Abstract
Objective
To determine whether copeptin-us can rule out diagnosis of non-ST-segment elevation myocardial infarction (NSTEMI) without prolonged monitoring and serial blood sampling in patients with high-sensitive cardiac troponin I (hs-cTnT) below the 99th centile at presentation to the emergency department (ED).
Design
Prospective, non-randomised, individual blinded diagnostic accuracy study.
Setting
Two EDs of a rural region of France.
Participants
Patients with chest pain suspected of NSTEMI with onset within the last 12 h were considered for enrolment.
Interventions
Serial clinical, electrographical and biochemical investigations were performed at admission and after 2, 4, 6 and 12 h. Hs-cTnT was measured using an assay with Dimension VISTA, Siemens. Copeptin was measured by the BRAHMS copeptin-us assay on the KRYPTOR Compact Plus system. The follow-up period was 90 days.
Primary and secondary outcome measures
Copeptin, troponin, myoglobin and creatine kinase values. Clinical and paraclinical events. The final diagnosis was adjudicated blinded to copeptin result.
Results
During 12 months, 102 patients were analysed. Final diagnosis was NSTEMI for 7.8% (n=8), unstable angina for 3.9% (n=4), cardiac but non-coronary artery disease for 8.8% (n=9), non-cardiac chest pain for 52% (n=53) and unknown for 27.5% (n=28). There was no statistical difference for copeptin values between patients with NSTEMI and others (respectively 5.5 pmol/L IQR (3.1–7.9) and 6.5 pmol/L IQR (3.9–12.1), p=0.49). Only one patient with NSTEMI had a copeptin value above the cut-off of 95th centile at admission.
Conclusions
In this study, copeptin does not add a diagnostic value at admission to ED for patients with suspected acute coronary syndrome without ST-segment elevation and with hs-cTnT below the 99th centile.
Trial registration number
Clinicaltrials.gov identifier: NCT01334645.
Keywords: Accident & Emergency Medicine
Strengths and limitations of this study.
To our knowledge, our prospective multicentric study is the only one that includes only patients with suspected non-ST-segment elevation myocardial infarction and high-sensitive cardiac troponin I below the 99th centile at presentation to the emergency department, to limit spectrum bias.
The main limitation of our study is the number of patients included. Indeed we are below the prevalence. This may be explained by the fact that our study included only patients with negative ultrasensitive troponin at admission. However, this is the only group of patients for which a multimarker rule-out strategy could add diagnostic value.
Moreover, we evaluated the sensitivity troponin and copeptin for all patients with the same assay technique which enabled to control the occurrence of methodological bias.
Introduction
Detection of a rise and/or fall of cardiac troponin with clinical symptoms of ischaemia or abnormal ECG or imaging findings remains the gold standard for the identification of myocardial infarction (MI).1 At an emergency department (ED), patients with non-ST-segment elevation MI (NSTEMI) working diagnosis require serial measurement of troponin.2 However, most of these patients do not have acute coronary syndrome (ACS). Identifying patients suffering from non-life-threatening diseases with only one blood sample is a challenge. Many biomarkers were evaluated, alone or in combination with troponin.3 4 Copeptin accuracy was explored recently in this rule-out diagnostic strategy. This glycopeptide, which is the C-terminal part of the arginine vasopressin (AVP) precursor, is secreted stoichiometrically with AVP from the neurohypophysis. AVP is a marker of endogenous stress but routine measurement of AVP is limited due to its instability and difficulty of the assay.5 Copeptin now appears to be an attractive alternative to AVP because of its stability and development of automated technique for reliable and reproducible dosage.6–8
Since the first publication making this indication in 2009, several studies have investigated copeptin.9–35 Some of these studies suggest that the association of troponin and copeptin at the first measurement has a powerful negative predictive value (NPV) to rule out patients without NSTEMI.
Interpretation of the copeptin diagnostic accuracy through these studies is not evident, first, because analysis comparisons are disrupted by the development of high-sensitivity cardiac troponin T and I assays and the availability of three commercial assays for copeptin (LUMItest, Copeptin Kryptor, Copeptin-us Kryptor). Furthermore, many protocols included patients with STEMI and patients with a high-sensitive cardiac troponin above the 99th centile at admission. For these patients, copeptin does not add diagnostic information, and urgent revascularisation or serial blood samples, respectively, remain necessary.
The aim of this study was to determine whether copeptin-us can rule out diagnosis of acute MI without prolonged monitoring and serial blood sampling in patients with suspected NSTEMI and high-sensitive cardiac troponin I (hs-cTnT) below the 99th centile at presentation to ED.
Methods
Study design and setting
This diagnostic test evaluation is a prospective non-randomised individual blinded multicentric cohort study. The Clermont-Ferrand University Hospital designed and coordinated the study. The duration of the study was 1 year, between March 2011 and March 2012 at the ED of two hospitals of Auvergne, a rural region of France (1.3 million people). The first one, Gabriel Montpied in Clermont-Ferrand, is a teaching hospital and provincial referral centre with 48 000 ED admissions/year. The second hospital, Henri Mondor in Aurillac, is a general hospital with 25 000 ED admissions/year. Each unit had a catheterisation laboratory available 24 h a day.
Population
Consecutive patients admitted with chest pain suspected of NSTEMI in the ED were considered for enrolment in the study. The inclusion criteria were the following: patients older than 18 years with chest pain suggestive of ACS of <12 h duration since its onset. Atypical presentations of NSTEMI are not uncommon,2 therefore the criteria for pain suggestive of ACS were those of usual clinical practice of investigators. It should be non-traumatic. Written informed consent was obtained from all participating patients. Patients with ST-segment elevation, legal incapacity, sepsis, shock, lung neoplasms, terminal kidney failure requiring dialysis and life expectancy of less than 6 months were excluded. After the result of the first blood sample, patients with hyponatraemia <135 mmol/L or hs-cTnT >0.045 μg/L were released of the study.
