Abstract
Context:
Of the individuals able to return to sport participation after an anterior cruciate ligament(ACL) injury, up to 25% will experience a second ACL injury. This population may be more sensitive to hormonal fluctuations, which may explain this high rate of second injury.
Objective:
To examine changes in 3-dimensional hip and knee kinematics and kinetics during a jump landing and to examine knee laxity across the menstrual cycle in women with histories of unilateral noncontact ACL injury.
Design
Controlled laboratory study.
Setting:
Laboratory.
Patients or Other Participants:
A total of 20 women (age = 19.6 ± 1.3 years, height = 168.6 ± 5.3 cm, mass = 66.2 ± 9.1 kg) with unilateral, noncontact ACL injuries.
Intervention(s)
Participants completed a jump-landing task and knee-laxity assessment 3 to 5 days after the onset of menses and within 3 days of a positive ovulation test.
Main Outcome Measure(s):
Kinematics in the uninjured limb at initial contact with the ground during a jump landing, peak kinematics and kinetics during the loading phase of landing, anterior knee laxity via the KT-1000, peak vertical ground reaction force, and blood hormone concentrations (estradiol-β-17, progesterone, free testosterone).
Results:
At ovulation, estradiol-β-17 (t = −2.9, P = .009), progesterone (t = −3.4, P = .003), and anterior knee laxity (t = −2.3, P = .03) increased, and participants presented with greater knee-valgus moment (Z = −2.6, P = .01) and femoral internal rotation (t = −2.1, P = .047). However, during the menses test session, participants landed harder (greater peak vertical ground reaction force; t = 2.2, P = .04), with the tibia internally rotated at initial contact (t = 2.8, P = .01) and greater hip internal-rotation moment (Z = −2.4, P = .02). No other changes were observed across the menstrual cycle.
Conclusions
Knee and hip mechanics in both phases of the menstrual cycle represented a greater potential risk of ACL loading. Observed changes in landing mechanics may explain why the risk of second ACL injury is elevated in this population.
Key Words: hormones, estrogen, vertical ground reaction force, knee-valgus moment
Key Points
Clinicians should be aware of the high rate of second injury and biomechanical consequences of many factors related to return to sport participation after anterior cruciate ligament (ACL) injury, including sensitivity to hormonal fluctuations and asymmetrical limb loading.
The biomechanical profiles of women with ACL injury changed during the preovulatory phase of the menstrual cycle, possibly increasing the risk of second ACL injury.
Women with ACL reconstructions should have their landing mechanics evaluated before returning to sport participation.
Anterior knee laxity and jump-landing biomechanics changed across the menstrual cycle in women with unilateral ACL injuries.
Both menstrual cycle phases had biomechanical variables associated with ACL loading.
The risk of sustaining a noncontact anterior cruciate ligament (ACL) injury is not equal across the menstrual cycle.1–4 The menstrual cycle consists of the follicular, ovulatory, and luteal phases, which have markedly different hormonal profiles. The follicular phase is associated with the lowest concentrations of estrogen, progesterone, and testosterone. Ovulation, which follows the follicular phase, occurs between days 9 and 20 and is associated with a spike in luteinizing hormone and then a spike in estrogen.5 This is the largest concentration of estrogen during the menstrual cycle. The final phase of the menstrual cycle is the luteal phase, which is associated with prolonged elevated estrogen levels. Progesterone also increases substantially during this phase. Researchers6,7 have reached consensus that the preovulatory phase (from the follicular phase to ovulation) of the menstrual cycle presents the highest risk for noncontact ACL injuries. The risk of injury is thought to result from hormonal fluctuations influencing tissue that, in turn, affects neuromuscular characteristics during dynamic tasks, such as landing from a jump.8,9 Differences across menstrual cycle phases have been identified in variables believed to be associated with joint stability, including laxity,5,10,11 muscle stiffness,9 strength,12–14 proprioception,15 and muscle-activation patterns.16 However, this area is not without controversy, with other researchers17–20 observing no change across the menstrual cycle in similar variables.
Reproductive hormones seem to influence ACL laxity in females with normal menstrual cycles and physiologic levels of estrogen and progesterone.5,11,21–25 Numerous authors have concluded that anterior laxity differs between sexes, with males having less laxity than females.25–31 Again, this area is not without controversy, as several authors have concluded that anterior knee laxity does not change across the menstrual cycle.18,31–36 However, negative correlations have been observed between ACL stiffness and estrogen concentration in active females, indicating that an increase in estrogen is associated with lower levels of ligament stiffness.21 Additionally, evidence8,37,38 has suggested that ACL laxity may influence muscular response during dynamic activity. Park et al8 collected biomechanical data on 26 participants and initially observed no change in kinematic and kinetic variables across the 3 phases of the menstrual cycle. However, when they reorganized participants based on their relative levels of knee laxity into low-, medium-, and high-laxity time points, the authors found that the high-laxity group had a 30% increase in adduction impulse, 20% increase in adduction moment, and 45% increase in external rotation compared with the medium- and low-laxity groups.8 This information demonstrates that knee laxity can influence joint loading and potentially influence noncontact ACL injury.
