Abstract
Background & Aims:
Measurements of α-fetoprotein (AFP) detect hepatocellular carcinoma (HCC) with low levels of sensitivity and specificity, and are therefore not recommended for use in liver cancer surveillance. However, AFP levels might accurately detect HCC in subgroups of patients. We performed a retrospective case–control study to identify features of patients with cirrhosis in whom levels of AFP correlated with HCC.
Methods:
We collected data from patients with cirrhosis, with HCC (n=452) or without (n=676), diagnosed at Parkland Hospital in Dallas, Texas from January 2005 through June 2012. We determined sensitivities and specificities with which different levels of AFP identified those with HCC; multivariate logistic regression was used to associate accurate identification of HCC with patient features (age, sex, race/ethnicity, alcohol intake, smoking, etiology of cirrhosis, presence of decompensation, and laboratory test results). We assessed overall accuracy of these factors in detecting HCC using receiver operator characteristic (ROC) curve analysis and the Delong method. We calculated levels of AFP that detect HCC with the highest levels of sensitivity and specificity in subgroups using ROC analysis.
Results:
The most common etiologies of cirrhosis hepatitis C virus (HCV) infection (60%) and alcohol induced (22%). Nearly 11% of patients were HIV-positive. Levels of AFP >20 ng/mL detected HCC with 70.1% sensitivity and 89.8% specificity. This AFP level identified patients with HCC with a c-statistic of 0.87 (95% confidence interval, 0.85–0.89); it was significantly more accurate in HCV-negative patients than HCV-positive patients (c-statistic 0.89 vs 0.83; P=.007). AFP levels ≥59 ng/mL most accurately detected HCC in patients with HCV-associated cirrhosis; levels of AFP ≥11 ng/mL accurately identified HCC in HCV-negative patients. Level of AFP identified early-stage HCC with a c-statistic of 0.62 (95% confidence interval, 0.58–0.66), and had a significantly higher level of accuracy for HIV-positive patients than HIV-negative patients (c-statistic 0.81 vs 0.59; P<.001).
Conclusion:
Based on retrospective analysis of data from patients with cirrhosis, with or without HCC, level of AFP most accurately detects HCC in patients without HCV infection. It detects HCC with a high level of accuracy in patients with cirrhosis and HIV infection.
Keywords: Biomarkers, liver disease, fibrosis, AIDS
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common cause of cancer and the third leading cause of cancer-related death worldwide(1). Within the United States and Europe, its incidence is rapidly increasing, largely driven by the current epidemic of hepatitis C virus (HCV) and non-alcoholic fatty liver disease (NAFLD) cases(2). Prognosis for patients with HCC depends on tumor stage at diagnosis, with curative options only available for patients diagnosed at an early stage.
Surveillance with ultrasound alone at six-month intervals is recommended in patients with cirrhosis to detect HCC at an early stage(3). However, ultrasound remains operator dependent, with a large gap between its efficacy and its effectiveness in clinical practice, creating a need for effective complementary biomarkers(4-7). Alpha fetoprotein (AFP), the best-studied serologic test, is attractive for surveillance, as it is relatively inexpensive and easily obtainable. However, the most recent guidelines from the American Association for the Study of Liver Diseases (AASLD) no longer recommend using AFP, citing poor sensitivity and specificity of AFP for early stage HCC. At a cut-off of 20ng/mL, the most commonly used cut-off in clinical practice, AFP has a sensitivity and specificity of approximately 60% and 80% for HCC, respectively(8).
However, most studies have assumed that AFP performs equally well in all patients, independent of liver disease etiology or severity. AFP has been shown to be elevated in several states of liver injury, including acute liver failure, suggesting decreased specificity in cases with high cell turnover(9, 10). Furthermore, the specificity of AFP may vary by patient characteristics, such as gender and race(6, 10-12). Many of the prior studies were limited by relatively small sample size, isolation to only patients with HCV or HBV infection, and the inclusion of patients without cirrhosis(10, 13, 14). However, the majority of HCC patients in the United States and Europe have underlying cirrhosis at the time of diagnosis(2, 15), and the inclusion of patients with milder degrees of liver disease, who carry a low risk of HCC, may have unfairly biased the results of prior studies against AFP. Therefore the primary aim of our study was to identify determinants for sensitivity, specificity, and overall accuracy of AFP in a cohort of patients with cirrhosis. A secondary aim of our study was to define new potential cut-offs for AFP in the subgroups of patients in whom accuracy varies.