ST-segment elevation, measured at the J point, was diagnosed according to the third universal definition of MI.1 It should be found in two contiguous leads with the cut-off points: ≥0.1 mV in all leads other than leads V2–V3 where the following cut points apply: ≥0.2 mV in men ≥40 years; ≥0.25 mV in men <40 years, or ≥0.15 mV in women.
Sepsis, shock, lung neoplasms, terminal kidney failure requiring dialysis and hyponatraemia are diseases in which the rate of vasopressin, and thus of copeptin, may be modified. These patients were not included to minimise confounding factors.
Study protocol
On admission, all patients underwent an initial clinical assessment, including medical history, temperature, respiratory rate, cardiac frequency, blood pressure, pulse oxymetry, 18-lead ECG, chest X-ray and screening blood test including C reactive protein, natraemia, creatinine, hs-cTnT and creatine kinase (CK). Risk factors and medical history were collected as stated by the patients, and also the treatment received. Family history of coronary artery disease (CAD) was noted if a member of the first-degree relatives had CAD before 65 years. Blood samples were collected for hs-cTnT and CK analysis and 18-lead ECG was performed after 2, 4, 6 and 12 h. At each time point, blood sample was centrifuged and plasma was frozen at −80°C for copeptin and myoglobin testing at the end of the study recruitment, blinded to final diagnosis. Further investigations and treatment of patients were not modified by the study. At 90 days, clinical events were collected from the patients, their general practitioners and the hospitals where they were examined.
Concentration of copeptin was measured by the BRAHMS copeptin-us immunoluminometric assay on the KRYPTOR Compact Plus system (Thermo Fisher Scientific). The detection limit as described by the manufacturer was signified as being 0.9 pmol/L and the lowest concentration measurable with a coefficient of variation (CV) <10% has been reported <4 pmol/L. The direct measuring range was 0.9–500 pmol/L. The 95th centile among healthy participants was <12.0 pmol/L and was specified for rapid exclusion of acute MI (AMI).
The hs-cTnT was measured using a chemiluminescence test (Dimension VISTA, Siemens Healthcare Diagnostics). The limit of blank of hs-cTnT was 0.015 μg/L, the 99th centile concentration was 0.045 μg/L and the lowest concentration measurable with a CV <10% was 0.040 μg/L according to the manufacturer. The 99th centile (0.045 μg/L) was used as the diagnostic cut-off to fulfil the AMI criteria.
Myoglobin was measured by Dimension VISTA (Siemens Healthcare Diagnostics). The measuring range extended from 0.5 to 1000 μg/L. The 95th centile concentration was 116 for men and 71 μg/L for women. At concentrations of 110 μg/L, the interassay CV was 4.9% and the intra-assay CV was 5%.
Natraemia, C reactive protein, creatinine and CK were measured using standardised methods.
Outcomes
The final diagnosis was adjudicated, blinded to copeptin results, by an expert committee of three cardiologists, four emergency physicians and two biochemists (whose one professor-practitioner of each specialty), with all available medical records from the time of ED presentation to 90-day follow-up. Each participant was classified in the following categories: NSTEMI, unstable angina (UA), cardiac but non-CAD (CNCAD), non-cardiac chest pain (NCCP) and unknown cause of chest pain. The diagnosis was determined according to the current guidelines and universal definition of MI.1 2 The diagnosis of NSTEMI, in these patients showing suspected symptoms of ACS, was defined by a rise and/or fall of hs-cTnT with at least one value above the 99th centile and with the following criteria: imaging evidence of new loss of viable myocardium or new regional wall motion abnormality or identification of an intracoronary thrombus by angiography. The criteria for UA diagnosis were the same as those defining the NSTEMI, but without troponin changes. Diagnosis of CNCAD was performed if a CAD was excluded by additional testing. Diagnosis of NCCP was performed if a cardiac aetiology was excluded. Unknown cause of chest pain diagnosis was defined when no sufficient further diagnostic procedures were performed.
Copeptin and myoglobin measurements were performed at the end of the study recruitment, blinded to the final diagnosis.
Statistical analysis
In order to show a different copeptin value between NSTEMI and non-NSTEMI participants, with an expected difference of 15 pmol/L, an SD of 20.7 pmol/L, a significance level of 5% and a power of 95%, 40 NSTEMI participants were needed.
Continuous variables were displayed either as means±SD or medians and IQR. Categorical variables were described by using frequencies and percentages.
The analysis of quantitative variables was performed using the two-tailed Student's t test after checking the assumption of equal variances (Levene test) and one-way analysis of variance for variables following a normal distribution. Otherwise, the Wilcoxon rank sum tests for continuous variables and Kruskal-Wallis tests were used. Categorical variables were analysed using χ2 analysis or the Fisher exact test (if needed). For all tests, a significant level of p<0.05 was used.
Statistical analysis was performed using SAS (V.9.3, SAS Institute Inc., Cary, North Carolina, USA).
Results
Patient characteristics
During 12 months, 147 patients were assessed for eligibility in both EDs. Nine presented 1 or more exclusion criteria, 6 did not give their informed consent for participation, 26 were released after the results of the first blood sample because they had hyponatraemia <135 mmol/L (n=3) or hs-cTnT >0.045 μg/L (n=23). For three patients, blood samples at presentation were not frozen for copeptin and myoglobin measurement. Only one patient was lost for follow-up. A total of 102 patients were analysed, 62 were recruited at the Clermont-Ferrand University Hospital ED and 40 at the Aurillac General Hospital ED (figure 1).
Figure 1.
Flow chart.
The adjudicated final diagnosis was NSTEMI for 7.8% (n=8), UA for 3.9% (n=4), CNCAD 8.8% (n=9), NCCP for 52% (n=53) and unknown for 27.5% (n=28).
CNCAD included pericarditis (3), supraventricular tachycardia (3), ventricular tachycardia (2) and left hypertrophy (1). Patients with adjudicated diagnosis of NCCP included patients with anxiety (3), stomach disease (4), herpes zoster (1), neoplasms (4), breast haematoma (1), cholecystitis (1) vasovagal syncope (1) and osteoarthritis (2).
