Abstract
Poly (ADP-ribose) polymerase 1 (PARP-1) is a constitutive enzyme, the major isoform of PARP family, which is involved in the regulation of DNA repair, cell death, metabolism, and inflammatory responses. Pharmacological inhibitors of PARP provide significant therapeutic benefits in various preclinical disease models associated with tissue injury and inflammation. However, our understanding the role of PARP activation in the pathophysiology of liver inflammation and fibrosis is limited. In this study we have investigated the role of PARP-1 in the liver inflammation and fibrosis using acute and chronic models of CCl4-induced liver injury and fibrosis, a model of bile duct ligation (BDL)-induced hepatic fibrosis in vivo, and isolated liver-derived cells ex vivo. Pharmacological inhibition of PARP with structurally distinct inhibitors or genetic deletion of PARP-1 markedly attenuated CCl4-induced hepatic cell death, inflammation, and fibrosis. Interestingly, the chronic CCl4-induced liver injury was also characterized by mitochondrial dysfunction and dysregulation of numerous genes involved in metabolism. Most of these pathological changes were attenuated by PARP inhibitors. PARP inhibition not only prevented CCl4-induced chronic liver inflammation and fibrosis, but was also able to reverse these pathological processes. PARP inhibitors also attenuated the development of BDL-induced hepatic fibrosis in mice. In liver biopsies of subjects with alcoholic or hepatitis B-induced cirrhosis, increased nitrative stress and PARP activation was noted. These results, taken together, suggest that the reactive oxygen/nitrogen species-PARP pathway plays a pathogenetic role in the development of liver inflammation, metabolism and fibrosis. Several PARP inhibitors are currently in clinical trials for oncological indications. The current results indicate that liver inflammation and liver fibrosis may be additional clinical indications where PARP inhibition may be of translational potential.
Introduction
Globally, alcohol consumption, viral hepatitis and steatohepatitis are the leading causes of chronic liver injury and inflammation, resulting in liver fibrosis, cirrhosis, eventually culminating in the development of hepatocellular carcinoma. Liver fibrosis is the major cause of morbidity and mortality amongst all chronic liver diseases(1). Liver fibrosis is characterized by increased deposition of extracellular matrix (ECM) (2), resulting in the disruption of cellular architecture. Derangement of the sinusoidal architecture not only affects hepatocytes, but also exerts adverse effects on other non-parenchymal cell types such as hepatic stellate cells (HSCs), myofibroblasts and endothelial cells, the function of which would be essential in the maintenance of liver structure and function(3, 4).Activation of HCSs plays a pivotal role in liver fibrogenesis, since these cells are the primary source of ECM deposition upon liver injury(4). Although our knowledge on the pathomechanisms of liver fibrosis has expanded over the past decade, the clinical options for the treatment of this devastating condition remain severely limited.
Poly (ADP-ribose) polymerase1 (PARP-1) is a constitutively expressed primarily nuclear enzyme, which plays important physiological roles in regulation of numerous cellular processes, such as DNA repair and the maintenance of chromatin structure. In addition, pathological over-activation of PARP-1, due to reactive oxygen and nitrogen species formation, promotes cell death, and stimulates pro-inflammatory mediator production(5). PARP-1 functions as a DNA damage sensor and signaling molecule, binding to both single- and double-stranded DNA breaks. Upon binding to damaged DNA, PARP-1 forms homodimers and catalyzes the cleavage of NAD+ into nicotinamide and ADP-ribose to form long branches of ADP-ribose polymers on target proteins such as histones and PARP-1 itself, which results in cellular energetic depletion, mitochondrial dysfunction, and ultimately necrosis. Numerous transcription factors (e.g. nuclear factor-κB), DNA replication factors, and signaling molecules have also been shown to become poly(ADP-ribosylated) by PARP-1(6, 7). By modulating these processed PARP inhibitors have been shown to exert tissue protective and anti-inflammatory effects in animal models of ischemia-reperfusion injury, circulatory shock, and various forms of inflammation(5, 8). It has also been recently suggested that PARP-1 and PARP-2 (a minor isoform of PARP enzyme family) play important roles in regulating important metabolic functions in rodents (e.g. mitochondrial function/biogenesis, and adipogenesis) in various organ systems, including in the liver, at least in part via modulation of NAD+ levels and consequently sirtuin 1 activity(9-12). Furthermore, PARP inhibition has recently been shown to improved mitochondrial function (respiration, enzyme activity, reactive oxygen species defense) in both C. elegans worms and in AML12 hepatocyte cell line, and promoted longevity in worms(13).
Recent studies have linked PARP-1 activation and up-regulation to the production of pro-fibrotic markers such as connective tissue growth factor (CTGF) (14) and transforming growth factor β (TGF-β)(15) in kidney tubular epithelial and vascular smooth muscle cells. In this study, we have investigated the role of PARP-1 in liver inflammation, metabolism and fibrosis using in vivo models of carbon tetrachloride (CCl4)-induced acute and chronic liver injury, a model of bile duct ligation (BDL)-induced hepatic fibrosis, isolated liver-derived cells, and samples from liver biopsies of cirrhotic human subjects. Our findings unveil a pathogenic role of PARP-1 in liver inflammation, metabolism and fibrosis, and indicate the potential therapeutic utility of PARP inhibitors for liver inflammatory diseases and fibrosis.
Materials and Methods
Animals
All the animal protocols conformed to the National Institutes of Health (NIH) guidelines and were approved by the Institutional Animal Care and use Committee of the National Institute on Alcohol Abuse and Alcoholism ((Bethesda, MD, USA) and/or INSERM (Créteil, France). 6- to 8-week-old male C57BL/6J mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). PARP1-/- mice on C57BL/6J were generated as previously described(16). Male PARP1-/- mice and their wild type controls (PARP1+/+) were used in the study (termed PARP-/- and WT/PARP+/+ mice in the figures).
Hepatic tissue samples from human subjects
Alcoholic and hepatitis B-associated cirrhotic liver samples (stage 3-4 fibrosis) were collected from donor livers during liver transplantation from the Liver Tissue Cell Distribution System (LTCDS), University of Minnesota. The LTCDs were supported by NIH Contract #N01-DK-7-0004 / HHSN267200700004C. Additional information on the sample preparation, age and gender of the donors is provided in the Supplemental Materials.
The details of the induction of liver injury and fibrosis by CCl4 and bile duct ligation (BDL) and the treatment protocols are described in the Supplemental Methods.
The determination of liver function, histology and immunohistochemistry, quantitative analysis of hepatic fibrosis are described in the Supplemental Methods.
The determination of hepatic PARP and myeloperoxidase activities, 4-hydroxynonenal (4-HNE), 3-nitrotyrosine (3-NT), and hydroxyproline contents, real-time PCR, Western immunoblot analysis are described in the Supplemental Methods.
