Abstract
IgA nephropathy (IgAN) is the most common primary glomerulonephritis worldwide, accounting for approximately 30–40% of patients undergoing renal biopsy in Asia. The characteristic and diagnostic lesion of IgAN is the deposition of glomerular IgA. The morphological lesions observed by light microscopy are extremely variable. A causal relationship between IgAN and burn injury has not been established, and the correlation between them is not clear if they appear at the same time. We have explored the cause of severe proteinuria of a Chinese patient with burns of 2nd or 3rd degree after a gas leakage accident 2 weeks ago. The diffuse proliferative glomerulonephritis of this patient revealed type I membranoproliferative glomerulonephritis-like symptoms. Moreover, this patient showed a sensitive response to prednisone. This case report demonstrates the intrinsic relationship between kidney disease and burn injury, which will facilitate a feasible treatment strategy for proteinuria after burn injury.
Key words: IgA nephropathy, Burn injury, Nephrotic syndrome
Introduction
Focal and diffuse mesangial proliferative glomerulonephritis reveal the most common histologic lesions observed on renal biopsies from patients with IgA nephropathy (IgAN) [1]. However, histologic variability is also observed in this disease, which is the same as with lupus nephritis. In some cases, endocapillary hypercellularity is accompanied by segmental duplication of the glomerular basement membrane, and there is rarely diffuse endocapillary proliferation with lobular accentuation and multiple double contours, resembling type I membranoproliferative glomerulonephritis (MPGN). Herein, we report such a case with MPGN-like IgAN. The etiology of IgAN needs to be further discussed. Tanaka and Waga [2] have reported a biopsied patient with acute IgAN following the formation of keloid scars due to a burn injury. However, further reports regarding the correlation between IgAN and burn injury are rare. This case here may explain such a correlation.
Case Report
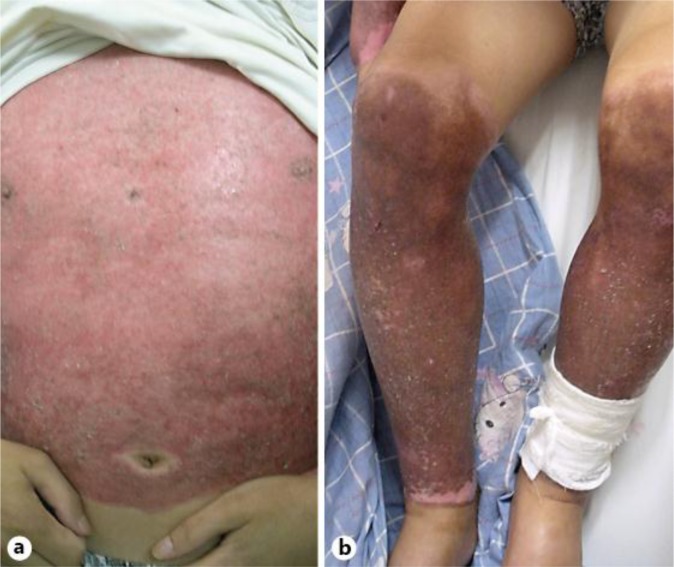
A 46-year-old male patient with scrotal and pedal edema was admitted to our department in August 2012. He had been burned in a gas leakage accident 2 weeks ago. Laboratory examination showed proteinuria (4+) and more than 775 red blood cells in the urine without casts. This patient had been treated for his 2nd- to 3rd-degree burn injuries in a local burn center. He had no inhalation injury or sepsis during the whole treatment process. At the admission time, he had a body temperature of 36.6°C, a pulse rate of 75 beats/min, a respiratory rate of 16/min, and a blood pressure of 118/80 mm Hg. Physical examination revealed cutaneous convalescence after full-thickness burns of both hands, the coronal plane of the body and both legs. The skin showed fuscous, slight exudation, bleeding cracks, mild scaling and itch during the treatment process (fig. 1). Severe edema in the bilateral lower extremities and scrotums were also observed.
Fig. 1.
The burned skin.
