Abstract
PECAM-1 (also known as CD31) is a cellular adhesion and signaling receptor comprised of six extracellular immunoglobulin (Ig) - like homology domains, a short transmembrane domain, and a 118 amino acid cytoplasmic domain that becomes serine and tyrosine phosphorylated upon cellular activation. PECAM-1 expression is restricted to blood and vascular cells. In circulating platelets and leukocytes, PECAM-1 functions largely as an inhibitory receptor that, via regulated sequential phosphorylation of its cytoplasmic domain, limits cellular activation responses. PECAM-1 is also highly expressed at endothelial cell intercellular junctions, where it functions as a mechanosensor, as a regulator of leukocyte trafficking, and in the maintenance of endothelial cell junctional integrity. In this review we will describe (1) the functional domains of PECAM-1 and how they contribute to its barrier-enhancing properties, (2) how the physical properties of PECAM-1 influence its subcellular localization and its ability to influence endothelial cell barrier function, (3) various stimuli that initiate PECAM-1 signaling and/or function at the endothelial junction, and (4) cross-talk of PECAM-1 with other junctional molecules, which can influence endothelial cell function.
Keywords: PECAM-1, CD31, vascular permeability, adhesion molecules
The vascular barrier
The vascular barrier is vitally important to cell and tissue homeostasis and it lies at the nexus of inflammatory responses. Breach of vascular integrity results in the accumulation of plasma, proteins and cells into the interstitial space, and is one of the cardinal signs of inflammation (Kumar et al. 2004). Tight regulation of the vascular permeability barrier is required to hold both acute and chronic inflammatory disease in check, and failure to restore barrier function in a timely manner can result in a catastrophic loss of vascular volume, as in septic shock (Cohen 2002), or contribute to the development of chronic inflammatory diseases like atherosclerosis (Vandenbroucke et al. 2008;Sima et al. 2009).
Endothelial cells constitute the inner lining of the blood vessel and serve as the gateway from the vasculature into sites of inflammation. In non-inflamed tissues, endothelial cells help to maintain and regulate blood flow, preserve the vascular barrier, and quiesce the activation and extravasation of leukocytes (Pober and Sessa 2007). In response to inflammatory mediators such as histamine, thrombin, and cytokines, endothelial cells retract from each other and intercellular junctions break down, allowing the extravasation of fluid, inflammatory cells, and proteins into the extravascular space (Pober and Sessa 2007). Fortunately, the increase in permeability is reversible, as the recovery of barrier function transpires after opened intercellular junctions are re-annealed and/or strengthening of adhesion between neighboring cells and the extracellular matrix (ECM) occurs (Mehta and Malik 2006). Consequently, molecules or proteins that hasten these processes or that strengthen adhesive bonds between cells or the ECM have the potential to prevent lethal increases in permeability that can lead to shock and death.
The endothelial vascular barrier is regulated by the coordinated opening and closing of intercellular junctions, which relies on a complex interplay of junctional adhesion components, cytoskeletal rearrangements, and cellular adhesive and counter-adhesive forces (Mehta and Malik 2006). The endothelial cell junction is made up of a variety of adhesion molecules and specialized junctional regions known as tight junctions and adherens junctions (Dejana 2004). Most adhesion molecules within endothelial cell-cell junctions interact in a homophilic manner with adhesion molecules on neighboring cells (Dejana 2004). Tight junctions, also known as zonula occludens, help regulate both paracellular permeability (barrier function) and cell polarity (fence function) (Bazzoni and Dejana 2004). Vessels that are less permeable to fluid (i.e. vessels in brain) have well-organized tight junctions, whereas leakier vessels (i.e. post-capillary venules) have poorly organized tight junctions (Bazzoni and Dejana 2004). Tight junction components include claudins, occludins, junctional adhesion molecules (JAM), and endothelial cell selective adhesion molecule (ESAM) (Dejana 2004). Adherens junctions, on the other hand, consist of vascular endothelial (VE)-cadherin and a variety of associated proteins, namely catenins and plakoglobin, which bind to its cytoplasmic tail (Dejana et al. 2008). The catenin proteins and plakoglobin help anchor VE-cadherin to the cytoskeleton, and these interactions are critical for full control of endothelial permeability and the stabilization of cell-cell junctions (Dejana et al. 2008). Outside of the specialized junctional components, other adhesion molecules are expressed at endothelial cell-cell junctions, the best known of which are S-endo-1 and PECAM-1 (Dejana 2004), the latter of whose biological properties will be discussed at length later in this review. As the assembly of adherens junctions was shown to be essential for the proper assembly of tight junctions (Taddei et al. 2008), and PECAM-1 has been reported to modulate the localization of VE-cadherin and β-catenin (Park et al. 2010;Biswas et al. 2006), it is likely that the various junctional components are intimately involved in the regulation of each other during junction formation and the coordination of junctional opening and closing.
The biology of PECAM-1
PECAM-1, depicted schematically in Figure 1, is a member of the Ig-superfamily of cell adhesion molecules and is a type I transmembrane glycoprotein that consists of six extracellular Ig-like homology domains, a 19-residue transmembrane domain, and a 118 residue cytoplasmic tail (Newman and Newman 2003). PECAM-1 is expressed on most non-erythroid cells of the hematopoietic lineage including platelets, monocytes, neutrophils, human T cell, and human and mouse B cell subsets (Newman and Newman 2003;Newman 1999;Newman 1997). PECAM-1 is also highly expressed on endothelial cells, where it is a major constituent of the endothelial cell intercellular junction in confluent vascular beds (Newman 1999;Newman 1997). Since its cloning in 1990 by three different groups (Newman et al. 1990;Simmons et al. 1990;Stockinger et al. 1990), there have been a plethora of studies that together provide mechanistic insights into how PECAM-1 regulates vascular barrier function. In this review we will describe (1) the functional domains of PECAM-1 and how they contribute to its barrier-enhancing properties, (2) how the physical properties of PECAM-1 influence its subcellular localization and its ability to influence endothelial cell barrier function, (3) various stimuli that initiate PECAM-1 signaling and/or function at the endothelial junction, and (4) cross-talk of PECAM-1 with other junctional molecules, which can influence endothelial cell function.
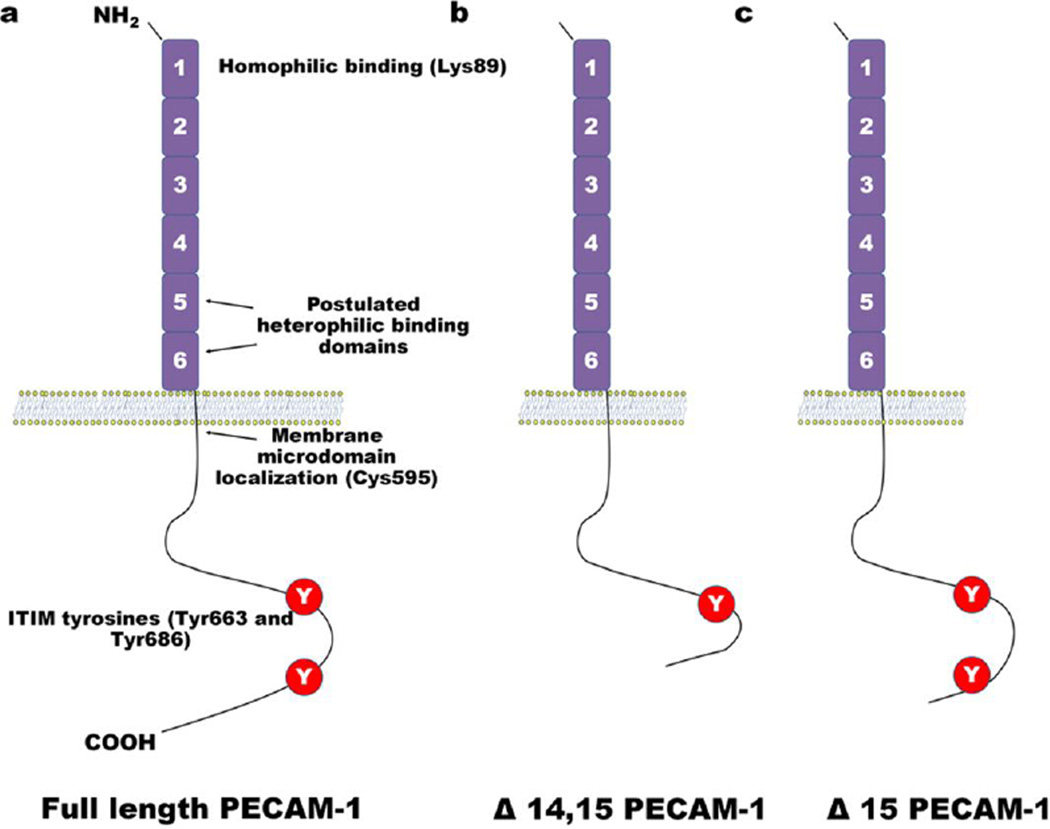
Fig. 1. Schematic of PECAM-1.
(a) Full-length PECAM-1 contains six Ig-like homology domains, a 19-residue transmembrane domain, and a 118 residue cytoplasmic tail. Residues important for homophilic binding include Lys89 in Ig-domain 1, residues important for modulating heterophilic binding interactions are located in Ig-domain 6. Post-translational palmitoylation of Cys595 can target PECAM-1 to membrane microdomains. Tyr663 and Tyr686 in exons 13 and 14 of the cytosolic domain comprise ITIMs that when phosphorylated create docking sites for cytosolic signaling molecules. (b) Δ14, 15 PECAM-1 does not contain exons 14 and 15, which removes Tyr686. This isoform of PECAM-1 no longer contains a functional ITIM. (c) Δ15 PECAM-1 does not contain exon 15, but still retains both tyrosine residues that make up the ITIM.