Other procedures such as isolation and treatments of murine hepatic hepatocytes and stellate cells, cell death determination by flow cytometer and activation of hepatic stellate cells are also described in the Supplemental Methods.
Statistical analysis
All the values were represented as mean ± S.E.M. Statistical analysis of the data was performed by ANOVA followed by Tukey's post-hoc test for multiple comparisons. The analysis was conducted using the GraphPad-Prism4 software. P<0.05 was considered statistically significant.
Results
Acute CCl4 treatment exerts a time-dependent liver injury and PARP activation
To determine the onset of PARP activation and liver injury by CCl4, we performed a time course experiment in an acute liver injury model induced by CCl4. CCl4 exerted a time-dependent hepatic injury peaking as early as 48 hours, as indicated by increased serum ALT activities (Suppl. Fig. 1A). This response was paralleled with enhanced hepatic nitrative and oxidative stress, characterized by increased 3-nitrotyrosine (3-NT;Suppl. Fig. 1B) and 4-hydroxynonenal (4-HNE;Suppl. Fig. 1C) content, and was also associated with a marked increase in PARP activity in the liver tissue (Suppl. Fig. 1D,E).
Genetic ablation of PARP1-/- or its pharmacological inhibition protects mice against acute CCl4 -induced liver injury
Acute liver injury was induced in either PARP1+/+(WT) or PARP1−/− mice with a single injection of CCl4 as described in the supplemental methods section. Mice were sacrificed and their hepatic tissues and serum were subjected to histological and biochemical investigations 48 hours later. First, histological examination revealed massive inflammation and necrosis (Suppl. Fig. 2A) and serum ALT levels revealed significant increase in WT mice treated with CCl4, when compared with WT mice treated with vehicle (Suppl. Fig. 2B). Similar trend was observed with hepatic myeloperoxidase (MPO) activities, marker of neutrophil infiltration (Suppl. Fig. 2C). Determination of inflammatory cytokines/chemokines mRNA expression also revealed enhanced expression of TNF-α, IL1-β, MIP1-α, MIP-2, MCP-1, respectively, in WT(PARP1+/+) mice treated with CCl4, when compared to corresponding vehicle controls (Suppl. Fig. 2C). These phenotypic changes were smaller in magnitude in PARP1-/- mice treated with CCl4 when compared to PARP1+/+ mice (Suppl. Fig. 2A-D). Likewise, treatment with either of the two PARP inhibitors used (PJ34 or AIQ) attenuated the CCl4-induced pathological alterations in WT mice (Suppl. Fig. 3A-C). Of note the biochemical parameters measured in the vehicle-treated PARP1-/- mice were comparable to those measured in WT(PARP1+/+) mice.
Genetic ablation of PARP1 protects against chronic CCl4-induced liver injury and inflammation in mice
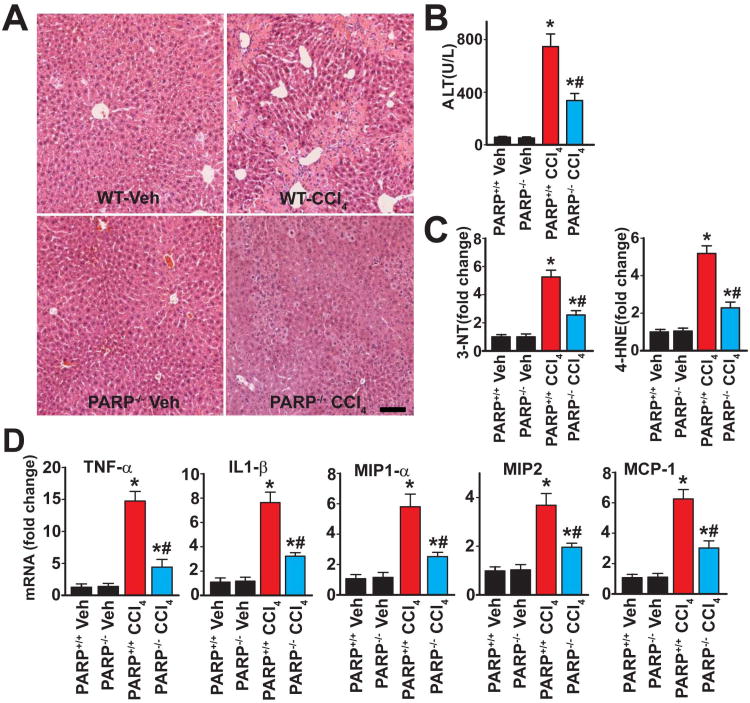
Chronic CCl4 treatment for 4 weeks (3 times/week) induced a significant degree of liver injury, characterized by inflammation and necrosis evidenced by histological examination (Fig. 1A) as well as elevated serum ALT levels (Fig. 1B). This liver injury was accompanied by an increase in hepatic oxidative/nitrative stress markers (3-NT and 4-HNE) in the CCl4 treated group (Fig. 1C) and was also associated with an increase in the mRNA levels for various pro-inflammatory cytokines and chemokines such as TNF-α, IL1-β, MIP1-α, MIP-2, MCP-1 (Fig. 1D), respectively in the CCl4 treated group. The CCl4-induced histological changes, oxidative/nitrative processes and pro-inflammatory responses were markedly attenuated in PARP1-/- mice, indicating that PARP1 plays a pathogenic role in CCl4 induced chronic liver injury (Fig. 1A-D).
Fig. 1. Genetic ablation of PARP1 attenuates CCl4-induced chronic liver injury and inflammation.
Livers of mice treated exposed to CCl4 for 4 weeks were excised and processed for histological and biochemical investigations. Part (A) shows representative H&E stained images of liver sections. The scale depicts 100 μm. (B): Serum ALT levels in the respective groups, n=6-8/group, *P<0.05 vs. WT (PARP1+/+)/PARP1-/- mice treated with vehicle; #P<0.05 vs. WT+CCl4. Part (C) depicts oxidative/nitrative stress markers 4-HNE and 3-NT, n=8/group, *P<0.05 vs. WT/PARP1-/- mice treated with vehicle; #P<0.05 vs. WT+CCl4. Part (D) shows the mRNA expression of pro-inflammatory cytokines (TNF- α, IL1- β) and chemokines (MIP1- α, MIP2, MCP-1) in hepatic tissues following 4 weeks of CCl4 administration. n=8/group, *P<0.05 vs. WT/PARP1-/- mice treated with vehicle; #P<0.05 vs. WT+CCl4.