Baseline characteristics of each population are shown in table 1.
Table 1.
Baseline characteristics
| Characteristics | All patients | NSTEMI | Non-NSTEMI | p Value |
|---|---|---|---|---|
| Patients, n (%) | 102 (100) | 8 (7.8) | 94 (92.2) | |
| Men, n (%) | 64 (62.7) | 7 (87.5) | 57 (55.9) | 0.25 |
| Age (years), mean (SD) | 59 (16) | 66 (16) | 59 (16) | 0.25 |
| Risk factors | ||||
| Body mass index, kg/m2 (SD) | 26.93 (4.9) | 27.1 (3.7) | 26.9 (5) | 0.94 |
| Family history of CAD, n (%) | 33 (32.3) | 3 (37.5) | 30 (31.9) | 0.71 |
| Hypertension, n (%) | 49 (48) | 5 (62.5) | 44 (46.8) | 0.48 |
| Hyperlipidaemia, n (%) | 51 (50) | 4 (50) | 47 (50) | 1.0 |
| Diabetes mellitus, n (%) | 17 (16.7) | 1 (12.5) | 16 (17) | 1.0 |
| Current smoking, n (%) | 26 (25.5) | 5 (62.5) | 21 (22.3) | 0.02 |
| History of smoking, n (%) | 30 (29.4) | 1 (12.5) | 29 (31.1) | 0.43 |
| History, n (%) | ||||
| CAD | 35 (34.3) | 4 (50) | 31 (33) | 0.44 |
| Previous myocardial infarction | 27 (26.5) | 4 (50) | 23 (24.5) | 0.20 |
| Previous revascularisation | 26 (25.5) | 3 (37.5) | 23 (24.5) | 0.42 |
| History of heart failure | 5 (4.9) | 0 | 5 (5.3) | 1.0 |
| Peripheral artery disease | 6 (5.9) | 2 (25) | 4 (4.3) | 0.07 |
| Previous stroke | 6 (5.9) | 1 (12.5) | 5 (5.3) | 0.4 |
| Clinical status | ||||
| Heart rate, bpm (SD) | 77 (17) | 81 (18) | 77 (17) | 0.5 |
| Systolic blood pressure, mm Hg (SD) | 141 (22) | 149 (28) | 140 (21) | 0.29 |
| Diastolic blood pressure, mm Hg (SD) | 83 (15) | 92 (13) | 82 (14) | 0.06 |
| Respiratory rate, respiratory cycles/min (SD) | 17 (4) | 16 (5) | 17 (4) | 0.53 |
| Temperature, °C (SD) | 36.7 (0.5) | 36.9 (0.2) | 36.7 (0.5) | 0.16 |
| Killip class 1, n (%) | 97 (95) | 8 (100) | 89 (94.7) | 1.0 |
| Killip class 2, n (%) | 5 (5) | 0 | 5 (5.3) | 1.0 |
| Time between pain onset and admission h:min (SD) | 3:48 (2:50) | 2:27 (1:39) | 3:55 (2:53) | 0.16 |
| Biochemical values at admission | ||||
| Natraemia, mmol/L (SD) | 140.3 (2.9) | 137.4 (2.3) | 140.5 (2.8) | 0.0022 |
| Creatinine, μmol/L (SD) | 80.4 (17.5) | 82.3 (18.7) | 80.2 (17.5) | 0.75 |
| MDRD, mL/min/1.73 m2 (SD) | 85.2 (23.5) | 84.1 (19.1) | 85.3 (23.9) | 0.9 |
| CRP, mg/L (SD) | 4.9 (7.7) | 4.6 (6.1) | 4.9 (7.8) | 0.93 |
| Electrocardiographic findings at admission | ||||
| Normal, n (%) | 43 (42.1) | 1 (12.5) | 42 (44.7) | 0.13 |
| Left bundle branch block, n (%) | 0 | 0 | 0 | |
| ST segment elevation, n (%) | 0 | 0 | 0 | |
| ST segment depression, n (%) | 9 (8.82) | 2 (25) | 7 (7.5) | 0.15 |
| T wave inversion, n (%) | 20 (19.6) | 3 (37.5) | 17 (18.1) | 0.19 |
| No significant abnormalities, n (%) | 30 (29.4) | 2 (25) | 28 (29.8) | 1.0 |
| Risk scores | ||||
| GRACE, score (SD) | 96 (31) | 107.8 (25.4) | 95.6 (31.3) | 0.29 |
| TIMI 0, n (%) | 29 (28.4) | 2 (25) | 28 (29.8) | 1.0 |
| TIMI 1, n (%) | 26 (25.5) | 0 | 26 (27.6) | 0.11 |
| TIMI 2, n (%) | 14 (13.7) | 2 (25) | 12 (12.8) | 0.30 |
| TIMI 3, n (%) | 21 (20.6) | 1 (12.5) | 20 (21.3) | 1.0 |
| TIMI 4, n (%) | 9 (8.8) | 3 (37.5) | 6 (6.4) | 0.02 |
| TIMI 5, n (%) | 2 (2) | 0 | 2 (2.1) | 1.0 |
| Explorations | ||||
| Echocardiography, n (%) | 61 (59.8) | 7 (87.5) | 54 (57.4) | 0.14 |
| Cardiac exercice test, n (%) | 47 (46) | 0 | 47 (50) | 0.007 |
| Coronary angiography, n (%) | 19 (18.6) | 7 (87.5) | 12 (12.8) | <0.0001 |
Values are presented as n (%) or mean±SD.
CAD, coronary artery disease; CRP, C reactive protein; GRACE, Global Registry of Acute Cardiac Events; MDRD, Modification of Diet in Renal Disease; NSTEMI, non-ST-segment elevation myocardial infarction; TIMI, thrombosis in myocardial infarction.