The first indication that PECAM-1 possesses adhesive activity came from a study in which PECAM-1 was observed to become strongly localized to cell-cell borders when adjacently transfected cells contact one another (Albelda et al. 1991). These findings led to the hypothesis that PECAM-1/PECAM-1 homophilic interactions are responsible for concentrating this molecule at endothelial cell intercellular junctions – a concept that was later proven in a sophisticated series of investigations employing chimeric proteins comprised of various mutant forms of the PECAM-1 extracellular domain fused to the Fc region of IgG (Sun et al. 1996b;Sun et al. 1996a;Newton et al. 1997). While collectively these studies determined that 4–5 amino acid residues on either face of Ig-homology domain 1 are required for PECAM-1/PECAM-1 homophilic interactions, how they interact to form stable, high-affinity PECAM-1/PECAM-1 adhesions is not yet known. Residues within IgD2 also appear to play a supporting role; however, whether this domain forms part of the homophilic binding interface, versus simply positioning IgD1 in such a way as to maximize its efficiency in forming homophilic interactions is also not known. Finally, PECAM-1 is heavily glycosylated, with nine consensus N-linked glycosylation sites within its extracellular domain (Newman et al. 1990;Newton et al. 1999), and Kitazume et al. recently reported that α2, 6 sialic residues may contribute importantly to the homophilic binding ability of endothelial PECAM-1(Kitazume et al. 2010). How, where and whether sialic acid residues contribute to the homophilic binding interface remains to be critically explored.
In addition to mediating homophilic PECAM-1/PECAM-1 interactions (Sun et al. 1996b;Sun et al. 1996a;Newton et al. 1997), a number of heterophilic binding partners for PECAM-1 have been reported, including glycosaminoglycans (GAG) (Delisser et al. 1993;Sun et al. 1998), the integrin αVβ3 (Piali et al. 1995;Buckley et al. 1996;Sun et al. 1996b), and CD38 on lymphocytes (Deaglio et al. 1998). None of these, however, have been either mapped to specific locations on PECAM-1 or shown to function as counter-receptors for PECAM-1 under physiological conditions. To date, the only heterophilic binding partner for PECAM-1 that has thus far been shown to be physiologically relevant is the neutrophil-specific CD177/PR3 complex of neutrophils (Kuckleburg and Newman 2013), which interacts with PECAM-1 IgD6 (Sachs et al. 2007). Such interactions, however, have to date not been shown to have any effect on endothelial barrier function, though PECAM-1 has been speculated to have a role in localizing the NB1/PR3 complex to endothelial cells, where PR3 can act, via protease-activated receptor 2 (PAR2) to reseal the endothelial cell junction following neutrophil transendothelial migration (Kuckleburg and Newman 2013)
The cytoplasmic domain of PECAM-1 (Figure 1) contains residues that serve as potential sites for palmitoylation, phosphorylation, and the docking of cytosolic signaling molecules (Newman and Newman 2003). When phosphorylated sequentially, first by a Src-family kinase (Ming et al. 2011;Paddock et al. 2011), and then by Csk (Tourdot et al. 2013), the two Immunoreceptor Tyrosine-based Inhibitory Motifs (ITIM) that encompass Tyr663 and Tyr686 of human PECAM-1 recruit Src homology 2 (SH2) domain-containing proteins, the best characterized of which is SH2 domain-containing protein tyrosine phosphatase (SHP)-2 (Jackson et al. 1997b;Newman and Newman 2003).
Mechanistic insights into PECAM-1-regulated barrier function
Due to its localization and expression on vascular endothelial cells and leukocytes that traverse the endothelial cell-cell junction during inflammatory processes, and its ability to modulate a variety of intracellular signaling processes, PECAM-1 is primed to serve a vital role in the regulation of the vascular barrier. Indeed, much recent work has implicated PECAM-1 in an array of biological processes for which the vascular barrier plays a central role. More specifically, PECAM-1 plays vital roles in leukocyte emigration (Muller et al. 1993;Thompson et al. 2001;Schenkel et al. 2004;Wang et al. 2005;Woodfin et al. 2009;Bixel et al. 2010), angiogenesis (Delisser et al. 1997;Zhou et al. 1999;Cao et al. 2002), and shear stress-induced atherosclerotic lesion development (Goel et al. 2008;Harry et al. 2008;Harrison et al. 2013); all physiologic processes that rely on junctional integrity and/or signaling from junctional components. Moreover, PECAM-1 has been shown to be important for the restoration of vascular integrity following barrier breach with histamine (Biswas et al. 2006), in the brain microvasculature of mice suffering from experimental autoimmune encephalomyelitis (Graesser et al. 2002), and during LPS-induced endotoxemia where the blood vessels of PECAM-1-deficient mice exhibit increased permeability and mice have an exaggerated loss of blood volume (Carrithers et al. 2005;Maas et al. 2005). In angiogenesis, PECAM-1 has been shown to play a prominent role in endothelial cell migration, junctional development, capillary morphogenesis, and the maturation of cell-matrix interactions (Delisser et al. 1997;Cao et al. 2002;RayChaudhury et al. 2001;Kondo et al. 2007;Wu and Sheibani 2003). PECAM-1 has additionally been demonstrated to be a “mechano-responsive” molecule that enables endothelial cells to respond to fluid shear stress, which modulates inflammatory signaling pathways, cytoskeletal architecture, and cell-matrix interactions (Tzima et al. 2005;Collins et al. 2012;Feaver et al. 2010). Table 1 summarizes the prominent role that PECAM-1 plays in the aforementioned physiologic processes. The rest of this review will help to elucidate the various mechanisms through which PECAM-1 regulates the vascular barrier during these biological processes.
Table 1.
| Process | Finding | Reference |
|---|---|---|
| Leukocyte diapedesis | PECAM-1 helps leukocytes transmigrate through the cell-cell junction and basement membrane | (Thompson et al. 2001;Wang et al. 2005;Woodfin et al. 2009;Duncan et al. 1999;Bixel et al. 2010) |
| ICAM-1-activated Src and eNOS signaling sequentially induce PECAM-1-mediated PMN transendothelial migration through Tyr686 phosphorylation and increased EC PECAM-1 surface expression | (Liu et al. 2012) | |
| PECAM-1 is part of the LBRC, a surface connected membrane compartment, that promotes leukocyte transmigration | (Mamdouh et al. 2003;Mamdouh et al. 2008;Mamdouh et al. 2009;Dasgupta et al. 2009;Sullivan et al. 2013) | |
| Monocyte diapedesis alters endothelial junctional organization to a more monocyte-permeable state (increased PECAM-1 and decreased VE-cadherin expression), which augments transmigratory activity | (Hashimoto et al. 2011) | |
| Mechanotransduction and atherogenesis | PECAM-1 transmits mechanical force in a mechanosensory complex with VE-cadherin and VEGFR2 to confer responsiveness to flow in endothelial cells and activate pro-inflammatory signaling pathways in response to disturbed flow | (Tzima et al. 2005) |
| PECAM-1 and Gαq11 are part of a sensory complex at EC junctions that respond to rapid changes in fluid shear stress | (Otte et al. 2009) | |
| Localized tensional forces on PECAM-1 result in activation of PI3K, cell-wide activation of integrins and the small GTPase RhoA, which facilitates changes in cytoskeletal architecture and focal adhesions | (Collins et al. 2012) | |
| PECAM-1 expression is correlated with more plaques in atherosusceptible regions of the aorta | (Harry et al. 2008;Stevens et al. 2008) | |
| Expression of PECAM-1 is correlated with decreased atherosclerotic lesion area in the total aorta with preferential protection in the aortic sinus, descending aorta, and the branching arteries of the aortic arch | (Goel et al. 2008) | |
| PECAM-1 mechanotransduction is essential for fibronectin gene expression and assembly into matrix fibrils in response to fluid shear stress | (Feaver et al. 2010) | |
| EC conditioned by shear stress recruit fewer flowing neutrophils after stimulation with TNF, a response that is less effective in the absence of PECAM-1. Expression of CD31 is not required for the shear-induced modification of wound closure | (Glen et al. 2012) | |
| Paracellular permeability | PECAM-1-specific antibody fragments augment albumin transit across endothelial cell junctions both in cultured cells and in mice, expression of PECAM-1 makes non-PECAM-1-expressing cells less permeable to albumin | (Ferrero et al. 1995) |
| Expression of PECAM-1 delays the onset of EAE by promoting vascular integrity and decreasing parenchymal inflammatory cell infiltration | (Graesser et al. 2002) | |
| Expression of PECAM-1 is associated with increased vascular integrity in LPS-induced endotoxemia | (Carrithers et al. 2005;Maas et al. 2005) | |
| PECAM-1 facilitates the dephosphorylation and stabilization of β-catenin through ITIM-mediated recruitment of SHP-2 and activation of GSK-3β thus promoting reconstitution of adherens junctions | (Biswas et al. 2006) | |
| PECAM-1 homophilic interactions are more important than its signaling function for maintaining the integrity of endothelial cell junctions | (Privratsky et al. 2011) | |
| CD44 regulates vascular permeability and integrity through a PECAM-1 dependent mechanism | (Flynn et al. 2013) | |
| Gamma radiation decreases endothelial barrier function, which is correlated with a transient loss of PECAM-1 and cell detachment | (Sharma et al. 2013) | |
| Reticular AJ act coordinately with PECAM-1 to maintain endothelial barrier function in regions of low actomyosin-mediated tension | (Fernandez-Martin et al. 2012) | |
| Angiogenesis | PECAM-1 engagement on the cell surface can transduce "outside-in" signals and activate MAPK/ERKs and small GTPases, impacting both cadherin-mediated cell-cell and integrin-mediated cell-matrix interactions | (Wang and Sheibani 2006) |
| PECAM-1 expression has a significant impact on endothelial cell-cell and cell-matrix interactions by augmenting cell migration and capillary morphogenesis by increasing eNOS expression and NO availability | (Park et al. 2010) | |
| PECAM-1 stimulates EC cell migration and the formation of filopodia through SHP-2- and paxillin-mediated MAPK pathway activation and the turnover of focal adhesions | (Zhu et al. 2010;O'Brien et al. 2004) | |
| PECAM-1 expression and its potential interactions with EphB4/ephrin B2 and eNOS are important for survival, migration, and functional organization of EC during retinal vascular development and angiogenesis | (Dimaio et al. 2008) | |
| PECAM-1 isoforms lacking ITIM, as opposed to isoforms containing the ITIM, in PECAM-1−/− bEND cells activated MAPK/ERKs, disrupted adherens junctions, and enhanced cell migration and capillary morphogenesis in Matrigel | (Dimaio and Sheibani 2008) | |
| PECAM-1-expression in EC is correlated with increased migration, more ability to undergo capillary morphogenesis, and more dense peripheral focal adhesions and peripheral cortical actin distribution | (Kondo et al. 2007) | |
| Sustained activation of MAPK/ERKs results in disruption of cadherin-mediated cell-cell adhesion, down-regulation of PECAM-1 expression, and enhanced cell migration in microvascular endothelial cells | (Wu and Sheibani 2003) | |
| Antibodies against PECAM-1 decrease angiogenesis in transplanted tumors, inhibit tube formation and migration of HUVEC, and block in vitro tube formation by rat capillary endothelial cells during cytokine-induced rat corneal neovascularization | (Cao et al. 2002;Delisser et al. 1997) | |
| PECAM-1 is strongly expressed at cell borders in confluent monolayers whereas little or no PECAM-1 immunostaining is detected in sparse or migrating cultured EC | (RayChaudhury et al. 2001) |
Adhesive interactions guiding subcellular localization
While a subpopulation of PECAM-1 associates with the cytoskeleton of activated platelets (Newman et al. 1992), very little of it can be found in the Triton X-100 insoluble cytoskeletal fraction of endothelial cells (Lampugnani et al. 1995). Consistent with these observations, Sun et al. demonstrated that homophilic binding Ig Domain 1 within the extracellular domain is sufficient for the molecule to become strongly localized to the cell-cell junctions of REN mesothelioma cells (Sun et al. 2000) – a finding that was more recently confirmed in cultured endothelium (Privratsky et al. 2011). The subcellular localization of PECAM-1 might also be able to be influenced by isoform switching in certain cell types. For example, mouse Δ15 PECAM-1 (contains exon 14) does not localize to cell-cell junctions in MDCK cells (Sheibani et al. 2000), but concentrates at cell-cell borders normally in endothelial-like bEND (Dimaio and Sheibani 2008) and Ren mesothelioma (Bergom et al. 2008) cells. Taken together, it appears that the homophilic binding of PECAM-1 to neighboring PECAM-1 molecules on adjacent cells is sufficient to enable junctional localization – a process that has been termed “diffusion trapping”.