Absence of PARP1 protects against chronic CCl4 -induced liver fibrosis in mice
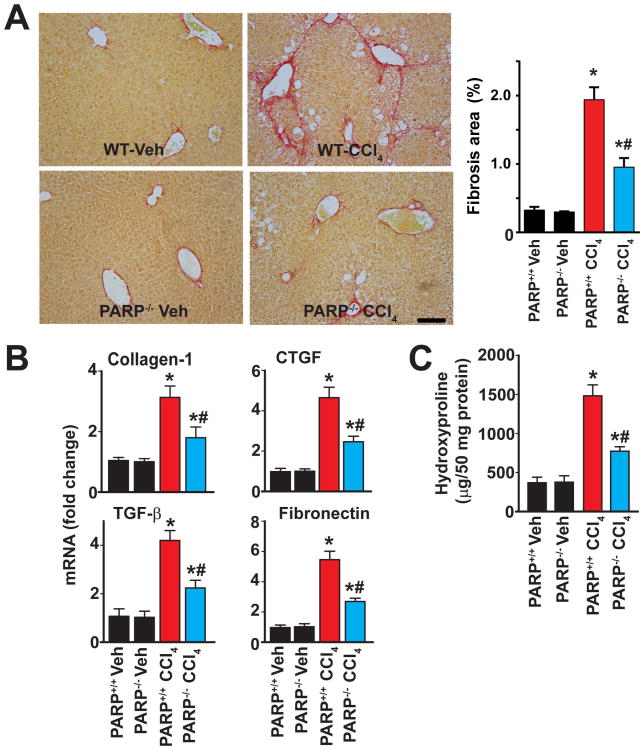
4 weeks of chronic CCl4 treatment induced development of significant liver fibrosis and stellate cell activation, as evidenced by enhanced Sirius red staining (Fig. 2A) and expression of alpha smooth muscle actin (α-SMA), markers of fibrosis and hepatic stellate action, respectively (Suppl. Fig. 4A,B), and mRNA expression of the prototypical pro-fibrotic markers such as collagen-1, connective tissue growth factor (CTGF), transforming growth factor-β (TGF-β) and fibronectin (Fig. 2B). The liver fibrosis was also evidenced by a significant increase in hydroxyproline levels, another marker of fibrosis (Fig. 2C). All of the above-mentioned pro-fibrotic changes were significantly abrogated in PARP1-/- mice (Fig. 2 and Suppl. Fig. 4A), suggesting that PARP1 plays a pathogenetic role in liver fibrosis.
Fig. 2. Genetic ablation of PARP1 attenuates CCl4 induced chronic liver fibrosis.
Part (A) shows representative images of paraffin embedded liver sections stained with Sirius red, a marker of fibrosis, and its quantification from n=4-7 livers/group. The scale depicts 100 μm. Part (B) shows mRNA expression of fibrosis markers (collagen-1, CTGF, TGF- β, and fibronectin) and part (C) denotes the levels of hydroxyproline in hepatic tissues from respective groups of mice exposed to CCl4 for 4 weeks, n=8/group, *P<0.05 vs. WT(PARP1+/+)/PARP1-/- mice treated with vehicle; # P<0.05 vs. WT+CCl4.
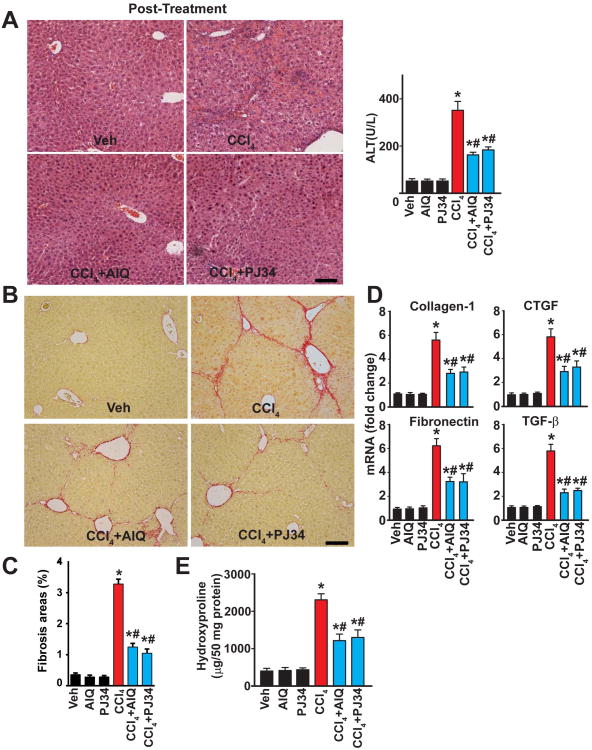
Pharmacological inhibition of PARP ameliorates CCl4-induced chronic liver injury, inflammation, mitochondrial injury/dysfunction, metabolic dysregulation, and fibrosis
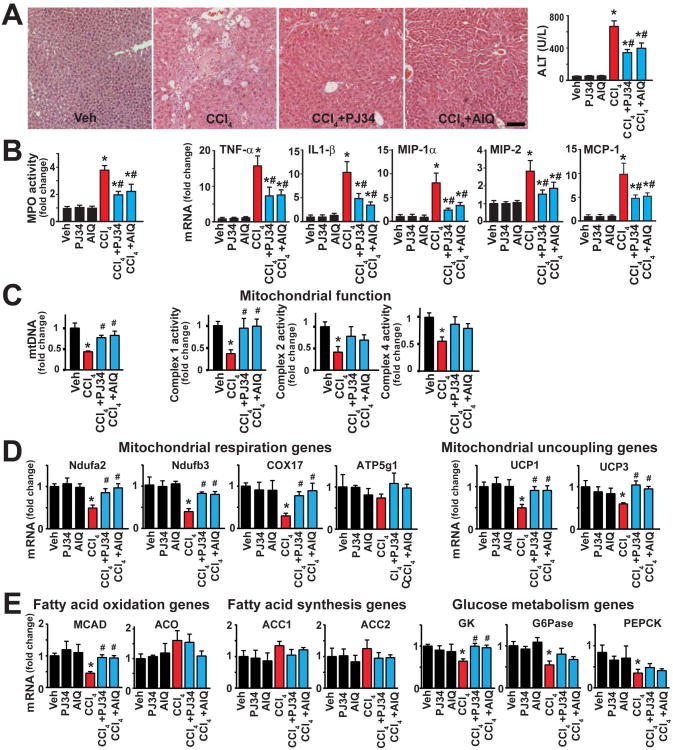
Similar to the effect of PARP1 genetic deficiency, treatment of mice with either of the two structurally distinct PARP inhibitors used (PJ34 and AIQ), markedly attenuated the 4 weeks of chronic CCl4 treatment-induced hepatic injury (evidenced by less histological injury and reduced serum ALT levels) (Fig. 3A), as well as attenuated inflammation (diminished hepatic myeloperoxidase (MPO) activity and reduced mRNA levels of the pro-inflammatory cytokines/chemokines) (Fig. 3B).