METHODS
Study Population
We conducted a retrospective case-control study of cirrhotic patients with and without HCC at Parkland Memorial Health and Hospital System, the safety-net system for Dallas County. With eleven primary care clinics in low-income neighborhoods, Parkland cares for a large proportion of patients with cirrhosis as well as patients with HCC in Dallas County. Furthermore, Parkland Hospital is one of the few safety-net hospitals with an integrated electronic medical record for the hospital and clinics, including primary care clinics.
We included all patients diagnosed with HCC at Parkland Hospital between January 2005 and June 2012. As previously described, patients were identified by a combination of ICD-9 codes for HCC (155.0 or 155.2), a prospectively maintained list of patients seen in a multidisciplinary liver tumor clinic, and tumor conference presentation lists(16). Two authors (A.S. and A.Y.) adjudicated all HCC cases to confirm they met diagnostic criteria, based on AASLD guidelines. We excluded patients who did not have an AFP level prior to HCC diagnosis.
Our control population consisted of patients with cirrhosis who were seen at Parkland Hospital between January 2010 and July 2011. Patients were initially identified using a previously validated combination of ICD-9 codes(17). Patients were required to have at least one outpatient appointment during this time period to suggest that Parkland Hospital was their medical home. We excluded patients with any suspicious liver mass on imaging and those who did not have an AFP level during the study period (January 2010 – July 2011). Patients were required to have at least six months of follow-up to confirm the absence of HCC. This study was approved by the Institutional Review Board of UT Southwestern Medical Center.
Data Collection
Patient demographics, clinical history, laboratory data and imaging results were obtained through review of computerized and paper medical records. Two investigators (A.S. and A.Y.) independently extracted information regarding HCC patients using standardized forms, with discrepancies resolved through consensus. Similarly, two investigators (M.N. and P.K.) independently extracted information regarding non-HCC patients using standardized forms, with a third investigator (A.S.) available to resolve discrepancies. Age, gender, race/ethnicity, and lifetime alcohol and smoking history were recorded, with active alcohol abuse defined as drinking more than 40 grams/day. Data regarding liver disease included underlying etiology and presence of decompensation (ascites or encephalopathy). We classified patients according to etiology of liver disease, including HCV, hepatitis B virus (HBV), alcohol-related liver disease, NAFLD, and other. Laboratory data of interest included platelet count, creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin, albumin, international normalized ratio (INR), and AFP. We assessed the latest laboratory values between January 2010 and July 2011 in non-HCC patients and the laboratory values prior to diagnosis in those with HCC. Tumor characteristics were determined by imaging studies, which had all been interpreted by radiologists at our institution. Early stage HCC was defined using the Milan criteria (one tumor less than 5 cm or 2-3 tumors, each less than 3 cm in diameter, without vascular invasion or distant metastases).
Statistical Analysis
Demographics and clinical features were compared between patients with and without HCC using Fisher exact and Mann-Whitney rank-sum tests for categorical and continuous variables, respectively.
We determined the sensitivity, specificity, positive predictive value, and negative predictive value of AFP for the detection of HCC. We dichotomized AFP at a cut-off of 20ng/mL, as this is the most commonly reported and used cut-off in clinical practice. We assessed overall accuracy, indicating the degree of correct classification, by the c-statistic using receiver operator characteristic (ROC) curve analysis. A c-statistic ranges from 0-1, with 1 indicating perfect prediction and 0.5 indicating prediction by chance alone; values between 0.7 and 0.8 are generally considered acceptable(18).
We determined predictors of sensitivity and specificity using Fisher exact and Mann-Whitney rank-sum tests for categorical and continuous variables, respectively. We assessed the following potential independent variables: age, gender, race, ethnicity, body mass index (BMI), etiology of liver disease, presence of hepatic decompensation, HIV serostatus, platelet count, creatinine, albumin, AST level, bilirubin, INR, and tumor stage. Variables significant on univariate analysis were included in multivariate logistic regression analysis. After Bonferoni adjustment, p-values of 0.05 and 0.025 were considered significant for univariate and multivariate analyses, respectively. We used the Delong method to compare c-statistics between groups and identify predictors of overall accuracy. Finally, we determined new optimal cut-offs to maximize sensitivity and specificity in these subgroups using ROC curve analysis. All data analysis was conducted using Stata 11 (College Station, TX).
RESULTS
Patient Characteristics
Between January 2005 and June 2012, 457 patients with cirrhosis were diagnosed with HCC. We excluded five patients who did not have an AFP level prior to HCC diagnosis. Between January 2010 and July 2011, 914 patients with cirrhosis were seen in an outpatient setting at Parkland Hospital, of whom 238 patients were excluded for a lack of AFP level or insufficient follow-up duration.