No lymphadenopathy, hepatosplenomegaly, or neurological abnormalities were shown. Electrocardiogram and chest X-ray were normal. Abdominal ultrasonography showed bilateral normal-sized kidneys. No antineutrophil cytoplasmic antibodies, antiglomerular basement membrane antibodies and antinuclear antibodies were detected. The laboratory test results of the urine and blood for this patient are listed in table 1. He was diagnosed as having nephrotic syndrome on the basis of a diminished serum albumin level of 23 g/l and an increased proteinuria excretion of 10.0 g/24 h. During the treatment course, microbiological examination of wound secretion was performed twice a week and no infection was observed.
Table 1.
Laboratory test data
| Blood cell counts | Serology | Urinalysis | |||
| WBC | 4.70×109/l (3.8–10.0) | IgG | 8.35 g/l (8–16) | pH | 7.0 |
| RBC | 3.17×1012/l (3.5–5.7) | IgA | 2.82 g/l (0.76–3.9) | SG | 1.026 (1.010–1.025) |
| Hb | 99.20 g/l (120–170) | IgM | 0.96 g/l (0.40–3.45) | Proteinuria | (3+) |
| Plt | 183.00×109/l (100–320) | IgE | 107.00 IU/ml (0–378) | Blood | (2+) |
| Blood biochemical analysis | C3 | 0.73 g/l (0.81–1.6) | Glucose-U | (−) | |
| Alt | 11.00 U/l (0–40) | C4 | 0.17 g/l (0.1–0.4) | Occult blood | (−) |
| Ast | 22.00 U/l (0–40) | ESR | 37 mm/H (0–15) | RBC | 775.1/μl (0–15) |
| BUN | 4.37 mmol/l (1.8–7.7) | HBsAg | (+) | WBC | 13.8/μl (0–14) |
| Cre | 91.50 μmol/l (54–133) | HBV-DNA | 1.66E5 copies/ml (<5×102) | Proteinuria | 10.0 g/day |
| TP | 41.00 g/l (60–80) | NAG | 59.2 IU/l (<18.5) | ||
| Alb | 23.20 g/l (35–55) | Hs-CRP | >5.0 mg/l (0–3) | α2-M | 2.03 mg/l (≤2.87) |
| Glb | 17.8 g/l (20–35) | CRP | 9.8 mg/l (0–10) | C3 | 3.06 mg/l (≤2.76) |
| TCH | 3.8 mmol/l (3.1–5.2) | D-Dimer | 3.98 mg/l (0–0.55) | RBP | 0.28 mg/l (≤0.5) |
| TG | 1.02 mmol/l (0.56–1.7) | Ccr | 61 ml/min (>80) | B-J protein | (−) |
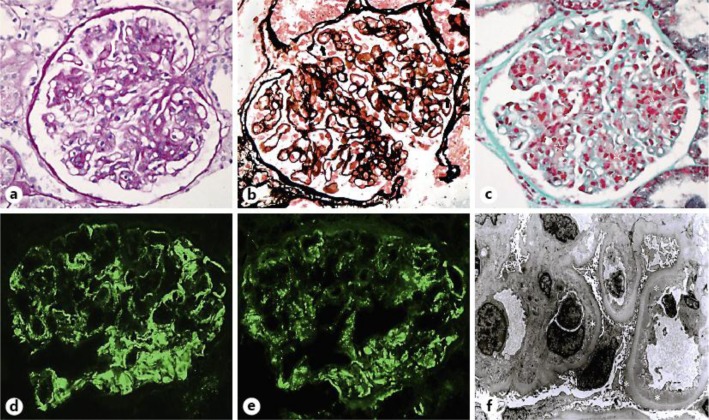
A percutaneous renal biopsy was performed on September 11, 2012 (day 57 after burn injury) to explore the cause of proteinuria. The examination under light microscopy revealed a diffuse and moderate proliferation of mesangial cells and endothelial cells without observable crescents in 20 glomeruli. Hyaline droplets containing proteins and lipids in tubules were observed. Meanwhile, red blood cells in some tubules were also observed. The interstitium showed diffuse edema and scattered infiltration of mononuclear cells. Arterioles presented as normal status. Neither tubular atrophy nor interstitial fibrosis was found, as shown in figure 2a–c.
Fig. 2.