PECAM-1 not only resides at the junction, but it is a constituent of a recycling compartment on endothelial cells, termed the lateral border recycling compartment (LBRC) that modulates the transmigration of leukocytes through the endothelial cell-cell junction. The LBRC is a surface-connected membrane network that is located at the borders between adjacent endothelial cells and is recycled and targeted to the region of the cell where paracellular or transcellular migration is occurring (Mamdouh et al. 2003;Mamdouh et al. 2009) (Figure 2). Leukocyte transmigration is facilitated by LBRC recycling mediated by endothelial microtubules and kinesin family molecular motors (Mamdouh et al. 2008). This membrane network contains PECAM-1, CD99, CD155 and junctional adhesion molecule (JAM)-A (Mamdouh et al. 2003;Mamdouh et al. 2009;Sullivan et al. 2013). PECAM-1/PECAM-1 homophilic interactions between leukocytes and endothelial cells appear to be important for leukocyte transmigration as monocytes were unable to transmigrate through the junction and endothelial PECAM-1 was not targeted to the zone around a transmigrating monocyte when PECAM-1 was blocked with an anti-PECAM-1 blocking antibody (Mamdouh et al. 2003). Interestingly, ITIM-mediated recruitment of SHP-2 is not essential for PECAM-1 to efficiently enter and exit the LBRC and to support targeted recycling of the LBRC (Dasgupta et al. 2009), though more recent studies have shown that endothelial CD155 regulates a step between those regulated by PECAM and CD99 and the recruitment of SHP-2 may be a common mechanism for the transmigration process (Sullivan et al. 2013). Thus, it is likely that the localization of PECAM-1, along with other adhesion molecules, to membrane compartments along the endothelial cell-cell border and their complex interplay with cytosolic signaling molecules and cytoskeletal elements is required for efficient leukocyte transendothelial migration and junctional integrity during this process. Whether PECAM-1 within the LBRC regulates the passage of fluid and proteins following stimuli that disrupt the paracellular endothelial barrier, however, remains to be determined. The cross-talk between elements of the LBRC and how they regulate the coordinated opening and closing of cell-cell junctions remains an active area of current research.
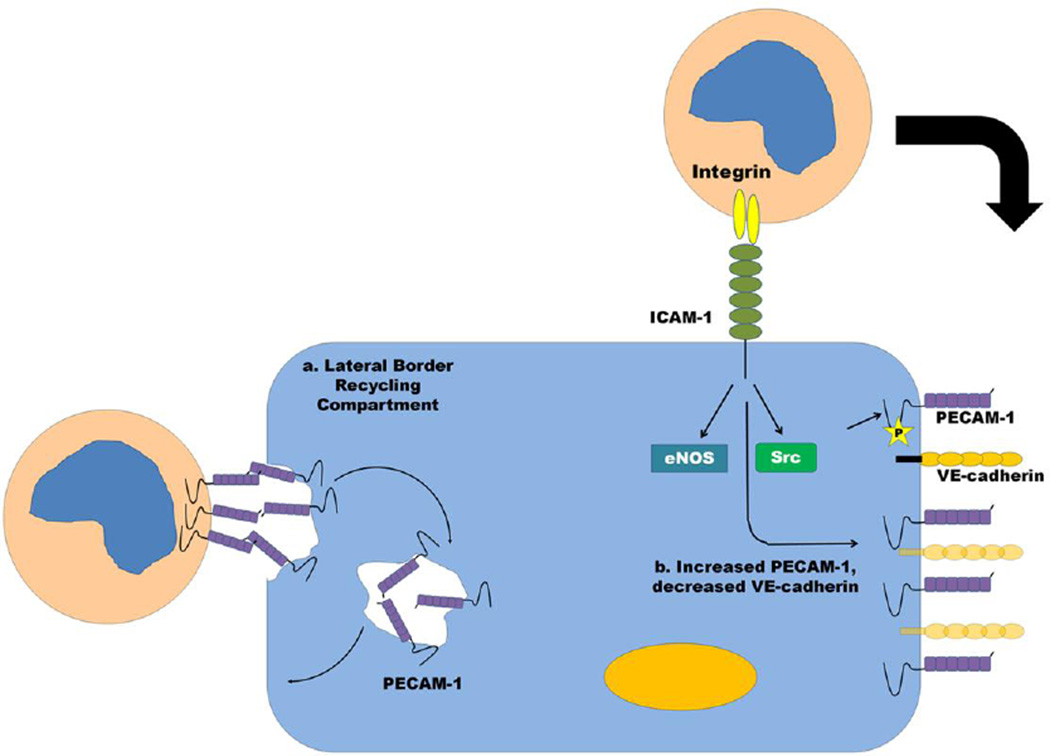
Fig. 2. Barrier regulation by PECAM-1 during leukocyte diapedesis.
(a) PECAM-1 is a constituent of a recycling compartment on endothelial cells, the LBRC, which modulates the transmigration of leukocytes through the endothelial cell-cell junction. The LBRC is a surface-connected membrane network that is located at the borders between adjacent endothelial cells and is recycled and targeted to the region of the cell where paracellular or transcellular migration is occurring. (b) Increased levels of PECAM-1 and decreased levels of VE-cadherin appear to be associated with a more “leukocyte-permeable” state as transmigrating monocytes appear to induce increased PECAM-1 and decreased VE-cadherin expression, which augments transmigratory activity. Mechanisms for this could include Src and eNOS activation downstream of ICAM-1 engagement on endothelial cells by adherent leukocytes, which have been shown to increase PECAM-1 expression.
Not only the localization, but the relative amount of PECAM-1 at the paracellular junction appears to modulate junctional integrity. Monocyte diapedesis has been demonstrated to alter endothelial junctional organization to a more monocyte-permeable state (increased PECAM-1 and decreased VE-cadherin expression), which augments transmigratory activity (Hashimoto et al. 2011) (Figure 2). Other reports have shown that ICAM-1 engagement by transmigrating leukocytes can activate Src and lead to increased PECAM-1 expression, which augments transmigration (Liu et al. 2012) (Figure 2). Thus, at least in the case of leukocyte transmigration, increased PECAM-1 expression is associated with a more leukocyte-permeable or “diapedesis-inducing” state. Whether the relative expression level of PECAM-1 affects junctional integrity in response to other inflammatory stimuli, however, remains to be determined.
PECAM-1/SHP-2 complexes have been proposed to regulate the phosphorylation state of β-catenin, and thereby β-catenin/VE-cadherin complexes (Ilan et al. 2000) (Figure 3). Recent studies, however, have shown that cells expressing a PECAM-1 variant incapable of binding SHP-2 exhibited normal to near-normal barrier integrity (Privratsky et al. 2011). More work needs to be done in this area, perhaps using a variety of agonists, as well as endothelial cells from different sources, to better understand cadherin-dependent versus –independent mechanisms of PECAM-1-mediated barrier protection.