The baseline characteristics of the remaining 1128 patients (452 HCC and 676 non-HCC) are shown in Table 1. The median age of patients was 55 years, and the majority of patients were male, with a higher proportion of males among HCC patients (78% vs. 66%, p<0.001). Our population was racially diverse, with 31% non-Hispanic Caucasians, 37% Hispanic Caucasians, and 27% African Americans. Non-HCC patients were significantly more likely to be non-Hispanic Caucasian than those with HCC (34% vs. 26%, p=0.01). The most common etiologies of cirrhosis were HCV (60%), alcohol-induced liver disease (22%), and NAFLD (10%). As expected, HCV cirrhosis was significantly more common among HCC patients than non-HCC patients (70% vs. 54%, p<0.001). Nearly 11% of patients with known HIV serostatus were HIV positive. Of the 80 HIV-positive patients, 59 (74%) had HCV co-infection and 14 (18%) had HBV co-infection.
Table 1.
Patient Characteristics
| Characteristic | HCC patients (n=452) |
Non-HCC patients (n=676) |
p-value |
|---|---|---|---|
| Age (years) | 56 (52 – 61) | 55 (49- 61) | 0.01 |
| Gender (% male) | 355 (78.4%) | 447 (66.2%) | < 0.001 |
| Race Caucasian Black Hispanic Other |
119 (26.4%) 160 (35.5%) 134 (29.7%) 38 (8.4%) |
231 (34.2%) 147 (21.8%) 278 (41.2%) 19 (2.8%) |
< 0.001 |
| BMI | 25.4 (22 – 29) | 28.7 (25 – 33) | < 0.001 |
| Etiology of Liver Disease Hepatitis C Hepatitis B Alcohol induced NASH Other |
314 (69.5%) 41 (9.1%) 56 (12.4%) 39 (8.6%) 2 (0.4%) |
362 (53.5%) 23 (3.4%) 192 (28.4%) 77 (11.4%) 22 (3.3%) |
< 0.001 |
| Presence of ascites | 211 (46.7%) | 260 (38.5%) | 0.007 |
| Presence of hepatic encephalopathy |
71 (15.7%) | 145 (21.5%) | 0.02 |
| HIV positive status** | 29 (9.0%) | 51 (11.9%) | 0.23 |
| Platelet count (×103/μL) | 129 (82 – 203) | 97 (66 – 138) | <0.001 |
| Creatinine (mg/dL) | 0.8 (0.7 – 1.0) | 0.8 (0.7 – 1.1) | 0.85 |
| Albumin (g/dL) | 3.0 (2.6 – 3.5) | 3.5 (2.9 – 3.9) | <0.001 |
| AST (U/L) | 110 (64 – 180) | 58 (39 – 92) | <0.001 |
| ALT (U/L) | 54 (35 – 83) | 40 (26 – 67) | <0.001 |
| Bilirubin (mg/dL) | 1.4 (0.8 – 2.8) | 1.1 (0.6 – 1.9) | <0.001 |
| INR | 1.2 (1.1 – 1.5) | 1.2 (1.1 – 1.4) | <0.001 |
| AFP level (ng/mL) | 198 (13 – 4114) | 4 (3 – 8) | <0.001 |
| Child Pugh Class Child Pugh A Child Pugh B |
168 (37.2%) 189 (41.8%) |
197 (29.1%) 403 (59.6%) |
<0.001 |
| Early Tumor Stage* (%) | 150 (33%) |
All values are expressed as median (interquartile range) unless otherwise specified. Percentages for categorical variables were calculated after accounting for missing data.
Early tumor stage was defined using Milan Criteria
HIV serostatus was available in 67% (n=751) of patients
AFP – alpha fetoprotein; ALT – alanine aminotransferase; AST – aspartate aminotransferase; BMI – Body mass index; HCC – hepatocellular carcinoma; HIV – human immunodeficiency virus; INR – international normalized ratio; NASH – nonalcoholic steatohepatitis
The majority (53%) of patients had Child-Pugh B cirrhosis, with another 32% having Child Pugh A cirrhosis. The median AFP level was 198ng/mL in the HCC patients and 4ng/mL in non-HCC patients. The HCC cohort was diverse with respect to tumor stage, with 150 (33%) patients having early stage tumors, as defined by Milan criteria.
Performance Characteristics of AFP
Table 2 shows the performance characteristics of AFP at a cut-off of 20 ng/mL. The sensitivity and specificity of AFP >20 ng/mL for the detection of HCC were 70.1% and 89.8%, respectively. AFP had high positive and negative predictive values of 82.2% and 81.5%, respectively, although the proportion of HCC patients in our study (40%) is substantially higher than that seen in usual clinical settings. The sensitivity of AFP, at a cut-off of 20ng/mL, for early stage HCC was significantly lower at 49.3%.
Table 2.