Renal pathology showed diffuse proliferative MPGN-like IgAN. a Slight lobular accentuation, intracapillary hypercellularity in the glomerular tuft, extensive mesangial involvement and capillary wall thickening. Periodic acid-Schiff staining, ×400. b Segmental duplication of glomerular basement membrane, thickening of glomerular capillary wall density, glomerular mesangial and subendothelial deposits. Periodic acid-silver metheramine staining, ×400. c Identification of discrete glomerular subendothelial and mesangial deposits. Masson trichrome staining, ×400. d Highly positive staining for IgA in a broken, wide and band-like pattern with some coarse granularity areas along capillary loops and mesangium. Segmental IgA staining were also observed in the mesangium. Immunofluorescence, ×400. e Highly positive staining for C3 in a coarse granular pattern along peripheral glomerular capillaries. Immunofluorescence, ×400. f Ultrastructural appearance during outward migration of mesangial cells, infiltration of inflammatory cells, margination of endothelial cells along the inside of capillary walls, and interposition between endothelium and glomerular basement membrane of these cells. Glomerular visceral epithelial cell foot process showed effacement. Numerous large discrete electron-dense osmiophilic deposits within the subendothelium, mesangium and segmental subepithelium were present. Uranyl acetate and lead citrate, ×3,000.
IgA and C3 are predominant immunoreactants for the diagnosis of IgAN. Immunofluorescence revealed IgA (3+), trace IgM, C3 (3+), IgG (–), C4 (–) and C1q (–) in a coarse granular pattern with mesangial and capillary distribution (fig. 2d, e), and inconspicuous staining for IgM along the glomerular capillary walls in a granular pattern. In addition, this patient showed negative staining of HBsAg and HBcAg.
Ultrastructural studies showed high-density deposits of mesangial and subendothelial cells. The mesangial hypercellularity and increased mesangial matrix were present between the glomerular basement membrane and glomerular endothelium. The thickened capillary wall was composed of basement membrane-like materials, interposed mesangial cells, and electron-dense immune-type deposits (fig. 2f).
Based on the proliferative properties of glomerular lesions, the patient was started on therapy with oral prednisone at a dose of 1 mg/kg/day. After treatment, the excretion of urinary protein revealed a significant decrease from 10.0 to 5.20 g/24 h in 2 weeks. Then, the patient was discharged and treatment was continued at the outpatient section. The patient had been followed up to date and showed normal renal function and mild proteinuria.
Discussion
The glomerular filtration rate is increased after burn injuries, which is accompanied by vascular dysfunction and increased cardiac output [3]. Renal disease and acute kidney injury remains prevalent and is associated with the increased mortality of patients with severe burn injuries [4]. This case shows the pattern of diffuse proliferative type I MPGN-like symptoms with mesangial interposition and double contours of the glomerular basement membrane present on periodic acid-Schiff and silver stains (fig. 2). All of those pathologic characteristics suggest that it is a secondary IgAN.
Consideration of these issues begins with the progress of this patient. The following reasons are considered as a close correlation between burn injury and the onset of IgAN. First, this patient did not have any urinary abnormalities or chronic kidney diseases before and at the early stage of burn injury. Second, nephrotic syndrome was observed at the 2nd week after the injury, and the patient did not have a history of infection such as inflammation of the respiratory tract although concurrent nephrotic syndrome in convalescence was observed. In addition, the patient suffered from skin problems with slight exudation, bleeding cracks, mild scaling and itch during the treatment process (fig. 1).
Although a causal relationship between IgAN and burn injury has not been established, an association of cutaneous lesions after burn injury with IgAN has been reported in a few patients [2, 4]. Tanaka and Waga [2] mentioned a biopsied patient with acute IgAN following the formation of keloid scars due to burn injury. The patient revealed a rapid recovery after complete removal of the scars without any medication. Wang et al. [5] have reported that acute IgAN is closely related to high-voltage electrical burn injury. In this study, the patient showed extracapillary segmental proliferation with fibrinoid necrosis in glomeruli. All those case reports revealed a close relationship between IgAN and burn injury. Because of trauma, infection, tissue necrosis, allogeneic plasma transfusion, increased concentrations of circulating immune complexes were observed [6]. This situation is similar to the immune dysfunction of IgAN [7]. Both have inherent genetic predisposition and external infections [8], which may induce IgAN.