Fig. 3. Pharmacological inhibition of PARP ameliorates CCl4-induced chronic liver injury, mitochondrial dysfunction, metabolic dysregulation and inflammation.
Part (A) shows representative H&E images from the respective groups (the scale depicts 100 μm) and serum ALT levels. Part (B) shows hepatic myeloperoxidase (MPO) activities (marker of neutrophil infiltration) and mRNA expression of pro-inflammatory cytokines (TNF-α, IL1-β) and chemokines (MIP1-α, MIP2, MCP-1) in hepatic tissues following 4 weeks of chronic CCl4 exposure, respectively. Part (C) shows determination of mitochondrial DNA content(left) and quantification of mitochondrial complex 1(I), 2(II) and 4(IV) activities(right). Part (D) shows expression of several mitochondrial respiration and uncoupling genes by Realtime PCR. Part (E) shows quantification of fatty acid oxidation (left), fatty acid synthesis (middle) and glucose metabolism (right) genes. All mRNA level is measured by Realtime PCR. n=6-12/group for experiments in panels A-E. *P<0.05 vs. vehicle; #P<0.05 CCl4 vs. CCl4+PJ34/AIQ.
Because, as mentioned in the introduction, PARP1 and PARP2 were recently implicated in regulation of key metabolic functions, including adipogenesis in the liver, we investigated the potential role of these effects in our 4 weeks CCl4 treatment-induced hepatic injury model. Following CCl4 administration we could not find significant changes in liver lipid accumulation as determining by Oil Red O staining and triglyceride content measurements neither with or without treatments with PARP inhibitors AIQ and PJ34 (Suppl. Fig. 6A,B), in contrast to the positive control livers from a chronic alcohol feeding model. Thus, the beneficial effect of PARP inhibition on adipogenesis is not likely to be involved in the anti-inflammatory and anti-fibrotic effects of PARP inhibitors observed in our study.
However, we found that chronic CCl4 treatment induced marked attenuation of mitochondrial DNA (mtDNA) number and mitochondrial function measured by complex 1, 2 and 4 activities from the livers (Fig. 3C). These pathological changes were attenuated by PARP inhibition.
Chronic CCl4 treatment was also associated with dysregulation of numerous genes involved in metabolism (e.g. mitochondrial respiration, fatty acid oxidation, and glucose metabolism; Fig. 3 D,E); and some of these pathological changes could be attenuated by PARP inhibitors (Fig 3 D,E, and Suppl, Methods for description of genes), supporting an important role of PARP in cellular metabolism, particularly in the mitochondria, which is in agreement with a recent study using worms and AML12 hepatocytes (13).
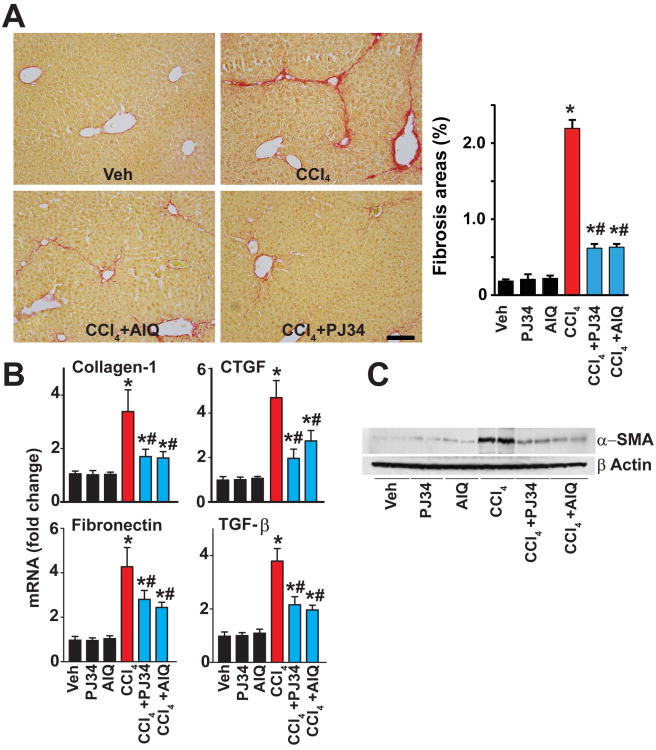
Moreover, PARP inhibition attenuated the chronic CCl4 treatment-induced fibrosis and stellate cell activation, as evidenced by attenuated Sirius red staining, reduced α-SMA protein expression (Fig. 4A; Suppl. Fig. 4B) and mRNA expressions of the pro-fibrotic markers collagen-1, connective tissue growth factor, TGF-β, fibronectin and α-SMA protein (Fig. 4B,C).
Fig. 4. Pharmacological inhibition of PARP mitigates CCl4-induced chronic liver fibrosis.
Part (A) shows representative images of paraffin embedded liver sections stained with Sirius red, a marker of fibrosis, and its quantification from n=4-7 livers/group. The scale depicts 100 μm. Part (B) shows mRNA expression of fibrosis markers (collagen-1, CTGF, TGF-β, and fibronectin) in the hepatic tissues following 4 weeks of chronic CCl4 exposure, n=8/group, *P<0.05 vs. vehicle/PJ34/AIQ alone; #P<0.05 CCl4 vs. CCl4+PJ34/AIQ. Part (C) is a representative immunoblot of α-SMA (marker of stellate cell activation) in the hepatic tissue lysates from the respective groups.
PARP inhibition or genetic deficiency of PARP1 protects against oxidant-induced cell death in primary hepatocytes in vitro
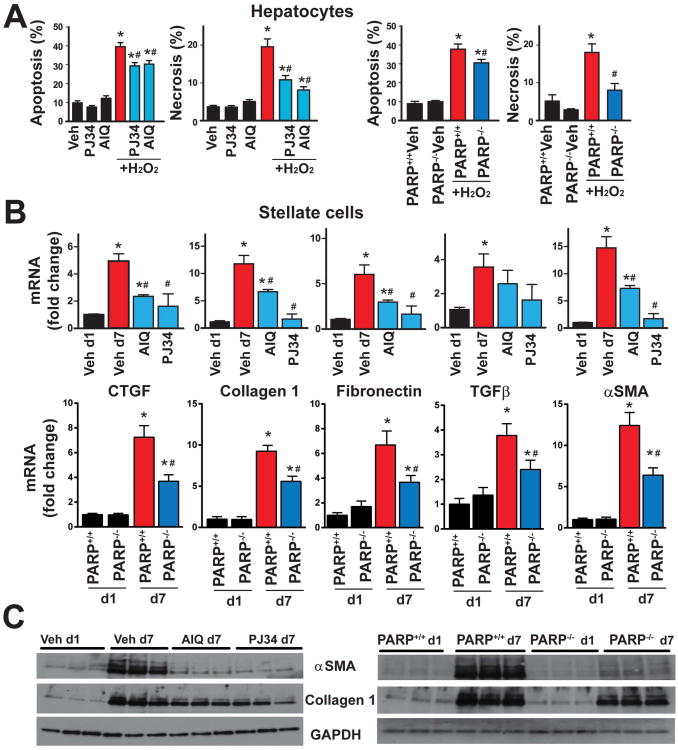
To gain functional insight into the mode of the regulation of hepatocyte death by PARP1, primary hepatocytes were isolated from WT (PARP1+/+) or PARP1-/- mice and treated with H2O2 either in the absence or presence of PARP inhibitors. Then cell death was determined by flow cytometry at 16h. Genetic deletion of PARP1 or its pharmacological inhibition provided marked protection, predominantly by reducing the percentage of H2O2 induced necrotic cell population (Fig. 5A), while only slightly affecting the percentage of the apoptotic cell population (Fig. 5A).