Performance Characteristics of AFP for detection of HCC at different cut-offs
| Sensitivity | Specificity | Percent Correctly Classified |
Positive Likelihood Ratio |
Negative Likelihood Ratio |
|
|---|---|---|---|---|---|
| 11 ng/mL | 78.1% | 81.8% | 80.3% | 4.3 | 0.27 |
| 20 ng/mL | 70.1% | 89.8% | 81.9% | 6.9 | 0.33 |
| 200 ng/mL | 50.0% | 99.4% | 79.6% | 84.4 | 0.50 |
| 400 ng/mL | 44.0% | 99.9% | 77.5 | 297 | 0.56 |
AFP – alpha fetoprotein; HCC – hepatocellular carcinoma
Predictors of specificity (i.e., proportion of patients with an AFP level < 20 ng/mL among those without HCC) included Black race, HCV etiology, and AST > 40 U/L on univariate analysis. On multivariate analysis, Black race (OR 0.47, 95% CI 0.27 – 0.81), HCV etiology (OR 0.18, 95%CI 0.08 – 0.41) and elevated AST levels (OR 0.06, 95%CI 0.01 – 0.42) were associated with lower specificity. Whereas only 7% of Caucasians and 8% of Hispanic patients had elevated AFP levels in the absence of HCC, false positive AFP results were found in 20% of African Americans. AFP >20ng/mL achieved a very high specificity in the other two subgroups, with a specificity of 98% among non-HCV patients and 99% among patients with normal AST levels.
Predictors of sensitivity (i.e., proportion of patients with an AFP level >20 ng/mL among those with HCC) on univariate analysis included early tumor stage, HIV status, viral etiology, AST > 40 U/L, platelet count > 100,000/μL, and bilirubin > 2mg/dL. On multivariate analysis, HIV status (OR 4.50, 95%CI 1.44 – 14.1) was significantly associated with higher sensitivity, and early tumor stage (OR 0.25, 95%CI 0.15 – 0.43) was associated with lower sensitivity. AFP had a sensitivity of 86% in HIV positive patients, compared to 67% in HIV negative patients.
Subgroup analyses, excluding HIV-positive patients, were performed for predictors of sensitivity and specificity. Predictors of specificity on multivariate analysis continued to include Black race (OR 0.43, 95%CI 0.24-0.77), HCV etiology (OR 0.20, 95%CI 0.09-0.45), and elevated AST levels (OR 0.07, 95%CI 0.01-0.50). Predictors of sensitivity were similar except viral etiology was no longer significant on univariate analysis (p=0.11). On multivariate analysis, early tumor stage (OR 0.23, 95%CI 0.15-0.37) and AST >40 U/L (OR 2.2, 95%CI 1.03-4.52) were significant predictors of sensitivity.
Overall Diagnostic Accuracy of AFP
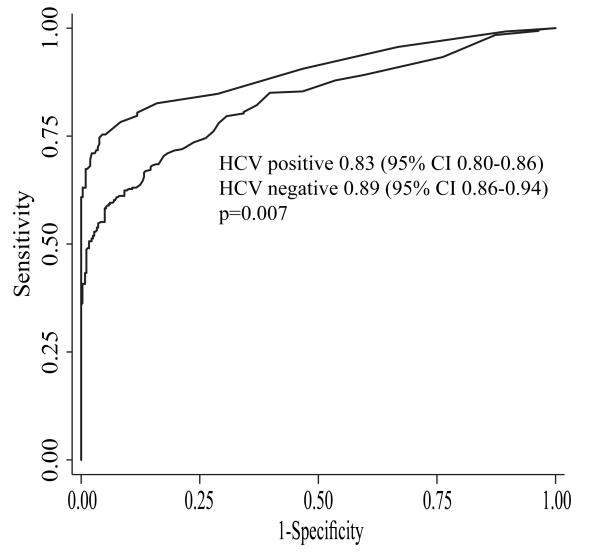
AFP had a c-statistic of 0.87 (95%CI 0.85 – 0.89) for the detection of HCC at any stage. The only predictor for overall accuracy of AFP on univariate analysis was HCV etiology. Although AFP had a c-statistic over 0.80 in both subgroups, it had significantly better overall accuracy in non-HCV patients (Figure 1). AFP had a c-statistic of 0.89 (95%CI 0.86-0.94) in non-HCV patients, which was significantly better than the c-statistic of 0.83 (95%CI 0.80-0.86) seen in those with HCV infection (p=0.007). AFP, at a cut-off of 20ng/mL, correctly classified 403 (89.4%) of 451 patients without HCV infection, compared to only 520 (76.9%) of 676 HCV-positive patients. In the subset of patients with NAFLD, AFP had a c-statistic of 0.87 (95%CI 0.77-0.94), with a sensitivity of 89.7% and specificity 85.1% at a cut-off of 20 ng/mL. The association between HCV infection and AFP accuracy persisted on subgroup analysis when excluding HIV-positive patients (c-statistic 0.89 vs. 0.83, p=0.02).