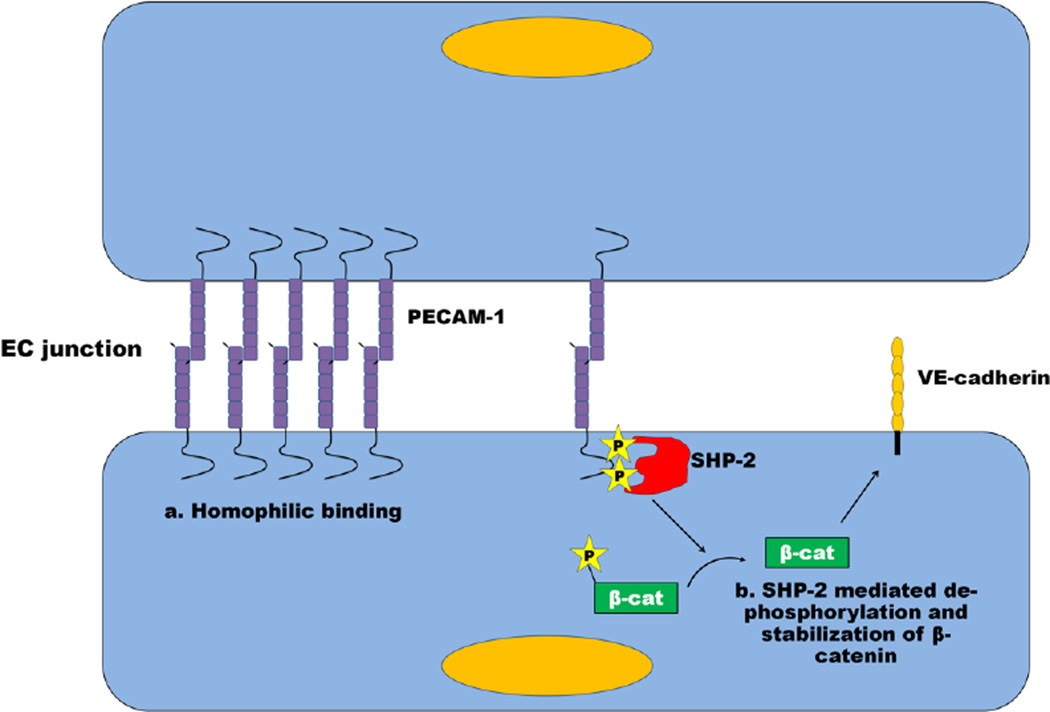
Fig. 3. Mechanisms by which PECAM-1 modulates paracellular permeability.
Homophilic PECAM-1/PECAM-1 interactions between neighboring endothelial cells have been demonstrated to localize PECAM-1 at the endothelial junction. These interactions have recently been shown to be more important than known signaling mechanisms for PECAM-1-mediated barrier protection. PECAM-1 has been shown in certain experimental systems to facilitate the dephosphorylation and stabilization of β-catenin through recruitment of SHP-2 to its ITIM, thus allowing β-catenin to re-associate with VE-cadherin, thus stabilizing the EC junction. In other experimental systems, the ability of PECAM-1 to fortify endothelial cell-cell junctions appears to be ITIM-, and therefore SHP-2, -independent. See text for details.
PECAM-1 isoforms and signaling
Another area of research revealing that the structure of PECAM-1 is important for junctional function is work that has centered on the properties of alternatively-spliced PECAM-1 isoforms and how cell type-specific expression of these isoforms can affect angiogenesis, leukocyte diapedesis, and endothelial junctional stability (Dimaio and Sheibani 2008;Kondo et al. 2007;Wang and Sheibani 2006;Wang et al. 2003a;Sheibani et al. 2000;Sheibani et al. 1997). The PECAM-1 gene consists of 16 exons, with the cytoplasmic domain being encoded from the end of exon 9 through exon 16 (Kirschbaum et al. 1994). Alternative splicing of the PECAM-1 cytoplasmic and transmembrane domains results in the production of numerous PECAM-1 isoforms, including a soluble form (Goldberger et al. 1994) and various isoforms that lack one or more cytoplasmic exons (Kirschbaum et al. 1994;Baldwin et al. 1994;Sheibani et al. 1997;Bergom et al. 2008;Yan et al. 1995;Robson et al. 2001;Sheibani et al. 1999;Sheibani et al. 2000;Wang and Sheibani 2002;Wu and Sheibani 2003;Wang et al. 2003a;Wang et al. 2003b;Wang et al. 2004;Dimaio and Sheibani 2008). Full length PECAM-1 is by far the predominant isoform expressed in humans in all cells (Wang et al. 2003b), whereas mRNA encoding PECAM-1 in mice tends to undergo more extensive alternative splicing with Δ14, 15 (loss of exons 14 and 15) being the predominant isoform expressed (Sheibani et al. 1999).
Isoform switching of PECAM-1 has the potential to alter the signaling properties of PECAM-1-expressing cells, as SHP-2 can only be recruited and activated by PECAM-1 isoforms that contain ITIMs encoded by exons 13 and 14 (Jackson et al. 1997a;Dimaio and Sheibani 2008;Wang and Sheibani 2006;Bergom et al. 2008) (Figure 4). To illustrate this concept, expression of a mouse PECAM-1 isoform containing exon 14, as opposed to one lacking exon 14, in heterologous Madin-Darby canine kidney (MDCK) cells led to activation of mitogen activated protein kinases (MAPK), extracellular signal-regulated kinases (ERK), and the small GTPases Rac1 and Rap1, resulting in loss of cell-cell contacts, de-stabilization of adherens junctions, and a change in the subcellular localization of cadherins and catenins (Wang and Sheibani 2006;Sheibani et al. 2000) (Figure 4). Early on, these effects were proposed to modulate the spatio-temporal disruption of adherens junctions downstream of PECAM-1 homophilic interactions during leukocyte transmigration (Wang and Sheibani 2006). It also helped to explain the more migratory phenotype of PECAM-1-expressing endothelial cells during angiogenesis, as exon 14-positive PECAM-1 isoforms were found to be preferentially expressed early in vascular development, and replaced later by isoforms lacking exon 14 (Wu and Sheibani 2003;Sheibani et al. 2000;Sheibani et al. 1997). Later experiments revealed that these effects might not be operative in endothelial cells, however, as expression of exon-14-containing murine PECAM-1 (contains the ITIM) in an immortalized mouse brain endothelial cell line (bEND) had minimal effects on the activation of MAPK/ERKs and resulted in a less migratory phenotype, which was hypothesized to be due to PECAM-1 ITIM-mediated SHP-2 inhibitory signaling (Dimaio and Sheibani 2008) (Figure 4). In addition, further work by this same group revealed that expression of the predominant form of murine PECAM-1, Δ14&15 (lacks the ITIM), in primary murine retinal endothelial cells was sufficient to restore migration and capillary morphogenesis of null cells to wild-type levels, whereas full-length PECAM-1 (contains the ITIM) had no effect on these cellular properties (Park et al. 2010). Thus, it appears that isoform switching can change the signaling properties of PECAM-1 through loss (or gain) of functional domains that bind signaling partners, but the effects of this is cell-type specific, and in the case of endothelial cells might result in more inhibitory, as opposed to activating, signaling if the PECAM-1 ITIM is present. As human endothelial cells express mostly full-length PECAM-1 (Wang et al. 2003b), it remains to be determined whether differential signaling induced by various PECAM-1 isoforms is relevant in humans.
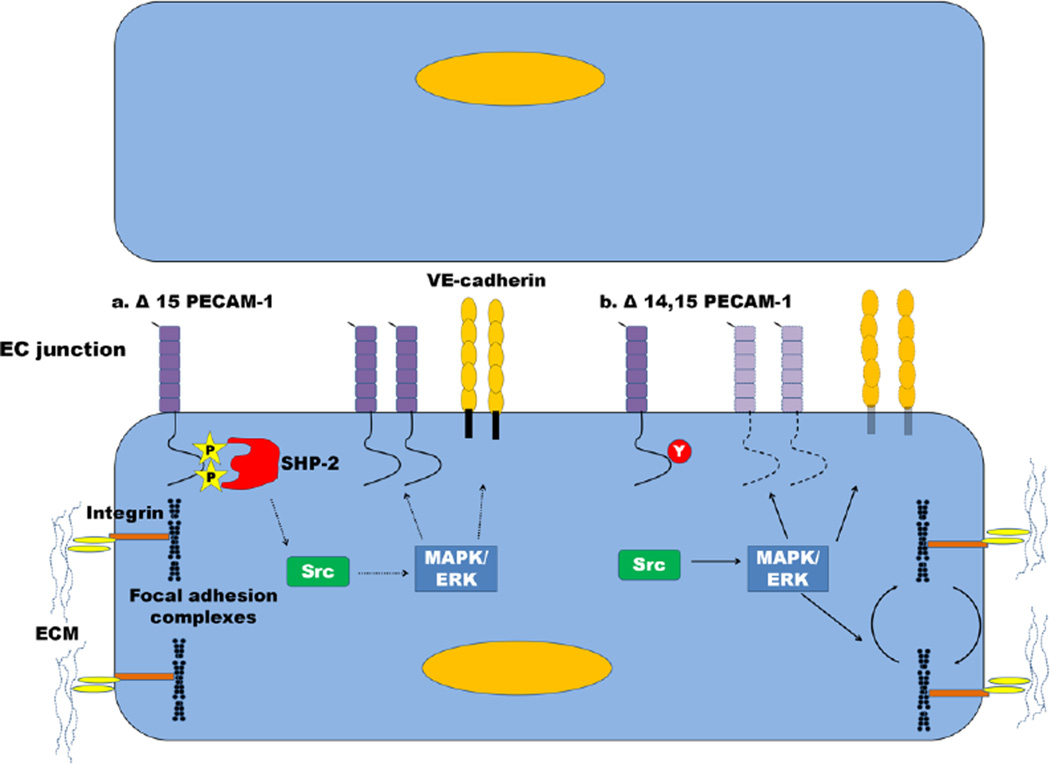
Fig. 4. Differential regulation of the vascular barrier by PECAM-1 isoforms.