Time between pain onset and admission was less than 3 h for 58 patients (56.9%). Twenty-four patients were admitted between 3 and 6 h after the onset of pain (23.5%), 13 patients between 6 and 9 h (12.7%) and 7 patients between 9 and 12 h (6.9%). All patients with a diagnosis of MI were admitted within the first 6 h after the chest pain onset, five of them in the first 3 h. The mean interval between chest pain onset and admission is 147.5 min±99 min for patients with NSTEMI and 235 min±173 min for patients without NSTEMI (p=0.16).
Main results
Serial blood testing
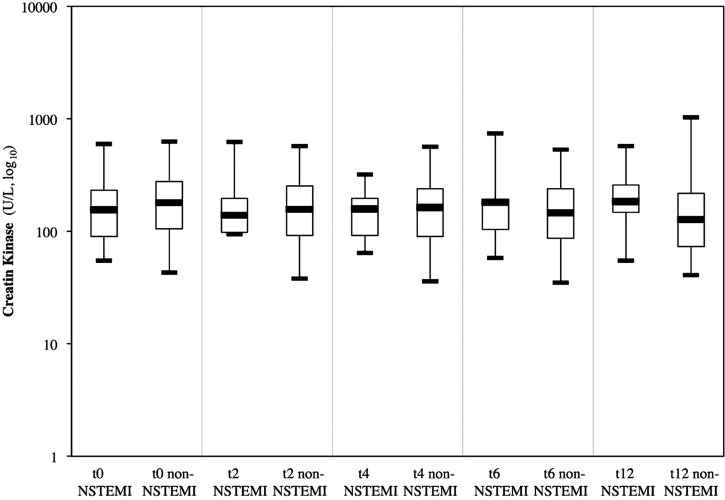
At admission, all patients were recruited for blood testing. Because of therapeutic necessities after inclusion, three patients with NSTEMI did not have all the required blood sampling. Thus, data of the eight patients with NSTEMI are available at H0, data of seven patients with NSTEMI are available at H2, H4 and H6, and data of six patients with NSTEMI at H12. Results of biomarkers are displayed in figures 2–5.
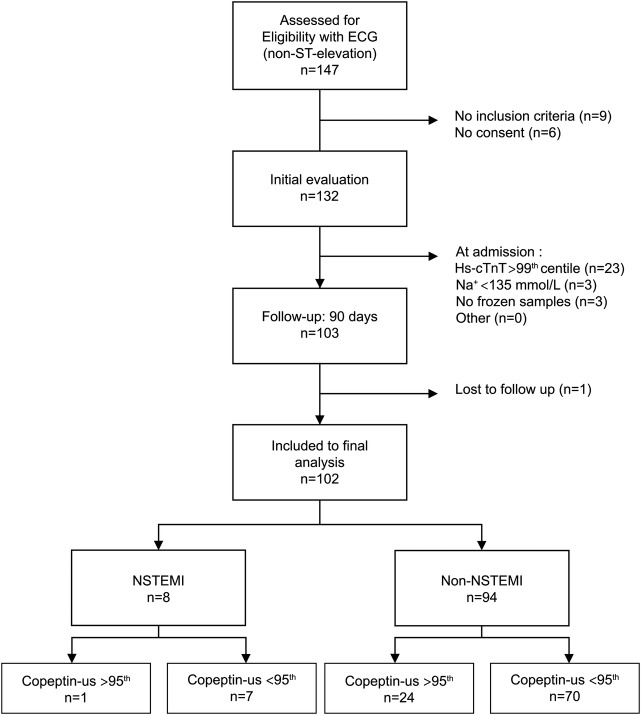
Figure 2.
Box plots (median, IQR, minimal and maximal values) illustrate troponin, copeptin, myoglobin and CK concentration in relation to time since admission for non-ST-segment elevation myocardial infarction (NSTEMI) and patients who are non-NSTEMI. *p<0.0001, **p=0.01, ***p=0.04, ****p=0.03. Hs-cTnT, high-sensitive cardiac troponin I.
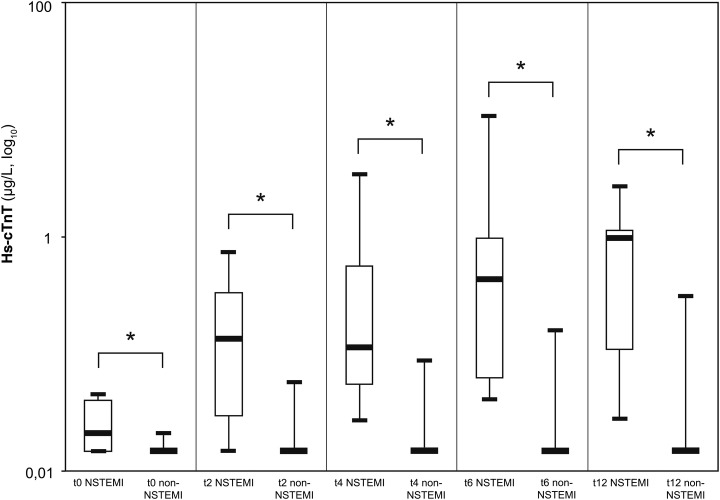
Figure 3.
Box plots (median, IQR, minimal and maximal values) illustrate Troponin, Copeptin, myoglobin and CK concentration in relation to time since admission for non-ST-segment elevation myocardial infarction (NSTEMI) and patients who are non-NSTEMI. *p<0.0001, **p=0.01, ***p=0.04, ****p=0.03.
