wilcox D. K., Widnell C. C., Youngner J. S. Interferon-mediated inhibition of virus penetration. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1083–1086. doi: 10.1073/pnas.80.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox D. K., Whitaker-Dowling P. A., Youngner J. S., Widnell C. C. Interferon treatment inhibits pinocytosis. Mol Cell Biol. 1983 Aug;3(8):1533–1536. doi: 10.1128/mcb.3.8.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]