(a) Δ15 PECAM-1 retains the ITIM tyrosines, which enables it to recruit SHP-2 and inhibit Src (dashed line). Src no longer activates MAPK/ERKs which stabilizes expression of PECAM-1 and VE-cadherin at the endothelial cell junction. (b) In contrast, Δ14, 15 PECAM-1 does not contain the ITIM, thus the inhibition on Src is relieved, which activates MAPK/ERKs (solid line) leading to decreased expression of PECAM-1 and VE-cadherin and turnover of focal adhesion complexes. This destabilizes the junction and creates a more migratory phenotype.
Stimulus-specific functions of PECAM-1
Though PECAM-1 modulates junctional signaling in a variety of physiological conditions, some studies have shown that PECAM-1 is only required for junctional integrity in response to certain stimuli, at least in the case of leukocyte transmigration. In vivo, PECAM-1 has been shown to be required for leukocyte transmigration in response to IL-1β, but not TNFα or certain chemokines (Thompson et al. 2001;Woodfin et al. 2009). IL-1β, which mainly activates endothelial cells as opposed to leukocytes, appears to make leukocyte transmigration dependent on the junctional adhesion molecules ICAM-1, JAM-A, and PECAM-1 in a sequential manner (Woodfin et al. 2009;Woodfin et al. 2007;Nourshargh et al. 2006). It is likely that interaction of these adhesion molecules on endothelial cells with their counterparts on leukocytes activates leukocyte integrins and allows them to traverse the junction. Other stimuli, such as TNFα and chemokines, however, bypass the need for these junctional adhesive interactions by directly activating leukocyte integrins and allowing them to transmigrate through junctions (Woodfin et al. 2009). Thus, it would not be surprising if integrin activation and cell-matrix adhesion in endothelial cells was also differentially regulated by PECAM-1 in response to various stimuli. For example, the expression of PECAM-1 has been shown to change the adhesive properties of endothelial cells rendering them more adherent to extracellular matrix (ECM) proteins, at least in the case of angiogenesis (Park et al. 2010). In addition, it has been shown PECAM-1/SHP-2 complexes can modulate actin stress fibers and focal adhesions (Wang and Sheibani 2006;O'Brien et al. 2004). Taken together, it is likely that in response to specific cytokine stimuli, PECAM-1 modulates the activation state of integrins, and other proteins important for adhesive interactions, which allows endothelial cells to turn over cell-cell and cell-matrix interactions that are required for efficient junctional function during processes such as leukocyte diapedesis and angiogenesis. Whether PECAM-1 changes the activation state of integrins important for endothelial cell-ECM interactions downstream of inflammatory stimuli, and whether this plays a prominent role in junctional integrity and localized movement of endothelial cells during leukocyte diapedesis or paracellular permeability remains to be determined.
PECAM-1 as a biosensor: cross-talk with other signaling molecules
The observation that PECAM-1 has both adhesive as well as signaling properties, together with the fact that it is expressed at high receptor density on the surface of vascular endothelial cells, suggested that it might function as a biosensor of local environmental changes. There now exists extensive evidence that PECAM-1 both senses flow and transmits tensile forces into endothelial cells. Early studies found that mechanical changes to endothelial cells brought about by hyper- or hypo-osmotic shock or changes in flow result resulted in rapid tyrosine phosphorylation of PECAM-1 (Harada et al. 1995;Osawa et al. 1997)(Osawa et al. 2002;Tai et al. 2005;Fleming et al. 2005), an observation that is now understood to be due to the presence of a multimolecular complex between PECAM-1 and VE-cadherin (Tzima et al. 2005;Collins et al. 2012;Conway et al. 2013) that, in response to flow activates Gα11, Src, and associated cytosolic and signaling molecules (Figure 5). The resulting phosphorylation of PECAM-1 ITIM tyrosines creates a scaffold for recruitment signaling molecules that have the potential to modulate responses of endothelial cells. In fact, PECAM-1-deficient endothelial cells exhibit defective activation of Src, MAPK/ERK, and PI3K/Akt in response to shear (Osawa et al. 2002;Tai et al. 2005;Tzima et al. 2005), with subsequent loss of NF- B-driven gene transcription (Tzima et al. 2005), and activation of endothelial cell nitric oxide synthase (eNOS) (Fleming et al. 2005;Tai et al. 2005). Functionally-related processes such as flow-mediated dilation, which is attributable to release from endothelial cells of vasodilatory factors, including prostacyclin, nitric oxide (NO) and endothelium-derived hyperpolarizing factor (Busse and Fleming 2003), are also markedly impaired in the absence of PECAM-1 (Bagi et al. 2005;Liu et al. 2005). Precisely how PECAM-1 and VE-cadherin interact remains uncertain, as they are concentrated in distinct, and different compartments that comprise endothelial cell-cell boundaries (Lampugnani et al. 1995;Dejana 2004). Thus, while VE-cadherin is found primarily within the adherens junction proper, PECAM-1 is enriched in distinctive actin/myosin/vinculin-depleted three-dimensional reticular networks that form in areas where adjacent endothelial cells overlap - sometimes referred to as the reticular adherens junction (Fernandez-Martin et al. 2012). Perhaps the biosensor function of PECAM-1 takes place at the intersection of these two specialized domains.
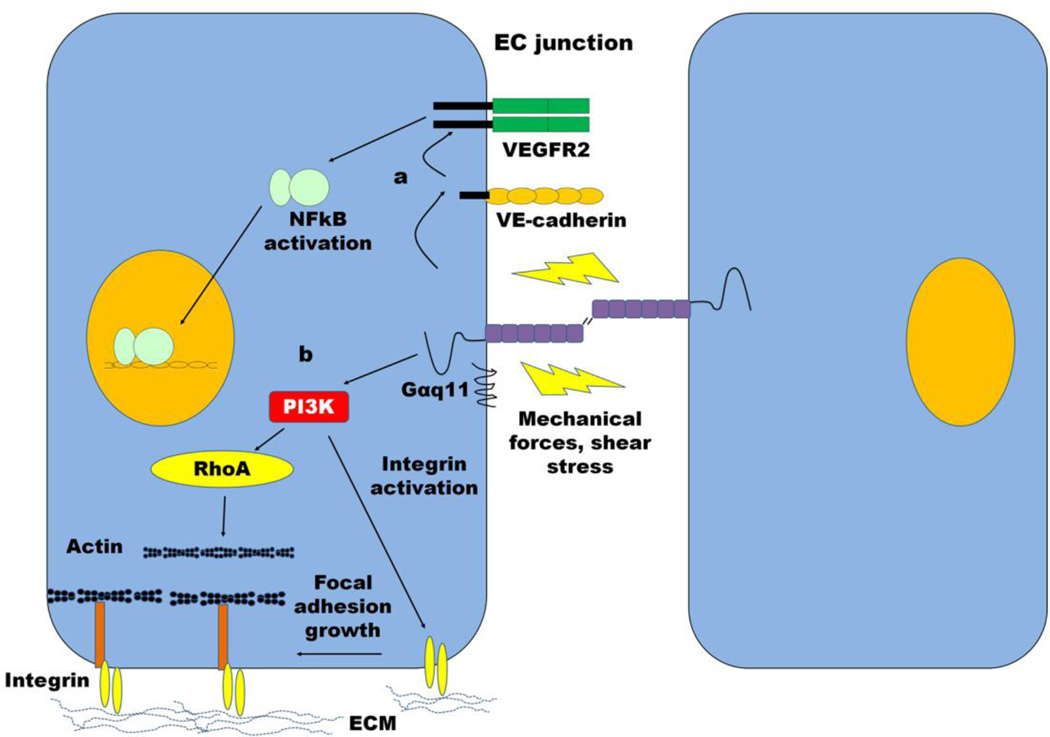
Fig. 5. Modulation of barrier function by PECAM-1-mediated mechanotransduction.
(a) PECAM-1 is associated with Gα11 and is part of a mechanosensory complex with VE-cadherin and VEGFR2. PECAM-1 senses mechanical force and transmits signals to VE-cadherin, which activates VEGFR2 leading to NFκB-mediated pro-inflammatory gene transcription. (b) Mechanical force on PECAM-1 also activates PI3K, which triggers RhoA activation leading to changes in cytoskeletal architecture and focal adhesion complexes.
Concluding Remarks
Owing to its unique distribution on platelets, leukocytes, and endothelial cells, PECAM-1 lies at the nexus of thrombosis and inflammation. There is growing appreciation that these two pathophysiological conditions are inextricably and mechanistically linked. PECAM-1, via its ability to inhibit platelet activation, suppress cytokine production and responsiveness, stimulate vessel wall production of prostacyclin, and support the integrity of endothelial cell-cell junctions, appears to play a significant role in each of these interrelated processes. Future studies aimed at understanding how the adhesive properties of PECAM-1 are regulated to support the integrity of the vascular endothelium should lead to an improved understanding of how PECAM-1, together with other cell surface receptors that are found at endothelial cell-cell junctions, functions to regulate inflammation, thrombosis, and the immune response.