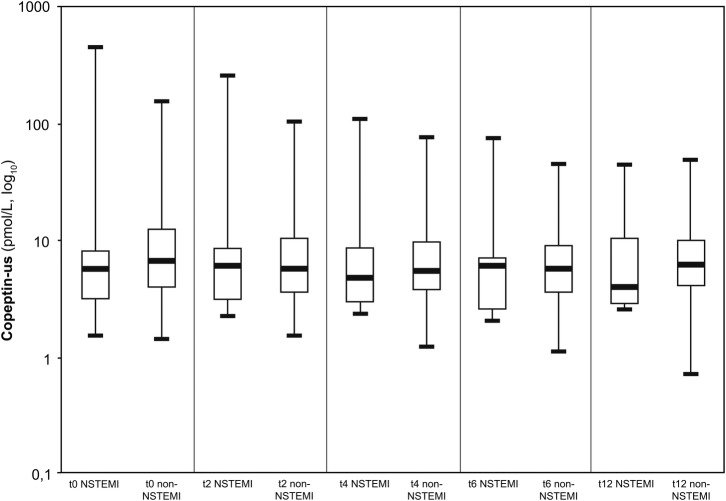
Figure 4.
Box plots (median, IQR, minimal and maximal values) illustrate Troponin, Copeptin, myoglobin and CK concentration in relation to time since admission for non-ST-segment elevation myocardial infarction (NSTEMI) and patients who are non-NSTEMI. *p<0.0001, **p=0.01, ***p=0.04, ****p=0.03.
Figure 5.
Box plots (median, IQR, minimal and maximal values) illustrate Troponin, Copeptin, myoglobin and CK concentration in relation to time since admission for non-ST-segment elevation myocardial infarction (NSTEMI) and patients who are non-NSTEMI. *p<0.0001, **p=0.01, ***p=0.04, ****p=0.03.
Troponin
According to the inclusion criteria, all patients had hs-cTnT ≤99th centile at admission. Troponin is the only marker studied which showed a significant difference between the two groups for each time tests were performed (0, 2, 4, 6 and 12 h), including at admission.
Copeptin
Median copeptin levels for NSTEMI and the other patients at admission were respectively 5.5 pmol/L IQR (3.1–7.9) and 6.5 pmol/L IQR (3.9–12.1), p=0.49. Only one patient with NSTEMI showed a copeptin value at admission above the cut-off of 12 pmol/L (435.2 pmol/L). This patient, who had a GRACE score of 151, was also the only patient who died during the follow-up. For all of the samples recruited during the 12 h following admission (2, 4, 6 and 12 h), there was no significant difference in the copeptin values between patients with NSTEMI and those with no NSTEMI, respectively, 5.9 pmol/L IQR (3.1–8.3) and 5.5 pmol/L IQR (3.5–10) at 2 h (p=0.86), 4.7 pmol/L IQR (2.9–8.4) and 5.4 pmol/L IQR (3.7–9.3) at 4 h (p=0.74), 5.9 pmol/L IQR (2.5–6.9) and 5.6 pmol/L IQR (3.5–8.8) at 6 h (p=0.77) and 3.9 pmol/L IQR (2.8–10.2) and 6.1 pmol/L IQR (4–9.7) at 12 h (p=0.49).
Myoglobin
At admission, the median myoglobin for patients with NSTEMI was 52.1 μg/L IQR (41.1–66.1) and 47.3 μg/L IQR (38–66.6) for patients with other diagnostics, p=0.71. At 2, 4 and 6 h, median myoglobin was significantly higher in patients with NSTEMI than in patients with other diagnosis, respectively, 72.9 and 48.6 μg/L (p=0.01), 102 and 47.8 μg/L (p=0.04), 107.5 and 49.5 μg/L (p=0.03).
Creatine kinase
At inclusion, median CK concentrations were 156.5 U/L IQR (90–231.5) in patients with NSTEMI and 182 U/L IQR (105–277) in non-NSTEMI (p=0.59). At 6 and 12 h, CK values of patients with NSTEMI were higher than those of other patients without significant difference, respectively 183 and 147 U/L (p=0.93), 186 and 128 U/L (p=0.26).
Diagnostic accuracy
For a cut-off level of 12 pmol/L, sensitivity of copeptin for NSTEMI diagnosis at admission was 12.5%, with a specificity of 74.5%, a predictive positive value of 4% and a NPV of 90.9%. None of the patients had a myoglobin value above the 95th centile at admission.
At the sixth hour, all of the eight patients with NSTEMI had at least one troponin above the 0.045 μg/L. One patient had troponin measured on the sample at the 6th hour already below this threshold and it continued to decrease until the 12th hour.
Limitations of the study
Despite the bicentric inclusions on a 1-year period, only eight patients with NSTEMI and hs-cTnT below the 99th centile at presentation were included. To show a significant difference between participants with NSTEMI and those who did not have NSTEMI with an expected difference of 15 pmol/L, as in the first study of Reichlin et al,9 the number of NSTEMI participants needed was 40. We were not able to include the expected number of patients within the time allowed by the design of the study and its permissions. Thus, the area under the ROC curve (AUC) and the net reclassification index could not be calculated.
We did not assess the pretest probability, which could increase the relevance of the biomarker in certain patient populations. However, there is no validated score to determine the clinical probability of ACS.
This study was conducted in France, with a prehospital system of medicalisation. Patients supported by a prehospital mobile medical team for a very suspicious chest pain, even without ST elevation, could be directly admitted to the cardiology department to perform immediate exploration, forming an incorporation bias. Probably, the results of this study cannot be extrapolated to EDs collaborating with other prehospital supports.
Twelve hours after admission, there was no significant difference between the two groups (NSTEMI vs non-NSTEMI) for myoglobin and CK. This may be due to the low infarcts size observed (hs-cTnT <99th centile at admission in the 6 h after the pain onset) but also due to the lack of 12 h blood samples for two patients with NSTEMI.
Discussion
Despite its limitations, our study complements the results of previously published data. In this prospective study, we used the latest generation of troponin I and copeptin assays. We have developed the protocol in a logical form. According to previous studies, copeptin can add a diagnostic value if there is no ST elevation and if troponin at admission is less than a threshold. Thus, we focused the study on this category of patients to reduce spectrum bias. Knowing that only 14 min are needed to get a copeptin-us result, this analysis can be requested or performed automatically when troponin is below the threshold, in a rational use of resources.