Fig.5. PARP inhibition or genetic deficiency of PARP1 protects against oxidant induced cell death in primary mouse hepatocytes and attenuates hepatic stellate cell activation.
Part (A): isolated mouse primary hepatocytes from either WT (PARP1+/+) or PARP1-/- mice were treated with hydrogen peroxide (H2O2; 3 mM) in the presence or absence of PARP inhibitors (AIQ and PJ34, 10 μM) for 16hr and normal cell population and populations of cells exhibiting the marks of either necrotic or apoptotic death were simultaneously determined by flow cytometry as described in Supplemental Methods. n=6-8/group, *P<0.05 vs. vehicle/PJ34/AIQ alone or PARP1+/+ cells treated with vehicle; #P<0.05 H2O2 vs. H2O2+PJ34/AIQ, respectively. (B): Hepatic stellate cells were isolated from WT (PARP1+/+) or PARP1-/- mice, and were maintained in the 10% FBS medium alone or with PARP inhibitors for 7 days and mRNA expression (Fig. 5B) or protein (Fig. 5C) of stellate cell activation and fibrosis markers (collagen-1, CTGF, TGF-β, and fibronectin) were determined, n=6-8/group, *P<0.05 vs. vehicle at Day 1 vs. Day 7 with or without PARP inhibitors; #P<0.05 vehicle at 7 days vs. AIQ/PJ34 at 7 days or PARP+/+ vs. PARP-/- cells at Day 7.
PARP inhibition or genetic deficiency of PARP1 attenuates murine hepatic stellate cell activation (HSC) in vitro
Activation of HSC and proliferation plays significant role in the liver fibrosis. Because previous studies suggested that PARP1 activation leads to up-regulation of the production of pro-fibrotic markers such as CTGF and TGF-β in kidney tubular epithelial and vascular smooth muscle cells (14, 15), and we found attenuation of chronic CCl4-induced fibrosis and expression of α-SMA by either PARP inhibition or deletion of PARP1, we examined role of PARP1 in the process of HSC activation. HSCs were isolated from murine livers and maintained for 7 days alone or in the presence or absence of PARP inhibitors, followed by the determination of HSC activation markers by reverse transcription - quantitative PCR and Western blotting. Both PARP inhibition with AIQ and PJ34 or genetic deletion of PARP1 significantly attenuated stellate cell activation, as evidenced by the significant attenuation of the mRNA expression of the HSC activation markers α-SMA, CTGF, collagen-1, fibronectin and TGFβ (Fig. 5B), and protein expression of α-SMA and collagen-1 (Fig. 5C). These data suggest that PARP1 plays an important role in the HSC activation.
Pharmacological inhibition of PARP reverses chronic CCl4-induced liver injury and fibrosis
In the next series of experiments we have delayed the start of PARP inhibitor treatment to 4 weeks after the start of CCl4 administration. This is a time point when significant fibrosis and stellate cell activation are already developed (Figs. 2,4; Suppl. Fig. 4). PARP inhibitor treatment continued for an additional 4-week period in the presence of CCl4. Using this protocol we were able to determine whether the development of fibrosis could be attenuated when PARP inhibition is applied to an on-going pro-fibrotic process. Treatment of mice with CCl4 for 8 weeks induced significant histopathological liver injury (Fig. 6A) and fibrosis (Fig. 6B-D), characterized by a significant elevation in ALT levels (Fig. 6A), increased Sirius red staining and α-SMA expression (Fig. 6B,C; Suppl. Fig. 5), as well as an enhanced mRNA expression of the pro-fibrotic markers collagen-1, CTGF, TGF-β and fibronectin (Fig. 6D) and hydroxyproline (Fig. 6E), all quantified at 8 weeks. When 4 weeks of chronic CCl4 treatment was followed by 4 weeks of CCl4 in the presence of either of the two PARP inhibitors (PJ34 or AIQ), the degree of liver injury, quantified at the end of the experiments (8 weeks after the start of CCl4) was markedly attenuated compared to the group that received CCl4 for 8 weeks without PARP inhibitors (Fig. 6). These findings indicate that PARP inhibition enhances the recovery of liner injury and attenuates the development of further fibrosis even when it is administered at a stage of the disease where established fibrosis is present and all relevant pro-fibrotic pathological processes continue.
Fig. 6. Pharmacological inhibition of PARP attenuates the established liver injury and fibrosis.
Initially mice were treated with CCl4 or vehicle for 4 weeks. After reaching 4 weeks of CCl4 or vehicle treatment mice continued to receive CCl4, vehicle, PARP inhibitors alone or PARP inhibitors in combination with CCl4 for additional 4 weeks. (A): Representative H&E images of liver sections and serum ALT levels. The scale depicts 100 μm. Part (B) shows the representative liver sections stained for Sirius red. The scale depicts 100 μm. Part (C) shows quantification of fibrosis based on Sirius red staining from 4-7livers/group. Part (D) depicts mRNA expression of fibrotic markers (collagen-1, CTGF, TGF-β, and fibronectin) and part (E) demonstrates the changes in hydroxyproline content in the hepatic tissues of mice exposed to CCl4 for 8 weeks or CCl4 for 8 weeks+PARP inhibitors (AIQ/PJ34) during the last 4 weeks of the CCl4 administration, n=8/group, *P<0.05 vs. vehicle/PJ34/AIQ alone; # P< 0.05 CCl4 vs. CCl4 + PJ34/AIQ.