Reference List
- Albelda SM, Muller WA, Buck CA, Newman PJ. Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell-cell adhesion molecule. J Cell Biol. 1991;114:1059–1068. doi: 10.1083/jcb.114.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagi Z, Frangos JA, Yeh JC, White CR, Kaley G, Koller A. PECAM-1 mediates NO-dependent dilation of arterioles to high temporal gradients of shear stress. Arterioscler Thromb Vasc Biol. 2005;25:1590–1595. doi: 10.1161/01.ATV.0000170136.71970.5f. [DOI] [PubMed] [Google Scholar]
- Baldwin HS, Shen HM, Yan HC, Delisser HM, Chung A, Mickanin C, Trask T, Kirschbaum NE, Newman PJ, Albelda SM. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31): alternatively spliced, functionally distinct isoforms expressed during mammalian cardiovascular development. Development. 1994;120:2539–2553. doi: 10.1242/dev.120.9.2539. [DOI] [PubMed] [Google Scholar]
- Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- Bergom C, Paddock C, Gao C, Holyst T, Newman DK, Newman PJ. An alternatively spliced isoform of PECAM-1 is expressed at high levels in human and murine tissues, and suggests a novel role for the C-terminus of PECAM-1 in cytoprotective signaling. J Cell Sci. 2008;121:1235–1242. doi: 10.1242/jcs.025163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas P, Canosa S, Schoenfeld D, Schoenfeld J, Li P, Cheas LC, Zhang J, Cordova A, Sumpio B, Madri JA. PECAM-1 affects GSK-3β-mediated β-catenin phosphorylation and degradation. Am J Pathol. 2006;169:314–324. doi: 10.2353/ajpath.2006.051112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixel MG, Li H, Petri B, Khandoga AG, Khandoga A, Zarbock A, Wolburg-Buchholz K, Wolburg H, Sorokin L, Zeuschner D, Maerz S, Butz S, Krombach F, Vestweber D. CD99 and CD99L2 act at the same site as, but independently of, PECAM-1 during leukocyte diapedesis. Blood. 2010;116:1172–1184. doi: 10.1182/blood-2009-12-256388. [DOI] [PubMed] [Google Scholar]
- Buckley CD, Doyonnas R, Newton JP, Blystone SD, Brown EJ, Watt SM, Simmons DL. Identification of alpha v beta 3 as a heterotypic ligand for CD31/PECAM-1. J Cell Sci. 1996;109(Pt 2):437–445. doi: 10.1242/jcs.109.2.437. [DOI] [PubMed] [Google Scholar]
- Busse R, Fleming I. Regulation of endothelium-derived vasoactive autacoid production by hemodynamic forces. Trends Pharmacol Sci. 2003;24:24–29. doi: 10.1016/s0165-6147(02)00005-6. [DOI] [PubMed] [Google Scholar]
- Cao G, O'Brien CD, Zhou Z, Sanders SM, Greenbaum JN, Makrigiannakis A, Delisser HM. Involvement of human PECAM-1 in angiogenesis and in vitro endothelial cell migration. Am J Physiol Cell Physiol. 2002;282:C1181–C1190. doi: 10.1152/ajpcell.00524.2001. [DOI] [PubMed] [Google Scholar]
- Carrithers M, Tandon S, Canosa S, Michaud M, Graesser D, Madri JA. Enhanced susceptibility to endotoxic shock and impaired STAT3 signaling in CD31-deficient mice. Am J Pathol. 2005;166:185–196. doi: 10.1016/S0002-9440(10)62243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- Collins C, Guilluy C, Welch C, O'Brien ET, Hahn K, Superfine R, Burridge K, Tzima E. Localized tensional forces on PECAM-1 elicit a global mechanotransduction response via the integrin-RhoA pathway. Curr Biol. 2012;22:2087–2094. doi: 10.1016/j.cub.2012.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway DE, Breckenridge MT, Hinde E, Gratton E, Chen CS, Schwartz MA. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr Biol. 2013;23:1024–1030. doi: 10.1016/j.cub.2013.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta B, Dufour E, Mamdouh Z, Muller WA. A novel and critical role for tyrosine 663 in platelet endothelial cell adhesion molecule-1 trafficking and transendothelial migration. J Immunol. 2009;182:5041–5051. doi: 10.4049/jimmunol.0803192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaglio S, Morra M, Mallone R, Ausiello CM, Prager E, Garbarino G, Dianzani U, Stockinger H, Malavasi F. Human CD38 (ADP-ribosyl cyclase) is a counter-receptor of CD31, an Ig superfamily member. J Immunol. 1998;160:395–402. [PubMed] [Google Scholar]
- Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008;121:2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- Delisser HM, Christofidou-Solomidou M, Strieter RM, Burdick MD, Robinson CS, Wexler RS, Kerr JS, Garlanda C, Merwin JR, Madri JA, Albelda SM. Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am J Pathol. 1997;151:671–677. [PMC free article] [PubMed] [Google Scholar]
- Delisser HM, Yan HC, Newman PJ, Muller WA, Buck CA, Albelda SM. Platelet/endothelial cell adhesion molecule-1 (CD31)-mediated cellular aggregation involves cell surface glycosaminoglycans. J Biol Chem. 1993;268:16037–16046. [PubMed] [Google Scholar]
- Dimaio TA, Sheibani N. PECAM-1 isoform-specific functions in PECAM-1-deficient brain microvascular endothelial cells. Microvasc Res. 2008;75:188–201. doi: 10.1016/j.mvr.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimaio TA, Wang S, Huang Q, Scheef EA, Sorenson CM, Sheibani N. Attenuation of retinal vascular development and neovascularization in PECAM-1-deficient mice. Dev Biol. 2008;315:72–88. doi: 10.1016/j.ydbio.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GS, Andrew DP, Takimoto H, Kaufman SA, Yoshida H, Spellberg J, Luis de la PJ, Elia A, Wakeham A, Karan-Tamir B, Muller WA, Senaldi G, Zukowski MM, Mak TW. Genetic evidence for functional redundancy of Platelet/Endothelial cell adhesion molecule-1 (PECAM-1): CD31-deficient mice reveal PECAM-1-dependent and PECAM-1-independent functions. J Immunol. 1999;162:3022–3030. [PubMed] [Google Scholar]
- Feaver RE, Gelfand BD, Wang C, Schwartz MA, Blackman BR. Atheroprone hemodynamics regulate fibronectin deposition to create positive feedback that sustains endothelial inflammation. Circ Res. 2010;106:1703–1711. doi: 10.1161/CIRCRESAHA.109.216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Martin L, Marcos-Ramiro B, Bigarella CL, Graupera M, Cain RJ, Reglero-Real N, Jimenez A, Cernuda-Morollon E, Correas I, Cox S, Ridley AJ, Millan J. Crosstalk between reticular adherens junctions and platelet endothelial cell adhesion molecule-1 regulates endothelial barrier function. Arterioscler Thromb Vasc Biol. 2012;32:e90–e102. doi: 10.1161/ATVBAHA.112.252080. [DOI] [PubMed] [Google Scholar]
- Ferrero E, Ferrero ME, Pardi R, Zocchi MR. The platelet endothelial cell adhesion molecule-1 (PECAM1) contributes to endothelial barrier function. FEBS Lett. 1995;374:323–326. doi: 10.1016/0014-5793(95)01110-z. [DOI] [PubMed] [Google Scholar]
- Fleming I, Fisslthaler B, Dixit M, Busse R. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J Cell Sci. 2005;118:4103–4111. doi: 10.1242/jcs.02541. [DOI] [PubMed] [Google Scholar]
- Flynn KM, Michaud M, Canosa S, Madri JA. CD44 regulates vascular endothelial barrier integrity via a PECAM-1 dependent mechanism. Angiogenesis. 2013 doi: 10.1007/s10456-013-9346-9. [DOI] [PubMed] [Google Scholar]
- Glen K, Luu NT, Ross E, Buckley CD, Rainger GE, Egginton S, Nash GB. Modulation of functional responses of endothelial cells linked to angiogenesis and inflammation by shear stress: differential effects of the mechanotransducer CD31. J Cell Physiol. 2012;227:2710–2721. doi: 10.1002/jcp.23015. [DOI] [PubMed] [Google Scholar]
- Goel R, Schrank BR, Arora S, Boylan B, Fleming B, Miura H, Newman PJ, Molthen RC, Newman DK. Site-specific effects of PECAM-1 on atherosclerosis in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2008 doi: 10.1161/ATVBAHA.108.172270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberger A, Middleton KA, Oliver JA, Paddock C, Yan HC, Delisser HM, Albelda SM, Newman PJ. Biosynthesis and processing of the cell adhesion molecule PECAM-1 includes production of a soluble form. J Biol Chem. 1994;269:17183–17191. [PubMed] [Google Scholar]
- Graesser D, Solowiej A, Bruckner M, Osterweil E, Juedes A, Davis S, Ruddle NH, Engelhardt B, Madri JA. Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM-1-deficient mice. J Clin Invest. 2002;109:383–392. doi: 10.1172/JCI13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N, Masuda M, Fujiwara K. Fluid flow and osmotic stress induce tyrosine phosphorylation of an endothelial cell 128 kDa surface glycoprotein. Biochem Biophys Res Commun. 1995;214:69–74. doi: 10.1006/bbrc.1995.2257. [DOI] [PubMed] [Google Scholar]
- Harrison M, Smith E, Ross E, Krams R, Segers D, Buckley CD, Nash GB, Rainger GE. The role of platelet-endothelial cell adhesion molecule-1 in atheroma formation varies depending on the site-specific hemodynamic environment. Arterioscler Thromb Vasc Biol. 2013;33:694–701. doi: 10.1161/ATVBAHA.112.300379. [DOI] [PubMed] [Google Scholar]