Figure 1.
Accuracy of AFP for Detection of HCC by Hepatitis C Viral Status
AFP had a c-statistic of 0.62 (95%CI 0.58 – 0.66) for the detection of early stage HCC. The only predictor for the accuracy of AFP to detect early stage HCC was HIV status, with significantly higher accuracy among HIV positive patients. AFP achieved a c-statistic of 0.81 (95%CI 0.70 – 0.91) for early stage HCC in HIV-positive patients, which was significantly higher than the c-statistic of 0.59 (95%CI 0.53 – 0.64) seen in HIV-negative patients (p<0.001). AFP, at a cut-off of 20ng/mL, was able to correctly classify 56 (84.8%) of 66 HIV positive patients, compared to 384 (81.0%) of 474 patients without HIV infection. On sensitivity analysis only including patients with viral hepatitis (HCV or HBV), HIV continued to be a predictor of AFP accuracy to detect early stage HCC. AFP achieved a c-statistic of 0.85 (95%CI 0.74 – 0.95) for early stage HCC in HIV-positive patients, which was significantly higher than the c-statistic of 0.65 (95%CI 0.59 – 0.71) seen in HIV-negative patients (p=0.001).
Finally, we derived new potential cut-off values for AFP in HCV-positive and HCV-negative patients using ROC curve analysis to optimize sensitivity and specificity for the detection of HCC (Table 4). In HCV positive patients, a cut-of 59ng/mL maximized the proportion of patients correctly classified, with a sensitivity of 59.6% and specificity of 93.9%, respectively. In HCV negative patients, a cut-off of 11ng/mL maximized the proportion of patients correctly classified, with a sensitivity of 74.6% and specificity of 96.2%, respectively.
Table 4.
Performance Characteristics of AFP for Detection of HCC by HCV Status
| Sensitivity | Specificity | Percent Correctly classified |
Positive Likelihood Ratio |
Negative Likelihood Ratio |
|
|---|---|---|---|---|---|
| HCV-positive patients | |||||
| 20 ng/mL | 70.4% | 82.6% | 76.9% | 4.0 | 0.36 |
| 59 ng/mL* | 59.6% | 93.9% | 78.0% | 9.8 | 0.43 |
| 200 ng/mL | 45.9% | 98.9% | 74.3% | 41.5 | 0.55 |
| HCV-negative patients | |||||
| 11 ng/mL* | 74.6% | 96.2% | 89.6% | 19.5 | 0.26 |
| 20 ng/mL | 69.6% | 98.0% | 89.4% | 36.3 | 0.31 |
| 200 ng/mL | 60.1% | 100% | 87.6% | 190 | 0.41 |
Newly derived optimal cut-off to maximize accuracy
AFP – alpha fetoprotein; HCV – hepatitis C virus
DISCUSSION
Although AFP was previously recommended as an adjunct surveillance test to ultrasound, the most recent AASLD guidelines no longer recommend using AFP, citing poor sensitivity and specificity(3). However, we found that AFP, at a cut-off of 20ng/mL, had acceptable performance characteristics in our population, with a sensitivity of 70.1% and specificity of 89.6%. AFP had high overall accuracy, with a c-statistic of 0.87 (95%CI 0.85 – 0.89). Most importantly, we found that patient characteristics influenced the performance of AFP and could be used to define subgroups in whom it performed particularly well. With further refinement, the presence or absence of certain patient characteristics may facilitate tailoring of surveillance so biomarkers are used in a subset of patients.
Our study suggests that AFP has significantly higher accuracy in non-HCV patients than those with HCV infection. This is particularly important given the rapidly rising incidence of NASH-related HCC in the United States and Europe(19, 20). Although HCV infection is the most common risk factor for HCC currently, the incidence of HCV is declining and the prevalence has likely peaked(1). With the growing epidemic of obesity and diabetes, NASH is anticipated to be the major etiology for HCC in the future. In addition to this shift in epidemiology, ultrasound may be less sensitive for the detection of HCC in obese patients, creating a need for effective biomarkers that can be used in combination(4, 7). Among patients with NAFLD in our study, AFP had a sensitivity and specificity above 80% and strong accuracy (c-statistic 0.81).