Although the copeptin NPV was 90.9% in our study, if NSTEMI diagnosis had been ruled out only by regarding copeptin value at admission, 7 of 8 patients with NSTEMI would have returned home without care. These results are consistent with COPED-MIRRO study which had a similar design but mostly used a fourth generation troponin.33
We identified other studies assessing the copeptin diagnostic accuracy that used a high-sensitive troponin. If we analyse the subgroups of patients with troponin below the 99th centile at presentation, our results are equivalent to those of most of these studies. Thus, in the latest study published, Sukul et al35 report that copeptin did not identify any additional patient with AMI in initial troponin-negative patients. Also, the CHOPIN (Copeptin Helps in the early detection Of Patients with acute myocardial INfarction) study, with 1967 patients analysed, had recruited 19 patients with NSTEMI with a negative troponin. In this group, copeptin added to troponin testing at admission did not identify nine patients with NSTEMI (sensitivity 53%).27 In the Rule Out Myocardial Infarction by Computed Tomography (ROMICAT) study, which did not separate the UA from the NSTEMI in its analysis, as well as in the Randomised Assessment of Panel Assay of Cardiac Markers (RATPAC) and Advantageous Predictors of Acute Coronary Syndromes Evaluation (APACE) trials, the authors report that copeptin did not provide additional significant diagnostic value to the high-sensitivity troponin.19 32 34 Charpentier et al28 report that the sensitivity and diagnostic accuracy were not acceptable for use in clinical practice. Moreover, for the patients from the FAST II and FASTER I studies, copeptin did not detect 18 of the 27 patients with NSTEMI with troponin below the 99th centile (sensitivity=33% in this subgroup). Bahrmann et al25 and Lotze et al14 found a NPV of 100%, but each of these studies included only one patient with NSTEMI with hs-cTnT below the cut-off defined. Thelin et al30 found a significant difference between sensitivities of single troponin versus the combination of troponin and copeptin. However, regarding published data, copeptin had identified six of nine patients with NSTEMI (sensitivity 67%) in patients presenting a negative troponin at admission.
The first studies analysing copeptin associated with a high-sensitive troponin revealed a significant diagnostic contribution of copeptin. Meune et al12 included 58 patients in a cardiology department where the prevalence of coronary syndromes is more important. The combination of copeptin and hs-cTnT had identified all patients with NSTEMI, but the status of the hs-cTnT for these patients is unknown. Keller et al showed a slight but significant improvement of the AUC for the subgroup of patients in the ED within 3 h after chest pain onset, but reported data do not permit to analyse the subgroup of patients with a negative troponin.
Consequently, copeptin seems to have insufficient sensitivity for patients with NSTEMI with troponin below the 99th centile at admission. This is probably due to important similarities between this group and patients with a diagnosis of UA, in which copeptin levels have not been shown as significantly different from those of patients with non-coronary chest pain in most of the previous studies. The hypothesis suggested in the first study on the diagnostic value of copeptin for ACS, could be that endogenous stress caused by UA could be lower than in patients with AMI and could be insufficient to cause a copeptin release.9 Moreover, the authors of the ROMICAT study, regarding their results, as they corroborated Kelly et al,36 suggest that copeptin is a reflection of left ventricular dysfunction and not of the coronary artery status.13 These assumptions are consistent with the physiological function of AVP and could explain the results of our study.
In our study, one patient had increased troponin level above the cut-off only at 6 h of admission. Still considering the sixth hour after inclusion, troponin level of one patient with NSTEMI had already begun its decline and was already below the threshold of the 99th centile. This observation is consistent with the precautionary statements of the Study Group on Biomarkers in Cardiology of the European Society of Cardiology Working Group on Acute Cardiac Care, advocating additional blood sampling in patients strongly suspected of having an AMI but no significant hs-cTnT increase after 3 h.37
A recent study suggests that undetectable Roche high-sensitive cardiac troponin T at admission could be considered to rule out patients with AMI.38 This algorithm could not be envisaged in our study population and the hs-cTnT used; three patients with NSTEMI had hs-cTnT undetectable at admission.
Finally, the only participant who died is the patient who had the highest value of copeptin, which is consistent with the highlight of the studies showing a prognostic role for copeptin.19 25 27 29
In conclusion, our study did not show a relevant diagnostic value of copeptin in patients with suspected ACS without ST-elevation and with hs-cTnT below the 99th centile at admission. Measurements of hs-cTnT at presentation and after 3 h, and after 6 h if necessary, remain the biochemical gold standard for NSTEMI diagnosis.1 37 Using a novel marker for NSTEMI diagnosis, alone or in a multimarker strategy, requires at least having as good sensitivity and NPV as serial troponin testing.
Supplementary Material
Acknowledgments
We thank the teams of emergency, cardiology and biochemistry departments of Clermont-Ferrand and Aurillac for their involvement in this study. Also, we thank Thermo Fisher Scientific and Siemens for providing the reagent for copeptin and troponin, respectively.
Footnotes
Collaborators: Marc Villemain; Philippe Evrard; Jean-Marc Philippe; Christophe Perrier; Thierry Mathevon; Benjamin André; Claire Billault; Christine Carrias; Nathalie Bailly-Glomot; Mathieu Lacroix; Catherine Maurin; Farès Moustafa; Daniel Pic; Jean-Luc Buisson; Catherine Rougier; Thomas Tatulli; Sandrine Tazé; Sébastien Dufraise; Thierry Cueto; Christelle Dejou; Bruno Laporte; Laura Luca; Mourad Chouaki; Guillaume Larroumets; Célia Nourrisson-Fage; Sylvain Ortigues; Christophe Sureau; Guillaume Weydenmeyer; Stéphane Bergzoll; Julien Raconnat; Aurélien Ponsoda; Guillaume Nguyen; Manuel Font; Marianne Brès.