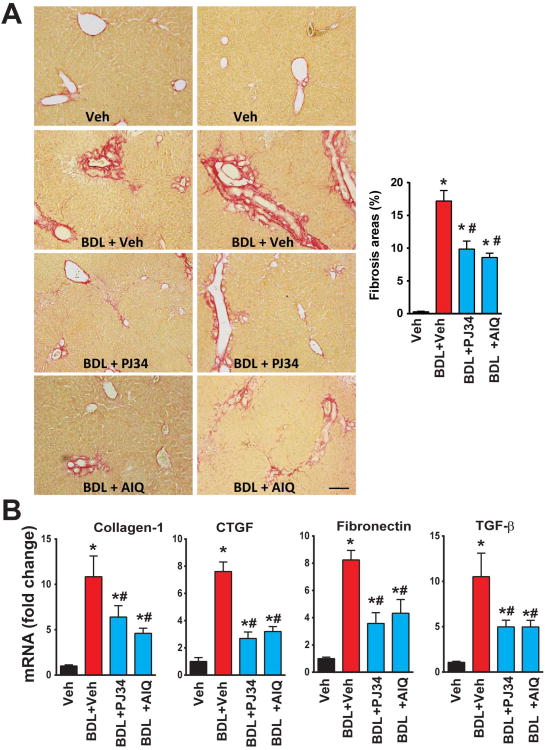
Pharmacological inhibition of PARP attenuates the bile duct ligation (BDL)-induced liver fibrosis
The limitation of the CCl4-induced hepatic fibrosis model is that it largely depends on repetitive parenchymal injury evoked by multiple doses of CCl4. Therefore, we also investigated the effects of PARP inhibition using the bile duct ligation (BDL) -induced liver fibrosis model, which is characterized by less parenchymal injury (Fig. 7). Two weeks following BDL there was marked increase in hepatic periductal fibrosis (Sirius red staining, Fig. 7A) and mRNA expressions of various pro-fibrotic mediators (Fig. 7B). These pathological changes were largely attenuated by treatment with PARP inhibitors (Fig. 7A,B), suggesting that PARP inhibition also exerts direct inhibitory effects on fibrotic process, in addition to attenuating parenchymal injury.
Fig. 7. Pharmacological inhibition of PARP attenuates the bile duct ligation (BDL)-induced liver fibrosis.
(A): Representative liver sections stained for Sirius red 2 weeks following the induction of BDL (left) and quantification of fibrosis staining (right) from n=4-7 livers/group. The scale depicts 100 μm. Part (B) depicts mRNA expression of fibrotic markers (collagen-1, CTGF, TGF-β, and fibronectin) in the hepatic tissues of mice exposed to BDL for 2 weeks or BDL for 2 weeks+PARP inhibitors (AIQ/PJ34) during the 2 weeks period, n=8/group, *P<0.05 vs. vehicle; # P< 0.05 BDL vs. BDL+ PJ34/AIQ.
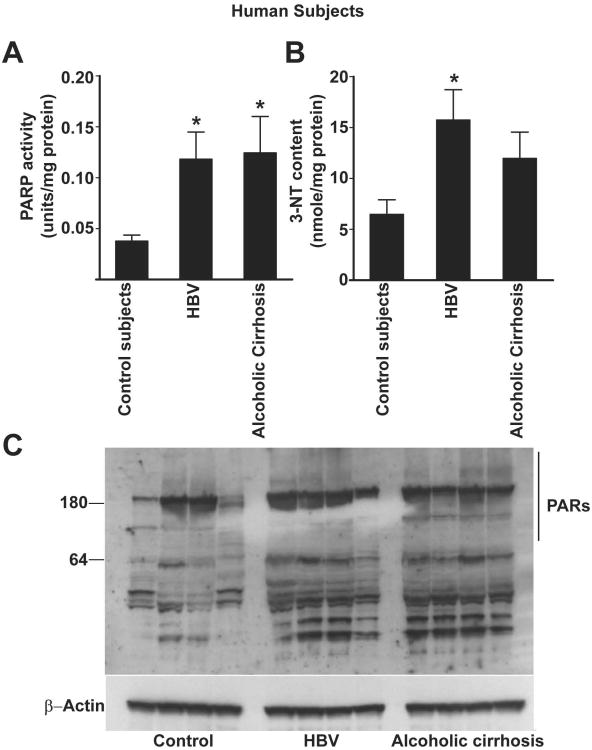
PARP activity is elevated in human subjects with alcoholic and viral hepatitis-induced cirrhosis
To determine whether human liver fibrosis is also associated with PARP-1 activation, we have measured PARP-1 activation in hepatic tissues obtained from subjects with either alcoholic- or viral hepatitis-induced cirrhosis (Fig. 8A). There was a significant increase in PARP activity (∼3 fold) in these tissues, when compared with control subjects, which was also associated with increased tissue levels of 3-NT (Fig. 8B). The increased PARP activity was also corroborated by the detection of increased amounts of poly(ADPribosyl)ated proteins in hepatic tissue lysates from subjects with liver fibrosis (Fig. 8C).
Fig. 8. PARP activation in hepatic tissues from human subjects with alcoholic and viral hepatitis B-induced cirrhosis.
Part (A) shows PARP activation in hepatic tissues obtained from human subjects with HBV-induced or alcoholic cirrhosis, n=6/group, P<0.05 vs control subjects. Part (B) depicts the amounts of the oxidative/nitrative marker 3-NT levels in the respective groups, n=6, P<0.05 vs. control subjects. Part (C) shows a representative immunoblot for poly(ADP-ribose) [PAR] proteins of the hepatic tissue lysates from the respective groups. Note the enhanced PAR proteins in the samples obtained from subjects with alcoholic cirrhosis or viral hepatitis.
Discussion
The novel findings arising from our study are: (a) pharmacological inhibition of PARP or genetic deletion of PARP1 ameliorates the acute and chronic CCl4 treatment-induced oxidative stress, liver injury and inflammation in murine models; (b) pharmacological inhibition of PARP or genetic ablation of PARP1 also attenuates liver fibrosis induced by chronic CCl4 exposure; (c) inhibition of PARP or genetic deletion of PARP1 in hepatocytes protects these cells from oxidant induced cell necrosis; (d) inhibition of PARP or genetic deletion of PARP1 abrogates HSCs activation; (e) PARP inhibitors facilitate the recovery of the liver after established liver fibrosis and also attenuate the development of fibrosis induced by BDL, which is less dependent on parenchymal injury; (f) PARP inhibitors facilitate the recovery of mitochondrial and various metabolic functions; (g) PARP1 activity and PAR accumulation is markedly enhanced in hepatic tissues obtained from patients with liver cirrhosis. These results indicate that oxidative stress and PARP1 play important pathogenetic roles in liver injury, metabolism, inflammation and fibrosis.