Implementing different AFP cut-offs for HCV-positive and HCV-negative patients could, in part, mitigate any difference in AFP accuracy. Patients without HCV infection appear to have less non-tumoral secretion of AFP, so even low-level AFP elevations in these patients should raise suspicion for the development of HCC. In contrast, patients with HCV infection often have elevated AFP levels in the absence of HCC so those with low-level elevations may simply be able to be closely monitored in most cases(10). Although we found cut-offs of 11ng/mL and 59ng/mL for HCV-negative and HCV-positive patients respectively, further studies are necessary to confirm our results and validate optimal cut-offs.
In addition to HCV infection, we found several other characteristics that influenced the sensitivity and/or specificity of AFP, including AST level, Black race, and HIV status. The association between AST levels and AFP specificity of is not surprising, as AFP can be secreted from non-tumoral cells in states of high cell turnover. Similar findings had been previously reported in an ancillary study from the HALT-C Trial, in which increased serum AST and ALT were associated with elevated AFP levels(10). Sterling and colleagues also reported an association between thrombocytopenia and elevated AFP levels(21); while present on univariate analysis in our study, this association was not significant on multivariate analysis. The etiology and clinical significance of the association between race and AFP specificity is less clear. Data from HALT-C similarly suggested racial differences, with African American patients with HCV being more than 2-times more likely to have elevations in AFP than others(10, 21). Nguyen and colleagues found that AFP had a lower sensitivity for HCC among African Americans than other races, although this was not replicated in our study(11). The higher rates of AFP among African Americans non-HCC patients correlate with epidemiologic data, which demonstrates higher incidence rates in Black patients compared to Caucasian patients.
Of interest, we found AFP had significantly higher sensitivity for HCC as well as significantly higher accuracy for early stage HCC in HIV-positive patients than HIV-negative patients. Prior studies have suggested that HIV status can influence the performance of non-invasive markers of fibrosis, such as the AST to platelet ratio index (APRI)(22). Although prior studies have found HIV-positive patients with HCC have higher AFP levels than HIV-negative HCC patients(23, 24), we believe this is the first to identify potential differences in the performance of AFP according to HIV serostatus. If confirmed in subsequent studies, this may be important given the growing burden of HCC in HIV-infected patients.
HBV infection is the most common underlying etiology for HCC worldwide but was only present in 9% of HCC patients and 3% of non-HCC patients in our study. In a prior large cross-sectional study of risk factors among HCC patients, 47% of patients were reported as having HCV infection and 15% HBV infection(25). We believe that HBV infection was less common in our population given our focus on patients with underlying cirrhosis. Patients with non-cirrhotic HBV, with or without HCC, were excluded from our study. We believe our population should be representative of the typical cirrhotic HCC and non-HCC populations seen in academic centers in the United States.
Our study has several limitations. Given its retrospective nature, our study was limited by possible unmeasured confounders and missing data. We unfortunately could not compare the performance of AFP to other biomarkers, such as AFP-L3 or DCP. Similarly, HIV status was not ascertained on all patients, particularly those without underlying viral hepatitis. Furthermore, our study is prone to verification bias, as some patients with cirrhosis may have had unrecognized HCC. We attempted to minimize this bias by excluding patients who did not have at least six months of follow-up after inclusion. Although it is possible that some patients were diagnosed with HCC at outside institutions, we believe this is unlikely given that Parkland Hospital, as the safety-net health system for Dallas County, is the only option for indigent patients. Another limitation of our study is that we only assessed the accuracy of AFP at one point in time. It is likely that longitudinal assessment of AFP levels, including change over time, may alter its performance characteristics(26, 27). Finally, our study is not generalizable to groups not represented in this study, including Asian patients. Overall, we believe that our study’s limitations are outweighed by its notable strengths, including its well-characterized cohort who all had underlying cirrhosis, its racially diverse population including both African American and Hispanic patients, and its relatively large sample size.
In conclusion, we found several patient characteristics influence the performance of AFP for the detection of HCC in patients with cirrhosis. The higher accuracy of AFP for detecting HCC among patients without HCV infection is particularly important given the rising incidence of NASH in the United States and Europe. A lower AFP cut-off should be used in this subgroup of patients to maximize its sensitivity and specificity. Further studies, specifically focusing on patients with NASH, are necessary to confirm our findings and further define the benefit of AFP when used in combination with ultrasound.
Table 3.