Contributors: JD, JS, GM, VS, NC and PM conceived the study and designed the trial. JD undertook recruitment of participating centres and patients, managed the data, supervised the conduct of the trial and drafted the manuscript. SU provided statistical advice on study design and analysed the data. SM has made monitoring and carried out biochemical assays. LC, ND, NC JS, GM, VS, PM, LD and JD were the expert committee to adjudicate the final diagnosis. ND, LC, SM and SU contributed substantially to the revision of the manuscript. JD is the guarantor.
Funding: Medical Association of Emergency Physician of Clermont Ferrand (ASMUC), University Hospital Gabriel Montpied of Clermont-Ferrand and General Hospital Henri Mondor of Aurillac have supported research. Kits for us-copeptin assays was provided by Thermo Fisher Scientific. Kits for troponin was provided by Siemens.
Competing interests: None.
Patient consent: Obtained.
Ethics approval: The study complied with the Declaration of Helsinki and was approved by the ethical committee Comité de Protection des Personnes Sud-Est VI (AU 871).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
Contributor Information
Collaborators: Marc Villemain, Philippe Evrard, Jean-Marc Philippe, Christophe Perrier, Thierry Mathevon, Benjamin André, Claire Billault, Christine Carrias, Nathalie Bailly-Glomot, Mathieu Lacroix, Catherine Maurin, Farès Moustafa, Daniel Pic, Jean-Luc Buisson, Catherine Rougier, Thomas Tatulli, Sandrine Tazé, Sébastien Dufraise, Thierry Cueto, Christelle Dejou, Bruno Laporte, Laura Luca, Mourad Chouaki, Guillaume Larroumets, Célia Nourrisson-Fage, Sylvain Ortigues, Christophe Sureau, Guillaume Weydenmeyer, Stéphane Bergzoll, Julien Raconnat, Aurélien Ponsoda, Guillaume Nguyen, Manuel Font, and Marianne Brès
References
- 1.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Eur Heart J 2012;33:2551–67 [DOI] [PubMed] [Google Scholar]
- 2.Hamm CW, Bassand J-P, Agewall S, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2011;32:2999–3054 [DOI] [PubMed] [Google Scholar]
- 3.Lin S, Yokoyama H, Rac VE, et al. Novel biomarkers in diagnosing cardiac ischemia in the emergency department: a systematic review. Resuscitation 2012;83:684–91 [DOI] [PubMed] [Google Scholar]
- 4.Lippi G. Biomarkers of myocardial ischemia in the emergency room: cardiospecific troponin and beyond. Eur J Intern Med 2013;24:97–9 [DOI] [PubMed] [Google Scholar]
- 5.Morgenthaler N, Struck J, Jochberger S. Copeptin: clinical use of a new biomarker. Trends Endocrinol Metab 2008;19:43–9 [DOI] [PubMed] [Google Scholar]
- 6.Morgenthaler NG, Struck J, Alonso C, et al. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem 2006;52:112–19 [DOI] [PubMed] [Google Scholar]
- 7.Nickel CH, Bingisser R, Morgenthaler NG. The role of copeptin as a diagnostic and prognostic biomarker for risk stratification in the emergency department. BMC Med 2012;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lippi G, Plebani M, Di Somma S, et al. Considerations for early acute myocardial infarction rule-out for emergency department chest pain patients: the case of copeptin. Clin Chem Lab Med 2012;50:243–53 [DOI] [PubMed] [Google Scholar]
- 9.Reichlin T, Hochholzer W, Stelzig C, et al. Incremental value of copeptin for rapid rule out of acute myocardial infarction. J Am Coll Cardiol 2009;54:60–8 [DOI] [PubMed] [Google Scholar]
- 10.Keller T, Tzikas S, Zeller T, et al. Copeptin improves early diagnosis of acute myocardial infarction. J Am Coll Cardiol 2010;55:2096–106 [DOI] [PubMed] [Google Scholar]
- 11.Chenevier-Gobeaux C, Freund Y, Claessens Y-E, et al. Copeptin for rapid rule out of acute myocardial infarction in emergency department. Int J Cardiol 2013;166:198–204 [DOI] [PubMed] [Google Scholar]
- 12.Meune C, Zuily S, Wahbi K, et al. Combination of copeptin and high-sensitivity cardiac troponin T assay in unstable angina and non-ST-segment elevation myocardial infarction: a pilot study. Arch Cardiovasc Dis 2011;104:4–10 [DOI] [PubMed] [Google Scholar]
- 13.Karakas M, Januzzi JL, Meyer J, et al. Copeptin does not add diagnostic information to high-sensitivity troponin T in low- to intermediate-risk patients with acute chest pain: results from the rule out myocardial infarction by computed tomography (ROMICAT) study. Clin Chem 2011;57:1137–45 [DOI] [PubMed] [Google Scholar]
- 14.Lotze U, Lemm H, Heyer A, et al. Combined determination of highly sensitive troponin T and copeptin for early exclusion of acute myocardial infarction: first experience in an emergency department of a general hospital. Vasc Health Risk Manag 2011;7:509–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giavarina D, Carta M, Fortunato A, et al. Copeptin and high sensitive troponin for a rapid rule out of acute myocardial infarction? Clin Lab 2011;57:725–30 [PubMed] [Google Scholar]
- 16.Giannitsis E, Kehayova T, Vafaie M, et al. Combined testing of high-sensitivity troponin T and copeptin on presentation at prespecified cutoffs improves rapid rule-out of non-ST-segment elevation myocardial infarction. Clin Chem 2011;57:1452–5 [DOI] [PubMed] [Google Scholar]
- 17.Freund Y, Chenevier-Gobeaux C, Leumani F, et al. Concomitant measurement of copeptin and high sensitivity troponin for fast and reliable rule out of acute myocardial infarction. Ann Emerg Med 2011;58:S190. [DOI] [PubMed] [Google Scholar]