Shiobara and colleagues have reported increased PARP1 expression, activity and PAR accumulation in hepatic tissues from patients with liver cirrhosis and hepatocellular carcinoma (17), which is in agreement with our results. The pathogenetic mechanisms involved in liver fibrogenesis, include oxidative stress and demise of hepatocytes, involvement of an inflammatory cascade resulting in Kupffer cells and HSCs activation, deposition of extracellular matrix, infiltration of inflammatory cells(3, 18). Given the important role of PARP1 in the promotion of oxidative stress-induced cell death, metabolic(9-12) and inflammatory processes(5-7) we hypothesized that PARP1 may represent a key checkpoint in liver fibrosis, and its inhibition could be of therapeutic potential. To gain functional insights on the role of PARP1 in hepatic inflammation, metabolism, and fibrogenesis, we have employed a well-established liver injury models induced by CCl4, and investigated whether PARP inhibition or genetic deletion of PARP1 ameliorates liver inflammation, extracellular matrix deposition and hepatic cellular injury resulting in fibrosis.
CCl4 is metabolized in the liver by cytochrome P450 to generate corresponding free radicals, that attacks the hepatocytes, induces the necrosis of parenchymal cells, augmenting the inflammatory cascade in the liver(18). Consistent with previous studies, we observed that CCl4 administration induced marked hepatic injury, oxidative stress, inflammatory cell infiltration, and impaired liver function. In line with the aforementioned role of PARP1 in mediating cell death and pro-inflammatory responses, here we found that loss of PARP1 or its inhibition was associated with attenuated CCl4-induced hepatic injury and inflammation.
Hepatic fibrogenesis develops on the basis of pre-existent and continuous liver injury, in part due to inflammatory cell infiltration(19). Chronic alterations in hepatic homeostasis are believed to transduce various pro-inflammatory and pro-oxidant signals involved in fibrogenesis(20). Activation of HSC are generally triggered by various cytokines/chemokines and oxidants, including potent mitogens such as platelet derived growth factor and epidermal growth factor(21, 22). Activated Kupffer cells also secret TGF-β, which causes HSC activation(3). Recently, the pivotal role linking PARP1 activation and the pro-fibrotic gene expression such as TGF-β and CTGF has been documented in vascular smooth muscle cells and renal proximal tubular epithelial cells in vitro(14, 15). Furthermore, PARP1 inhibition has been found to abrogate, unilateral ureter obstruction induced CTGF expression and renal interstitial fibrosis(14). CTGF and TGF-β have been also shown to be pivotal mediators of hepatic fibrogenesis(23, 24). Consistent with these observations, we have observed that PARP inhibition/genetic deletion abrogated the CCl4 induced HSC activation and fibrogenesis.
Necrosis of hepatic parenchymal cells is both the consequence of liver injury, as well as an active player in the promotion of local pro-inflammatory processes, which may contribute to the activation of HSCs(2). Profibrotic responses can also be triggered by hepatocyte cell death(25). Thus, pharmacological agents, which could selectively block the death of hepatocytes, may also prevent Kupffer cell and HSC activation and consequent fibrosis. In fact, our in vitro and in vivo experiments show that PARP inhibition or genetic deletion of PARP1 attenuates hepatocyte necrosis and inflammatory response. It is likely that the net effect of these processes is the interruption of multiple positive feed-forward cycles of pro-inflammatory mediator production, pro-fibrotic mediator production, cell death and oxidative and metabolic stress. The attenuation of oxidative and nitrative markers by PARP1 deficiency and by PARP1 inhibitors, in fact, points to the existence of such feed-forward cycles, and their interruption in the absence of functional PARP1. However, our results, demonstrating that PARP inhibition also attenuates the BDL-induced hepatic fibrosis in vivo, which is less dependent on parenchymal injury, coupled with attenuation of stellate cell activation by genetic deletion of PARP1 or its pharmacological inhibition in vitro, strongly suggest that PARP1 may also have direct regulatory effects on fibrotic processes, independent on protecting from parenchymal injury and inflammation.
Recently studies also revealed an important role of PARP1, and the significantly less abundant PARP2, in regulating cellular metabolism (e.g. mitochondrial function, mitochondrial biogenesis, adipogenesis, among others) in multiple organ systems (including in the liver) via modulation of cellular NAD+ supply, and consequently NAD+-dependent deacetylase enzyme functions(9-12). Our observation that PARP inhibition attenuated the CCl4-induced mitochondrial dysfunction, decline in mitochondrial number, dysregulation of various key genes involved in metabolism, also in agreement with important role of PARP1 in metabolic regulation. Furthermore, these results also provide the in vivo proof of the concept support for an elegant recent study demonstrating that PARP inhibition through increased cellular NAD+ levels led to improved mitochondrial homeostasis in worms and various mammalian cells, which was dependent on the activation of the worm sirtuin homolog sir-2.1 and involved induction of mitonuclear protein imbalance, as well as activation of stress signaling via the mitochondrial unfolded protein response with consequent nuclear translocation and activation of FOXO transcription factor DAF-16, promoting longevity(13).
PARP inhibitors also exert beneficial effects in preclinical and clinical models of cancers via multiple mechanisms involving attenuation of cancer cell proliferation and migration, decrease of angiogenesis, modulation of the tumor pro-inflammatory environment, and promotion of cancer cell demise. The selective promotion of apoptotic cell death in cancer, but not in normal cells by PARP inhibitors is based on the novel approach of “ synthetic lethality ” in cancer therapy, because in certain cancers with selective defects in homologous recombination repair (cancer cells frequently harbor defects in DNA repair pathways leading to genomic instability) inactivation of PARP1, and possibly other minor isoforms of PARP, directly causes cell death. Because of this, several classes of ultrapotent PARP inhibitors are currently in clinical trials for the experimental therapy of various malignancies, including triple-negative breast and ovarian cancers(26). The initial concern with chronic PARP inhibition was the potential genomic instability and “ premature aging ” of cells. However, the recent study demonstrating that reduced PARP activity extends lifespan in worms(13), coupled with improved cardiovascular function and energetics in aging rats chronically treated with PARP inhibitors(27, 28), argues that PARP inhibitors will be well tolerated even during prolonged use in humans.
Our results, demonstrating the pivotal pathogenetic role of PARP1 in liver fibrosis, and the potential of PARP1 inhibitors in restoring liver function after fibrosis, coupled with the clinical availability of PARP1 inhibitors, suggest that re-purposing of PARP1 inhibitors for the treatment of liver diseases associated with injury, inflammation and fibrosis may be of future potential clinical utility.
Supplementary Material
Acknowledgments
This study was supported by the Intramural Program of the NIH/NIAAA to PP and BG. CS and AHB were supported by a grant from the NIH P50GM060338 and HL072889. Authors are grateful to Laura Nagy for helpful discussions.