Predictors of AFP Sensitivity and Specificity for Detection of HCC
| Univariate Analysis | Multivariate Analysis | Values | |||
|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | ||
| SENSITIVITY | |||||
| HIV status | 3.11 | 1.05 – 9.18 | 4.50 | 1.44 – 14.1 | 86% vs. 67% |
| Early stage tumor* | 0.25 | 0.16 – 0.38 | 0.25 | 0.15 – 0.43 | 49% vs. 80% |
| Viral etiology | 1.53 | 0.95 – 2.44 | 1.19 | 0.63 – 2.23 | 72% vs. 62% |
| AST > 40 U/L | 2.50 | 1.28 – 4.88 | 1.93 | 0.81 – 4.58 | 71% vs. 50% |
| Platelets >100,000/μL | 1.64 | 1.08 – 2.49 | 1.25 | 0.74 – 2.13 | 73% vs. 62% |
| Bilirubin > 2 mg/dL | 1.67 | 1.07 – 2.61 | 1.23 | 0.70 – 2.16 | 77% vs. 66% |
| SPECIFICITY | |||||
| HCV etiology | 0.11 | 0.05 – 0.25 | 0.18 | 0.08 – 0.41 | 83% vs. 98% |
| AST > 40 U/L | 0.24 | 0.13 – 0.43 | 0.06 | 0.01 – 0.42 | 87% vs. 99% |
| Black race | 0.31 | 0.18 – 0.52 | 0.47 | 0.27 – 0.81 | 80% vs. 93% |
Early stage HCC was defined using Milan Criteria (one tumor < 5 cm in diameter or 2-3 tumors, each less than 3 cm in diameter, without vascular invasion or distant metastases)
AFP – alpha fetoprotein; AST – aspartate aminotransferase; CI – confidence interval; HCC – hepatocellular carcinoma; HCV – hepatitis C virus; HIV – human immunodeficiency virus; OR odds ratio
Abbreviations
- AASLD
American Association for the Study of Liver Diseases
- AFP
alpha fetoprotein
- ALT
alanine aminotransferase
- APRI
AST to platelet ratio index
- AST
aspartate aminotransferase
- BMI
body mass index
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- INR
international normalized ratio
- NAFLD
nonalcoholic fatty liver disease
- ROC
receiver operator characteristic
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures: This work was conducted with support from UT-STAR, NIH/NCATS Grant Number KL2 TR000453, NIH/NCATS Grant UL1-TR000451, and the ACG Junior Faculty Development Award awarded to Dr. Singal. Dr. Waljee’s research is funded by a VA HSR&D CDA-2 Career Development Award 1IK2HX000775. The content is solely the responsibility of the authors and does not necessarily represent the official views of UT-STAR, the University of Texas Southwestern Medical Center and its affiliated academic and health care centers, the National Center for Advancing Translational Sciences, the Veterans Affairs, or the National Institutes of Health.
Conflicts of Interest: None of the authors have any conflicts of interest to disclose
Author Contributions
Purva Gopal involved in study concept and design, interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content.
Adam Yopp involved in study concept and design, interpretation of data, and critical revision of the manuscript for important intellectual content.
Akbar Waljee involved in study concept and design, interpretation of data, and critical revision of the manuscript for important intellectual content.
Jason Chiang involved in acquisition of data and critical revision of the manuscript for important intellectual content.
Mahendra Nehra involved in acquisition of data and critical revision of the manuscript for important intellectual content.
Pragathi Kandunoori involved in acquisition of data and critical revision of the manuscript for important intellectual content.
Amit Singal involved in study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis, and study supervision.
REFERENCES
- 1.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273. doi: 10.1053/j.gastro.2011.12.061. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Serag HB, Lau M, Eschbach K, et al. Epidemiology of hepatocellular carcinoma in Hispanics in the United States. Arch Intern Med. 2007;167:1983–9. doi: 10.1001/archinte.167.18.1983. [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M. Management of Hepatocellular Carcinoma: An Update. Hepatology. 2010;53:1–35. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finberg HJ. Whither (wither?) the ultrasound specialist? J Ultrasound Med. 2004;23:1543–7. doi: 10.7863/jum.2004.23.12.1543. [DOI] [PubMed] [Google Scholar]
- 5.Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30:37–47. doi: 10.1111/j.1365-2036.2009.04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singal AG, Conjeevaram HS, Volk ML, et al. Effectiveness of Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis. Cancer Epidemiol Biomarkers Prev. 2012;21:793–9. doi: 10.1158/1055-9965.EPI-11-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singal AG, Nehra M, Adams-Huet B, et al. Detection of hepatocellular carcinoma at advanced stages among patients in the HALT-C trial: where did surveillance fail? Am J Gastroenterol. 2013;108:425–32. doi: 10.1038/ajg.2012.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta S, Bent S, Kohlwes J. Test characteristics of alpha-fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C. A systematic review and critical analysis. Ann Intern Med. 2003;139:46–50. doi: 10.7326/0003-4819-139-1-200307010-00012. [DOI] [PubMed] [Google Scholar]