- Harry BL, Sanders JM, Feaver RE, Lansey M, Deem TL, Zarbock A, Bruce AC, Pryor AW, Gelfand BD, Blackman BR, Schwartz MA, Ley K. Endothelial cell PECAM-1 promotes atherosclerotic lesions in areas of disturbed flow in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:2003–2008. doi: 10.1161/ATVBAHA.108.164707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Kataoka N, Nakamura E, Hagihara K, Hatano M, Okamoto T, Kanouchi H, Minatogawa Y, Mohri S, Tsujioka K, Kajiya F. Monocyte trans-endothelial migration augments subsequent transmigratory activity with increased PECAM-1 and decreased VE-cadherin at endothelial junctions. Int J Cardiol. 2011;149:232–239. doi: 10.1016/j.ijcard.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Ilan N, Cheung L, Pinter E, Madri JA. Platelet-endothelial cell adhesion molecule-1 (CD31), a scaffolding molecule for selected catenin family members whose binding is mediated by different tyrosine and serine/threonine phosphorylation. J Biol Chem. 2000;275:21435–21443. doi: 10.1074/jbc.M001857200. [DOI] [PubMed] [Google Scholar]
- Jackson DE, Kupcho KR, Newman PJ. Characterization of phosphotyrosine binding motifs in the cytoplasmic domain of platelet/endothelial cell adhesion molecule-1 (PECAM-1) that are required for the cellular association and activation of the protein-tyrosine phosphatase, SHP-2. J Biol Chem. 1997a;272:24868–24875. doi: 10.1074/jbc.272.40.24868. [DOI] [PubMed] [Google Scholar]
- Jackson DE, Ward CM, Wang R, Newman PJ. The protein-tyrosine phosphatase SHP-2 binds platelet/endothelial cell adhesion molecule-1 (PECAM-1) and forms a distinct signaling complex during platelet aggregation. Evidence for a mechanistic link between PECAM-1- and integrin-mediated cellular signaling. J Biol Chem. 1997b;272:6986–6993. doi: 10.1074/jbc.272.11.6986. [DOI] [PubMed] [Google Scholar]
- Kirschbaum NE, Gumina RJ, Newman PJ. Organization of the gene for human platelet/endothelial cell adhesion molecule-1 shows alternatively spliced isoforms and a functionally complex cytoplasmic domain. Blood. 1994;84:4028–4037. [PubMed] [Google Scholar]
- Kitazume S, Imamaki R, Ogawa K, Komi Y, Futakawa S, Kojima S, Hashimoto Y, Marth JD, Paulson JC, Taniguchi N. Alpha2,6-sialic acid on platelet endothelial cell adhesion molecule (PECAM) regulates its homophilic interactions and downstream antiapoptotic signaling. J Biol Chem. 2010;285:6515–6521. doi: 10.1074/jbc.M109.073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Scheef EA, Sheibani N, Sorenson CM. PECAM-1 isoform-specific regulation of kidney endothelial cell migration and capillary morphogenesis. Am J Physiol Cell Physiol. 2007;292:C2070–C2083. doi: 10.1152/ajpcell.00489.2006. [DOI] [PubMed] [Google Scholar]
- Kuckleburg CJ, Newman PJ. Neutrophil proteinase 3 acts on protease-activated receptor-2 to enhance vascular endothelial cell barrier function. Arterioscler Thromb Vasc Biol. 2013;33:275–284. doi: 10.1161/ATVBAHA.112.300474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Abbas A, Fausto N. Robbins and Cotran Pathologic Basis of Disease. Philadelphia: Saunders; 2004. [Google Scholar]
- Lampugnani MG, Corada M, Caveda L, Breviario F, Ayalon O, Geiger B, Dejana E. The molecular organization of endothelial cell to cell junctions: differential association of plakoglobin, betacatenin, and alpha-catenin with vascular endothelial cadherin (VE-cadherin) J Cell Biol. 1995;129:203–217. doi: 10.1083/jcb.129.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Place AT, Chen Z, Brovkovych VM, Vogel SM, Muller WA, Skidgel RA, Malik AB, Minshall RD. ICAM-1-activated Src and eNOS signaling increase endothelial cell surface PECAM-1 adhesivity and neutrophil transmigration. Blood. 2012;120:1942–1952. doi: 10.1182/blood-2011-12-397430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Bubolz AB, Shi Y, Newman PJ, Newman DK, Gutterman DD. Peroxynitrite reduces the endothelium derived hyperpolarizing factor component of coronary flow-mediated dilation in PECAM-1-knock out mice. Am J Physiol Regul Integr Comp Physiol. 2005 doi: 10.1152/ajpregu.00424.2005. [DOI] [PubMed] [Google Scholar]
- Maas M, Stapleton M, Bergom C, Mattson DL, Newman DK, Newman PJ. Endothelial cell PECAM-1 confers protection against endotoxic shock. Am J Physiol Heart Circ Physiol. 2005;288:H159–H164. doi: 10.1152/ajpheart.00500.2004. [DOI] [PubMed] [Google Scholar]
- Mamdouh Z, Chen X, Pierini LM, Maxfield FR, Muller WA. Targeted recycling of PECAM from endothelial surface-connected compartments during diapedesis. Nature. 2003;421:748–753. doi: 10.1038/nature01300. [DOI] [PubMed] [Google Scholar]
- Mamdouh Z, Kreitzer GE, Muller WA. Leukocyte transmigration requires kinesin-mediated microtubule-dependent membrane trafficking from the lateral border recycling compartment. J Exp Med. 2008;205:951–966. doi: 10.1084/jem.20072328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamdouh Z, Mikhailov A, Muller WA. Transcellular migration of leukocytes is mediated by the endothelial lateral border recycling compartment. J Exp Med. 2009;206:2795–2808. doi: 10.1084/jem.20082745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- Ming Z, Hu Y, Xiang J, Polewski P, Newman PJ, Newman DK. Lyn and PECAM-1 function as interdependent inhibitors of platelet aggregation. Blood. 2011;117:3903–3906. doi: 10.1182/blood-2010-09-304816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993;178:449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman PJ. The biology of PECAM-1. J Clin Invest. 1997;100:S25–S29. [PubMed] [Google Scholar]
- Newman PJ. Switched at birth: a new family for PECAM-1. J Clin Invest. 1999;103:5–9. doi: 10.1172/JCI5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman PJ, Berndt MC, Gorski J, White GC, Lyman S, Paddock C, Muller WA. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990;247:1219–1222. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- Newman PJ, Hillery CA, Albrecht R, Parise LV, Berndt MC, Mazurov AV, Dunlop LC, Zhang J, Rittenhouse SE. Activation-dependent changes in human platelet PECAM-1: phosphorylation, cytoskeletal association, and surface membrane redistribution. J Cell Biol. 1992;119:239–246. doi: 10.1083/jcb.119.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman PJ, Newman DK. Signal transduction pathways mediated by PECAM-1: new roles for an old molecule in platelet and vascular cell biology. Arterioscler Thromb Vasc Biol. 2003;23:953–964. doi: 10.1161/01.ATV.0000071347.69358.D9. [DOI] [PubMed] [Google Scholar]
- Newton JP, Buckley CD, Jones EY, Simmons DL. Residues on both faces of the first immunoglobulin fold contribute to homophilic binding sites of PECAM-1/CD31. J Biol Chem. 1997;272:20555–20563. doi: 10.1074/jbc.272.33.20555. [DOI] [PubMed] [Google Scholar]
- Newton JP, Hunter AP, Simmons DL, Buckley CD, Harvey DJ. CD31 (PECAM-1) exists as a dimer and is heavily N-glycosylated. Biochem Biophys Res Commun. 1999;261:283–291. doi: 10.1006/bbrc.1999.1018. [DOI] [PubMed] [Google Scholar]
- Nourshargh S, Krombach F, Dejana E. The role of JAM-A and PECAM-1 in modulating leukocyte infiltration in inflamed and ischemic tissues. J Leukoc Biol. 2006;80:714–718. doi: 10.1189/jlb.1105645. [DOI] [PubMed] [Google Scholar]
- O'Brien CD, Cao G, Makrigiannakis A, Delisser HM. Role of immunoreceptor tyrosine-based inhibitory motifs of PECAM-1 in PECAM-1-dependent cell migration. Am J Physiol Cell Physiol. 2004;287:C1103–C1113. doi: 10.1152/ajpcell.00573.2003. [DOI] [PubMed] [Google Scholar]
- Osawa M, Masuda M, Harada N, Lopes RB, Fujiwara K. Tyrosine phosphorylation of platelet endothelial cell adhesion molecule-1 (PECAM-1, CD31) in mechanically stimulated vascular endothelial cells. Eur J Cell Biol. 1997;72:229–237. [PubMed] [Google Scholar]
- Osawa M, Masuda M, Kusano K, Fujiwara K. Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: is it a mechanoresponsive molecule? J Cell Biol. 2002;158:773–785. doi: 10.1083/jcb.200205049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte LA, Bell KS, Loufrani L, Yeh JC, Melchior B, Dao DN, Stevens HY, White CR, Frangos JA. Rapid changes in shear stress induce dissociation of a G{alpha}q/11/PECAM-1 Complex. J Physiol. 2009 doi: 10.1113/jphysiol.2009.172643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock C, Lytle BL, Peterson FC, Holyst T, Newman PJ, Volkman BF, Newman DK. Residues within a lipid-associated segment of the PECAM-1 cytoplasmic domain are susceptible to inducible, sequential phosphorylation. Blood. 2011;117:6012–6023. doi: 10.1182/blood-2010-11-317867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Dimaio TA, Scheef EA, Sorenson CM, Sheibani N. PECAM-1 regulates proangiogenic properties of endothelial cells through modulation of cell-cell and cell-matrix interactions. Am J Physiol Cell Physiol. 2010;299:C1468–C1484. doi: 10.1152/ajpcell.00246.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piali L, Hammel P, Uherek C, Bachmann F, Gisler RH, Dunon D, Imhof BA. CD31/PECAM-1 is a ligand for v 3 integrin involved in adhesion of leukocytes to endothelium. J Cell Biol. 1995;130:451–460. doi: 10.1083/jcb.130.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- Privratsky JR, Paddock CM, Florey O, Newman DK, Muller WA, Newman PJ. Relative contribution of PECAM-1 adhesion and signaling to the maintenance of vascular integrity. J Cell Sci. 2011;124:1477–1485. doi: 10.1242/jcs.082271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RayChaudhury A, Elkins M, Kozien D, Nakada MT. Regulation of PECAM-1 in endothelial cells during cell growth and migration. Exp Biol Med (Maywood) 2001;226:686–691. doi: 10.1177/153537020222600715. [DOI] [PubMed] [Google Scholar]