References
- 1.El–Serag HB, Rudolph KL. Hepatocellular Carcinoma: Epidemiology and Molecular Carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Muhanna N, Doron S, Wald O, Horani A, Eid A, Pappo O, Friedman SL, et al. Activation of hepatic stellate cells after phagocytosis of lymphocytes: A novel pathway of fibrogenesis. Hepatology. 2008;48:963–977. doi: 10.1002/hep.22413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott LF. Mechanisms of Hepatic Fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman SL. Hepatic Stellate Cells: Protean, Multifunctional, and Enigmatic Cells of the Liver. Physiological Reviews. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pacher P, Szabo C. Role of the Peroxynitrite-Poly(ADP-Ribose) Polymerase Pathway in Human Disease. Am J Pathol. 2008;173:2–13. doi: 10.2353/ajpath.2008.080019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassa PO, Hottiger MO. The functional role of poly(ADP-ribose)polymerase 1 as novel coactivator of NF-kappaB in inflammatory disorders. Cellular and molecular life sciences: CMLS. 2002;59:1534–1553. doi: 10.1007/s00018-002-8527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hottiger MO, Boothby M, Koch-Nolte F, Luscher B, Martin NM, Plummer R, Wang ZQ, et al. Progressin the function and regulation of ADP-Ribosylation. Science Signaling. 2011;4:mr5. doi: 10.1126/scisignal.2001645. [DOI] [PubMed] [Google Scholar]
- 8.Jagtap P, Szabo C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005;4:421–440. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]
- 9.Bai P, Canto C, Oudart H, Brunyanszki A, Cen Y, Thomas C, Yamamoto H, et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metabolism. 2011;13:461–468. doi: 10.1016/j.cmet.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bai P, Canto C, Brunyanszki A, Huber A, Szanto M, Cen Y, Yamamoto H, et al. PARP-2 regulates SIRT1 expression and whole-body energy expenditure. Cell Metabolism. 2011;13:450–460. doi: 10.1016/j.cmet.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erener S, Mirsaidi A, Hesse M, Tiaden AN, Ellingsgaard H, Kostadinova R, Donath MY, et al. ARTD1 deletion causes increased hepatic lipid accumulation in mice fed a high-fat diet and impairs adipocyte function and differentiation. FASEB J. 2012;26:2631–2638. doi: 10.1096/fj.11-200212. [DOI] [PubMed] [Google Scholar]
- 12.Erener S, Hesse M, Kostadinova R, Hottiger MO. Poly(ADP-ribose)polymerase-1 (PARP1) controls adipogenic gene expression and adipocyte function. Molec Endocrinol. 2012;26:79–86. doi: 10.1210/me.2011-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Canto C, Mottis A, et al. The NAD(+)/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell. 2013;154:430–441. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okada H, Inoue T, Kikuta T, Kato N, Kanno Y, Hirosawa N, Sakamoto Y, et al. Poly(ADP-Ribose) Polymerase-1 Enhances Transcription of the Profibrotic CCN2 Gene. J Am Soc Nephrol. 2008;19:933–942. doi: 10.1681/ASN.2007060648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang D, Wang Y, Wang L, Zhang F, Deng S, Wang R, Zhang Y, et al. Poly(ADP-ribose) Polymerase 1 Is Indispensable for Transforming Growth Factor-β Induced Smad3 Activation in Vascular Smooth Muscle Cell. PLoS ONE. 2011;6:e27123. doi: 10.1371/journal.pone.0027123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukhopadhyay P, Horváth B, Kechrid M, Tanchian G, Rajesh M, Naura AS, Boulares AH, et al. Poly(ADP-ribose) polymerase-1 is a key mediator of cisplatin-induced kidney inflammation and injury. Free Radical Biology and Medicine. 2011;51:1774–1788. doi: 10.1016/j.freeradbiomed.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiobara M, Miyazaki M, Ito H, Togawa A, Nakajima N, Nomura F, Morinaga N, et al. Enhanced polyadenosine diphosphate-ribosylation in cirrhotic liver and carcinoma tissues in patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2001;16:338–344. doi: 10.1046/j.1440-1746.2001.02378.x. [DOI] [PubMed] [Google Scholar]
- 18.Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. Journal Clinical Investig. 2007;117:539–548. doi: 10.1172/JCI30542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao B, Jeong WI, Tian Z. Liver: An organ with predominant innate immunity. Hepatology. 2008;47:729–736. doi: 10.1002/hep.22034. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez-Gea V, Friedman SL. Pathogenesis of Liver Fibrosis. Annual Review of Pathology. 2011;6:425–456. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- 21.Pinzani M, Gesualdo L, Sabbah GM, Abboud HE. Effects of platelet-derived growth factor and other polypeptide mitogens on DNA synthesis and growth of cultured rat liver fat-storing cells. J Clinical Investig. 1989;84:1786–1793. doi: 10.1172/JCI114363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinnman N, Goria O, Wendum D, Gendron MC, Rey C, Poupon R, Housset C. Hepatic Stellate Cell Proliferation is an Early Platelet-Derived Growth Factor-Mediated Cellular Event in Rat Cholestatic Liver Injury. Lab Invest. 2001;81:1709–1716. doi: 10.1038/labinvest.3780384. [DOI] [PubMed] [Google Scholar]
- 23.Tong Z, Chen R, Alt DS, Kemper S, Perbal B, Brigstock DR. Susceptibility to liver fibrosis in mice expressing a connective tissue growth factor transgene in hepatocytes. Hepatology. 2009;50:939–947. doi: 10.1002/hep.23102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inagaki Y, Okazaki I. Emerging insights into Transforming growth factor β Smad signal in hepatic fibrogenesis. Gut. 2007;56:284–292. doi: 10.1136/gut.2005.088690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhan SS, Jiang JX, Wu J, Halsted C, Friedman SL, Zern MA, Torok NJ. Phagocytosis of apoptotic bodies by hepatic stellate cells induces NADPH oxidase and is associated with liver fibrosis in vivo. Hepatology. 2006;43:435–443. doi: 10.1002/hep.21093. [DOI] [PubMed] [Google Scholar]
- 26.Curtin NJ, Szabo C. Therapeutic applications of PARP inhibitors: Anticancer therapy and beyond. Mol Aspects Med. 2013 doi: 10.1016/j.mam.2013.01.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pacher P, Mabley JG, Soriano FG, Liaudet L, Komjati K, Szabo C. Endothelial dysfunction in aging animals: the role of poly(ADP-ribose) polymerase activation. Br J Pharmacol. 2002;135:1347–1350. doi: 10.1038/sj.bjp.0704627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pacher P, Vaslin A, Benko R, Mabley JG, Liaudet L, Hasko G, Marton A, et al. A new, potent poly(ADP-ribose) polymerase inhibitor improves cardiac and vascular dysfunction associated with advanced aging. J Pharmacol Exper Therap. 2004;311:485–491. doi: 10.1124/jpet.104.069658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.