If the pathogenesis of IgAN is not correlated with the concurrent burn injury, what is the cause of the nephrotic sydrome? We hypothesize that an autoimmune dysregulation due to a sequestered antigen induced by skin infection and pruritus after burn injury may have an important role in the pathogenesis of IgAN. The persistent skin lesions with dry, itch and crusting properties can trigger IgA deposition and promote the depositions in the mesangium and subendothelium of the patient.
After serious burns, the immune system is disordered, the immune complex is increased in the circulation, and macrophages are excessively activated. All of these disorders will lead to gradual glomerular injury. Numerous studies have provided evidence that burns upregulate inflammatory cytokines such as TNF-α and IL-1β [9]. Those cytokines can interfere with the hemodynamics of intraglomerular microcirculation, the coagulation-fibrinolysis system and the infiltration of inflammatory cells after burns.
Nephritis is a rare complication of burn injury, and once this happens, it will affect the recovery and prognosis of the patient. Regular urine analysis will contribute to the early detection and diagnosis of glomerular diseases while treating burn injury. It is difficult to measure the blood pressure of burned patients, but blood pressure should be monitored as early as possible. Because of wound exudation, infection and other reasons, the patients with large burned areas often have some degree of hypoalbuminemia. Once persistent hypoalbuminemia exist, the burned patients need further examination to diagnose whether they have chronic glomerulonephritis or nephrotic syndrome. If the burned patient has nephrotic syndrome, enough glucocorticoid should be administered in order to alleviate proteinuria effectively and for the recovery of burn wounds.
The casual relationship between the onset of IgAN and burn injury remains speculative and needs to be explored further.
Disclosure Statement
The authors have no conflicts of interest to disclose.
Acknowledgement
This work was supported by grants from the National Science Foundation of China (81100519 to Jili Zhu; 81370800 to Huiming Wang).
References
- 1.Zeng CH, Le W, Ni Z, et al. A multicenter application and evaluation of the Oxford classification of IgA nephropathy in adult Chinese patients. Am J Kidney Dis. 2012;60:812–820. doi: 10.1053/j.ajkd.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka H, Waga S. Acute IgA nephropathy following keloid scar formation due to burn injury. Clin Nephrol. 2003;60:440–441. doi: 10.5414/cnp60440. [DOI] [PubMed] [Google Scholar]
- 3.Zdolsek HJ, Kågedal B, Lisander B, Hahn RG. Glomerular filtration rate is increased in burn patients. Burns. 2010;36:1271–1276. doi: 10.1016/j.burns.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Mosier MJ, Pham TN, Klein MB, et al. Dysfunction and higher mortality in severely burned adults. J Burn Care Res. 2010;31:83–92. doi: 10.1097/BCR.0b013e3181cb8c87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Tang HT, Xia ZF, et al. Acute IgA nephropathy following high-voltage electrical burn injury. Clin Nephrol. 2009;71:588–589. doi: 10.5414/cnp71588. [DOI] [PubMed] [Google Scholar]
- 6.Teplova SN, Zveniatskovskaia ER, Nikushkina KV. The complement system and circulating immune complexes in burn patients. Zh Mikrobiol Epidemiol Immunobiol. 1999;3:61–65. [PubMed] [Google Scholar]
- 7.Hashimoto A, Suzuki Y, Suzuki H, et al. Determination of severity of murine IgA nephropathy by glomerular complement activation by aberrantly glycosylated IgA and immune complexes. Am J Pathol. 2012;181:1338–1347. doi: 10.1016/j.ajpath.2012.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sobouti B, Fallah S, Ghavami Y, et al. Serum immunoglobulin levels in pediatric burn patients. Burns. 2013;39:473–476. doi: 10.1016/j.burns.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Salgado RM, Alcántara L, Mendoza-Rodríguez CA, et al. Post-burn hypertrophic scars are characterized by high levels of IL-1β mRNA and protein and TNF-α type I receptors. Burns. 2012;38:668–676. doi: 10.1016/j.burns.2011.12.012. [DOI] [PubMed] [Google Scholar]