- Robson P, Stein P, Zhou B, Schultz RM, Baldwin HS. Inner cell mass-specific expression of a cell adhesion molecule (PECAM-1/CD31) in the mouse blastocyst. Dev Biol. 2001;234:317–329. doi: 10.1006/dbio.2001.0274. [DOI] [PubMed] [Google Scholar]
- Sachs UJ, Andrei-Selmer CL, Maniar A, Weiss T, Paddock C, Orlova VV, Choi EY, Newman PJ, Preissner KT, Chavakis T, Santoso S. The neutrophil-specific antigen CD177 is a counter-receptor for platelet endothelial cell adhesion molecule-1 (CD31) J Biol Chem. 2007;282:23603–23612. doi: 10.1074/jbc.M701120200. [DOI] [PubMed] [Google Scholar]
- Schenkel AR, Chew TW, Muller WA. Platelet endothelial cell adhesion molecule deficiency or blockade significantly reduces leukocyte emigration in a majority of mouse strains. J Immunol. 2004;173:6403–6408. doi: 10.4049/jimmunol.173.10.6403. [DOI] [PubMed] [Google Scholar]
- Sharma P, Templin T, Grabham P. Short term effects of gamma radiation on endothelial barrier function: uncoupling of PECAM-1. Microvasc Res. 2013;86:11–20. doi: 10.1016/j.mvr.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Sheibani N, Newman PJ, Frazier WA. Thrombospondin-1, a natural inhibitor of angiogenesis, regulates platelet-endothelial cell adhesion molecule-1 expression and endothelial cell morphogenesis. Mol Biol Cell. 1997;8:1329–1341. doi: 10.1091/mbc.8.7.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheibani N, Sorenson CM, Frazier WA. Tissue specific expression of alternatively spliced murine PECAM-1 isoforms. Dev Dyn. 1999;214:44–54. doi: 10.1002/(SICI)1097-0177(199901)214:1<44::AID-DVDY5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Sheibani N, Sorenson CM, Frazier WA. Differential modulation of cadherin-mediated cell-cell adhesion by platelet endothelial cell adhesion molecule-1 isoforms through activation of extracellular regulated kinases. Mol Biol Cell. 2000;11:2793–2802. doi: 10.1091/mbc.11.8.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sima AV, Stancu CS, Simionescu M. Vascular endothelium in atherosclerosis. Cell Tissue Res. 2009;335:191–203. doi: 10.1007/s00441-008-0678-5. [DOI] [PubMed] [Google Scholar]
- Simmons DL, Walker C, Power C, Pigott R. Molecular cloning of CD31, a putative intercellular adhesion molecule closely related to carcinoembryonic antigen. J Exp Med. 1990;171:2147–2152. doi: 10.1084/jem.171.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens HY, Melchior B, Bell KS, Yun S, Yeh JC, Frangos JA. PECAM-1 is a critical mediator of atherosclerosis. Dis Model Mech. 2008;1:175–181. doi: 10.1242/dmm.000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger H, Gadd SJ, Eher R, Majdic O, Schreiber W, Kasinrerk W, Strass B, Schnabl E, Knapp W. Molecular characterization and functional analysis of the leukocyte surface protein CD31. J Immunol. 1990;145:3889–3897. [PubMed] [Google Scholar]
- Sullivan DP, Seidman MA, Muller WA. Poliovirus Receptor (CD155) Regulates a Step in Transendothelial Migration between PECAM and CD99. Am J Pathol. 2013;182:1031–1042. doi: 10.1016/j.ajpath.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Paddock C, Shubert J, Zhang HB, Amin K, Newman PJ, Albelda SM. Contributions of the extracellular and cytoplasmic domains of platelet-endothelial cell adhesion molecule-1 (PECAM-1/CD31) in regulating cell-cell localization. J Cell Sci. 2000;113(Pt 8):1459–1469. doi: 10.1242/jcs.113.8.1459. [DOI] [PubMed] [Google Scholar]
- Sun J, Williams J, Yan HC, Amin KM, Albelda SM, Delisser HM. Platelet endothelial cell adhesion molecule-1 (PECAM-1) homophilic adhesion is mediated by immunoglobulin-like domains 1 and 2 and depends on the cytoplasmic domain and the level of surface expression. J Biol Chem. 1996a;271:18561–18570. doi: 10.1074/jbc.271.31.18561. [DOI] [PubMed] [Google Scholar]
- Sun QH, Delisser HM, Zukowski MM, Paddock C, Albelda SM, Newman PJ. Individually distinct Ig homology domains in PECAM-1 regulate homophilic binding and modulate receptor affinity. J Biol Chem. 1996b;271:11090–11098. doi: 10.1074/jbc.271.19.11090. [DOI] [PubMed] [Google Scholar]
- Sun QH, Paddock C, Visentin GP, Zukowski MM, Muller WA, Newman PJ. Cell surface glycosaminoglycans do not serve as ligands for PECAM-1. PECAM-1 is not a heparin-binding protein. J Biol Chem. 1998;273:11483–11490. doi: 10.1074/jbc.273.19.11483. [DOI] [PubMed] [Google Scholar]
- Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, Potente M, Daly C, Dimmeler S, Dejana E. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol. 2008;10:923–934. doi: 10.1038/ncb1752. [DOI] [PubMed] [Google Scholar]
- Tai LK, Zheng Q, Pan S, Jin ZG, Berk BC. Flow activates ERK1/2 and endothelial nitric oxide synthase via a pathway involving PECAM1, SHP2, and Tie2. J Biol Chem. 2005;280:29620–29624. doi: 10.1074/jbc.M501243200. [DOI] [PubMed] [Google Scholar]
- Thompson RD, Noble KE, Larbi KY, Dewar A, Duncan GS, Mak TW, Nourshargh S. Plateletendothelial cell adhesion molecule-1 (PECAM-1)-deficient mice demonstrate a transient and cytokinespecific role for PECAM-1 in leukocyte migration through the perivascular basement membrane. Blood. 2001;97:1854–1860. doi: 10.1182/blood.v97.6.1854. [DOI] [PubMed] [Google Scholar]
- Tourdot BE, Brenner MK, Keough KC, Holyst T, Newman PJ, Newman DK. Immunoreceptor tyrosine-based inhibitory motif (ITIM)-mediated inhibitory signaling is regulated by sequential phosphorylation mediated by distinct nonreceptor tyrosine kinases: a case study involving PECAM-1. Biochemistry. 2013;52:2597–2608. doi: 10.1021/bi301461t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- Vandenbroucke E, Mehta D, Minshall R, Malik AB. Regulation of endothelial junctional permeability. Ann N Y Acad Sci. 2008;1123:134–145. doi: 10.1196/annals.1420.016. [DOI] [PubMed] [Google Scholar]
- Wang S, Dangerfield JP, Young RE, Nourshargh S. PECAM-1, alpha6 integrins and neutrophil elastase cooperate in mediating neutrophil transmigration. J Cell Sci. 2005;118:2067–2076. doi: 10.1242/jcs.02340. [DOI] [PubMed] [Google Scholar]
- Wang Y, Repyak K, Sheibani N. Expression pattern of alternatively spliced PECAM-1 isoforms in retinal vasculature. Mol Vis. 2004;10:103–111. [PubMed] [Google Scholar]
- Wang Y, Sheibani N. Expression pattern of alternatively spliced PECAM-1 isoforms in hematopoietic cells and platelets. J Cell Biochem. 2002;87:424–438. doi: 10.1002/jcb.10321. [DOI] [PubMed] [Google Scholar]
- Wang Y, Sheibani N. PECAM-1 isoform-specific activation of MAPK/ERKs and small GTPases: implications in inflammation and angiogenesis. J Cell Biochem. 2006;98:451–468. doi: 10.1002/jcb.20827. [DOI] [PubMed] [Google Scholar]
- Wang Y, Su X, Sorenson CM, Sheibani N. Modulation of PECAM-1 expression and alternative splicing during differentiation and activation of hematopoietic cells. J Cell Biochem. 2003a;88:1012–1024. doi: 10.1002/jcb.10451. [DOI] [PubMed] [Google Scholar]
- Wang Y, Su X, Sorenson CM, Sheibani N. Tissue-specific distributions of alternatively spliced human PECAM-1 isoforms. Am J Physiol Heart Circ Physiol. 2003b;284:H1008–H1017. doi: 10.1152/ajpheart.00600.2002. [DOI] [PubMed] [Google Scholar]
- Woodfin A, Voisin MB, Imhof BA, Dejana E, Engelhardt B, Nourshargh S. Endothelial cell activation leads to neutrophil transmigration as supported by the sequential roles of ICAM-2, JAM-A, and PECAM-1. Blood. 2009;113:6246–6257. doi: 10.1182/blood-2008-11-188375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodfin A, Voisin MB, Nourshargh S. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler Thromb Vasc Biol. 2007;27:2514–2523. doi: 10.1161/ATVBAHA.107.151456. [DOI] [PubMed] [Google Scholar]
- Wu J, Sheibani N. Modulation of VE-cadherin and PECAM-1 mediated cell-cell adhesions by mitogen-activated protein kinases. J Cell Biochem. 2003;90:121–137. doi: 10.1002/jcb.10600. [DOI] [PubMed] [Google Scholar]
- Yan HC, Baldwin HS, Sun J, Buck CA, Albelda SM, Delisser HM. Alternative splicing of a specific cytoplasmic exon alters the binding characteristics of murine platelet/endothelial cell adhesion molecule-1 (PECAM-1) J Biol Chem. 1995;270:23672–23680. doi: 10.1074/jbc.270.40.23672. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Christofidou-Solomidou M, Garlanda C, Delisser HM. Antibody against murine PECAM-1 inhibits tumor angiogenesis in mice. Angiogenesis. 1999;3:181–188. doi: 10.1023/a:1009092107382. [DOI] [PubMed] [Google Scholar]
- Zhu JX, Cao G, Williams JT, Delisser HM. SHP-2 phosphatase activity is required for PECAM-1-dependent cell motility. Am J Physiol Cell Physiol. 2010;299:C854–C865. doi: 10.1152/ajpcell.00436.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]