- 9.Takikawa Y, Suzuki K. Is AFP a new reliable marker of liver regeneration in acute hepatic failure? J Gastroenterol. 2002;37:681–2. doi: 10.1007/s005350200111. [DOI] [PubMed] [Google Scholar]
- 10.Di Bisceglie AM, Sterling RK, Chung RT, et al. Serum alpha-fetoprotein levels in patients with advanced hepatitis C: results from the HALT-C Trial. J Hepatol. 2005;43:434–41. doi: 10.1016/j.jhep.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen MH, Garcia RT, Simpson PW, et al. Racial differences in effectiveness of alpha-fetoprotein for diagnosis of hepatocellular carcinoma in hepatitis C virus cirrhosis. Hepatology. 2002;36:410–7. doi: 10.1053/jhep.2002.34744. [DOI] [PubMed] [Google Scholar]
- 12.Volk ML, Hernandez JC, Su GL, et al. Risk factors for hepatocellular carcinoma may impair the performance of biomarkers: a comparison of AFP, DCP, and AFP-L3. Cancer Biomark. 2007;3:79–87. doi: 10.3233/cbm-2007-3202. [DOI] [PubMed] [Google Scholar]
- 13.Cedrone A, Covino M, Caturelli E, et al. Utility of alpha-fetoprotein (AFP) in the screening of patients with virus-related chronic liver disease: does different viral etiology influence AFP levels in HCC? A study in 350 western patients. Hepatogastroenterology. 2000;47:1654–8. [PubMed] [Google Scholar]
- 14.Trevisani F, D'Intino PE, Morselli-Labate AM, et al. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol. 2001;34:570–5. doi: 10.1016/s0168-8278(00)00053-2. [DOI] [PubMed] [Google Scholar]
- 15.Yang JD, Kim WR, Coelho R, et al. Cirrhosis is present in most patients with hepatitis B and hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9:64–70. doi: 10.1016/j.cgh.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singal AG, Yopp AC, Gupta S, et al. Failure Rates in the Hepatocellular Carcinoma Surveillance Process. Cancer Prev Res (Phila) 2012;5:1124–1130. doi: 10.1158/1940-6207.CAPR-12-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nehra MS, Ma Y, Clark C, et al. Use of administrative claims data for identifying patients with cirrhosis. J Clin Gastroenterol. 2013;47:e50–4. doi: 10.1097/MCG.0b013e3182688d2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mossman D, Somoza E. ROC curves, test accuracy, and the description of diagnostic tests. J Neuropsychiatry Clin Neurosci. 1991;3:330–3. doi: 10.1176/jnp.3.3.330. [DOI] [PubMed] [Google Scholar]
- 19.Bellentani S, Scaglioni F, Marino M, et al. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28:155–61. doi: 10.1159/000282080. [DOI] [PubMed] [Google Scholar]
- 20.Starley BQ, Calcagno CJ, Harrison SA Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–32. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 21.Sterling RK, Wright EC, Morgan TR, et al. Frequency of elevated hepatocellular carcinoma (HCC) biomarkers in patients with advanced hepatitis C. Am J Gastroenterol. 2012;107:64–74. doi: 10.1038/ajg.2011.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singal AG, Thomassen LV, Gretch DR, et al. Use of the AST to platelet ratio index in HCV/HIV co-infected patients. Aliment Pharmacol Ther. 2011;33:566–77. doi: 10.1111/j.1365-2036.2010.04560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brau N, Fox RK, Xiao P, et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: a U.S.-Canadian multicenter study. J Hepatol. 2007;47:527–37. doi: 10.1016/j.jhep.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Yopp AC, Subramanian M, Jain MK, et al. Presentation, treatment, and clinical outcomes of patients with hepatocellular carcinoma, with and without human immunodeficiency virus infection. Clin Gastroenterol Hepatol. 2012;10:1284–90. doi: 10.1016/j.cgh.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Bisceglie AM, Lyra AC, Schwartz M, et al. Hepatitis C-related hepatocellular carcinoma in the United States: influence of ethnic status. Am J Gastroenterol. 2003;98:2060–3. doi: 10.1111/j.1572-0241.2003.t01-1-07641.x. [DOI] [PubMed] [Google Scholar]
- 26.Lee E, Edward S, Singal AG, et al. Improving screening for hepatocellular carcinoma by incorporating data on levels of alpha-fetoprotein, over time. Clin Gastroenterol Hepatol. 2013;11:437–40. doi: 10.1016/j.cgh.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 27.Singal AG, Waljee A, Mukherjee A, et al. Machine Learning Algorithms Outperform Conventional Regression Models in Identifying Risk Factors for Hepatocellular Carcinoma in Patients with Cirrhosis. Gastroenterology. 2012;142:S984. doi: 10.1038/ajg.2013.332. [DOI] [PMC free article] [PubMed] [Google Scholar]