- 18.Dupuy A-M, Chastang E, Cristol J-P, et al. Analytical performances of the newly developed, fully automated Kryptor Copeptin assay: which impact factor for myocardial infarction rules out in the emergency department? Clin Lab 2012;58:635–44 [PubMed] [Google Scholar]
- 19.Potocki M, Reichlin T, Thalmann S, et al. Diagnostic and prognostic impact of copeptin and high-sensitivity cardiac troponin T in patients with pre-existing coronary artery disease and suspected acute myocardial infarction. Heart 2012;98:558–65 [DOI] [PubMed] [Google Scholar]
- 20.Ray P, Charpentier S, Chenevier-Gobeaux C, et al. Combined copeptin and troponin to rule out myocardial infarction in patients with chest pain and a history of coronary artery disease. Am J Emerg Med 2012;30:440–8 [DOI] [PubMed] [Google Scholar]
- 21.Charpentier S, Maupas-Schwalm F, Cournot M, et al. Combination of copeptin and troponin assays to rapidly rule out non-ST elevation myocardial infarction in the emergency department. Acad Emerg Med 2012;19:517–24 [DOI] [PubMed] [Google Scholar]
- 22.Eggers KM, Venge P, Lindahl B. High-sensitive cardiac troponin T outperforms novel diagnostic biomarkers in patients with acute chest pain. Clin Chim Acta 2012;413:1135–40 [DOI] [PubMed] [Google Scholar]
- 23.Folli C, Consonni D, Spessot M, et al. Diagnostic role of copeptin in patients presenting with chest pain in the emergency room. Eur J Intern Med 2013;24:189–93 [DOI] [PubMed] [Google Scholar]
- 24.Dedic A, ten Kate GJ, Rood PP, et al. Copeptin in acute chest pain: identification of acute coronary syndrome and obstructive coronary artery disease on coronary CT angiography. Emerg Med J 2013;30:910–13 [DOI] [PubMed] [Google Scholar]
- 25.Bahrmann P, Bahrmann A, Breithardt O-A, et al. Additional diagnostic and prognostic value of copeptin ultra-sensitive for diagnosis of non-ST-elevation myocardial infarction in older patients presenting to the emergency department. Clin Chem Lab Med 2013;51:1307–19 [DOI] [PubMed] [Google Scholar]
- 26.Ledochowski S, Fayet JM, Collin-Chavagnac D, et al. Évaluation de la copeptine dans les syndromes coronaires aigus non ST. Ann Fr Med Urgence 2013;3:138–44 [Google Scholar]
- 27.Maisel A, Mueller C, Neath S-X, et al. Copeptin helps in the early detection of patients with acute myocardial infarction: primary results of the CHOPIN trial (Copeptin Helps in the early detection Of Patients with acute myocardial INfarction). J Am Coll Cardiol 2013;62:150–60 [DOI] [PubMed] [Google Scholar]
- 28.Charpentier S, Lepage B, Maupas-Schwalm F, et al. Copeptin improves the diagnostic performance of sensitive troponin I-Ultra but cannot rapidly rule out non-ST-elevation myocardial infarction at presentation to an emergency department. Ann Emerg Med 2013;61:549–58e1 [DOI] [PubMed] [Google Scholar]
- 29.Afzali D, Erren M, Pavenstädt HJ, et al. Impact of copeptin on diagnosis, risk stratification, and intermediate-term prognosis of acute coronary syndromes. Clin Res Cardiol 2013;102:755–63 [DOI] [PubMed] [Google Scholar]
- 30.Thelin J, Borna C, Erlinge D, et al. The combination of high sensitivity troponin T and copeptin facilitates early rule-out of ACS: a prospective observational study. BMC Cardiovasc Disord 2013;13:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sebbane M, Lefebvre S, Kuster N, et al. Early rule out of acute myocardial infarction in ED patients: value of combined high-sensitivity cardiac troponin T and ultrasensitive copeptin assays at admission. Am J Emerg Med 2013;31:1302–8 [DOI] [PubMed] [Google Scholar]
- 32.Balmelli C, Meune C, Twerenbold R, et al. Comparison of the performances of cardiac troponins, including sensitive assays, and copeptin in the diagnostic of acute myocardial infarction and long-term prognosis between women and men. Am Heart J 2013;166:30–7 [DOI] [PubMed] [Google Scholar]
- 33.Llorens P, Sánchez M, Herrero P, et al. The utility of copeptin in the emergency department for non-ST-elevation myocardial infarction rapid rule out: COPED-MIRRO study. Eur J Emerg Med 2013. Published Online First: 17 July 2013. doi:10.1097/MEJ.0b013 e3283632f8b. http://journals.lww.com/euro-emergencymed/Abstract/publishahead/The_utility_of_copeptin_in_the_emergency.99604.aspx [DOI] [PubMed] [Google Scholar]
- 34.Collinson P, Gaze D, Goodacre S. Comparison of contemporary troponin assays with the novel biomarkers, heart fatty acid binding protein and copeptin, for the early confirmation or exclusion of myocardial infarction in patients presenting to the emergency department with chest pain. Heart 2014;100:140–5 [DOI] [PubMed] [Google Scholar]
- 35.Sukul D, Bonaca MP, Ruff CT, et al. Diagnostic performance of copeptin in patients with acute nontraumatic chest pain: BWH-TIMI ED chest pain study. Clin Cardiol 2014. Published Online First: 22 January 2014. doi:10.1002/clc.22244. http://onlinelibrary.wiley.com/doi/10.1002/clc.22244/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelly D, Squire IB, Khan SQ, et al. C-terminal provasopressin (copeptin) is associated with left ventricular dysfunction, remodeling and clinical heart failure in survivors of myocardial infarction. J Card Fail 2008;14:739–45 [DOI] [PubMed] [Google Scholar]
- 37.Thygesen K, Mair J, Giannitsis E, et al. How to use high-sensitivity cardiac troponins in acute cardiac care. Eur Heart J 2012;33:2252–7 [DOI] [PubMed] [Google Scholar]
- 38.Body R, Carley S, McDowell G, et al. Rapid exclusion of acute myocardial infarction in patients with undetectable troponin using a high-sensitivity assay. J Am Coll Cardiol 2011;58:1332–9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.