Besides knee laxity, other biomechanical factors are associated with ACL loading and ACL injury during jumping and landing. Landing with decreased sagittal-plane motion or moment (knee and hip extension) and increased frontal- and rotational-plane motion of the hip (adduction and internal rotation) and knee (valgus and internal rotation) contribute to ACL loading.39 Researchers8,40,41 have examined changes in jump-landing mechanics across the menstrual cycle in healthy female populations without histories of ACL injury. These authors observed no change in jump-landing hip and knee mechanics across the menstrual cycle, leading them to conclude that injury rates were most likely due to other factors, including strength or ligament properties.41 One limitation of these studies is that some women may be more responsive to hormonal fluctuations than others (ie, responders versus nonresponders).5,11 We theorize that females with histories of ACL injury may be responsive to hormonal fluctuations, and this increased sensitivity may have a greater effect on tissue and ultimately landing mechanics. Additionally, up to 25% of individuals who sustain primary ACL ruptures will have second ACL injuries, with many second injuries occurring in the contralateral limb.42–44 In a recent paper on second ACL injuries, Paterno et al42 examined athletes who were returning to high-level sports and observed that 75% of second ACL injuries occurred in the contralateral limb and 88% of individuals sustaining these injuries were females. The rate of second injury is particularly high for individuals returning to sport participation even after successfully completing rehabilitation programs.44 This warrants further investigation because underlying risk factors, such as hormones, could play a role in the rates of second injury in the contralateral limb. Therefore, the purpose of our study was to examine anterior knee laxity and 3-dimensional hip and knee kinematics and kinetics across the menstrual cycle in a population of women with previous unilateral, noncontact ACL injuries. We hypothesized that biomechanical variables assessed during a jump landing would be altered at ovulation in ways that would increase ACL loading and laxity compared with menses. We based this theory on research in which investigators5,38 have identified increased ligamentous laxity at ovulation. Additionally, we hypothesized that hip and knee kinematics and kinetics during a jump landing would change in ways associated with increased ACL loading.5,38
METHODS
Participants
Twenty-four women were enrolled in the study, with 20 participants (age = 19.6 ± 1.3 years, height = 168.6 ± 5.3 cm, mass = 66.2 ± 9.1 kg) completing the protocol. Three participants had anovulatory cycles, and 1 withdrew because of scheduling conflicts. To be included in the study, participants had to satisfy the following criteria: 18 to 25 years of age; no history of pregnancy or neurologic disorder; self-reported normal menstrual cycle at the time of injury and at the time of testing; no use of hormone-altering contraception for 6 months before testing; no use of hormone-altering contraception at the time of ACL injury; sustained a unilateral, noncontact ACL injury, which was defined as “forces applied to the knee at the time of injury resulted from the athlete's own movements and did not involve contact with another athlete or object”45; and cleared by a physician to return to participation. All data were collected from the nonreconstructed or contralateral limb to eliminate reconstruction as an influence and the unknown state of the hormone receptors in the reconstructed ACL.
Participants described their injury histories, mechanisms of injury (eg, landing, cutting), and surgical repair methods to the primary investigator (Table 1). The time from ACL injury to testing ranged from 7 to 52 months (average = 25.7 ± 12.6 months), menstrual cycle length ranged from 25 to 34 days (average = 28.9 ± 2.3 days), and time from the onset of menses to a positive ovulation test ranged from 8 to 21 days (average = 13.6 ± 3.5 days). The types of grafts used for reconstruction varied; the bone-patellar tendon-bone autograft (n = 9) was the most common, followed by the semitendinosus-gracilis autograft (n = 6) and allograft (n = 4). One participant was ACL deficient. Soccer was the most common sporting activity in which participants sustained their injuries, and cutting was the most common mechanism of injury. Participants had an average International Knee Documentation Committee (IKDC) 2000 subjective score of 83.9 ± 8.2. All participants provided written informed consent, and the study was approved by the Institutional Review Board of the University of North Carolina at Chapel Hill.
Table 1. .
Anterior Cruciate Ligament Injury History for Each Participant (N = 20)
| Injured Limb |
Time From Reconstruction to Testing, mo |
Mechanism of Injury |
Sport Participation at Time of Injury |
Graft Type |
| Right | 18 | Landing | Gymnastics | Hamstrings |
| Right | 52 | Pivoting | Softball | Bone-patellar tendon-bone |
| Left | 42 | Pivoting | Soccer | Bone-patellar tendon-bone |
| Right | 16 | Landing | Basketball | Hamstrings |
| Right | 7 | Cutting | Soccer | Bone-patellar tendon-bone |
| Right | 34 | Cutting | Soccer | Allograft |
| Right | 33 | Cutting | Soccer | Allograft |
| Left | 12 | Cutting | Flag football | Bone-patellar tendon-bone |
| Right | 45 | Landing | Basketball | Bone-patellar tendon-bone |
| Left | 17 | Landing | Long jump | Bone-patellar tendon-bone |
| Left | 29 | Cutting | Soccer | Hamstrings |
| Left | 14a | Cutting | Field hockey | Not applicableb |
| Left | 24 | Cutting | Flag football | Allograft |
| Right | 26 | Landing | Volleyball | Allograft |
| Right | 38 | Cutting | Soccer | Bone-patellar tendon-bone |
| Left | 17 | Cutting | Soccer | Bone-patellar tendon-bone |
| Right | 36 | Tumbling or landing | Gymnastics | Hamstrings |
| Right | 13 | Cutting | Handball | Hamstrings |
| Right | 14 | Cutting | Soccer | Hamstrings |
| Right | 26 | Cutting | Flag football | Bone-patellar tendon-bone |
Indicates time from injury to testing.
Participant was anterior cruciate ligament deficient.
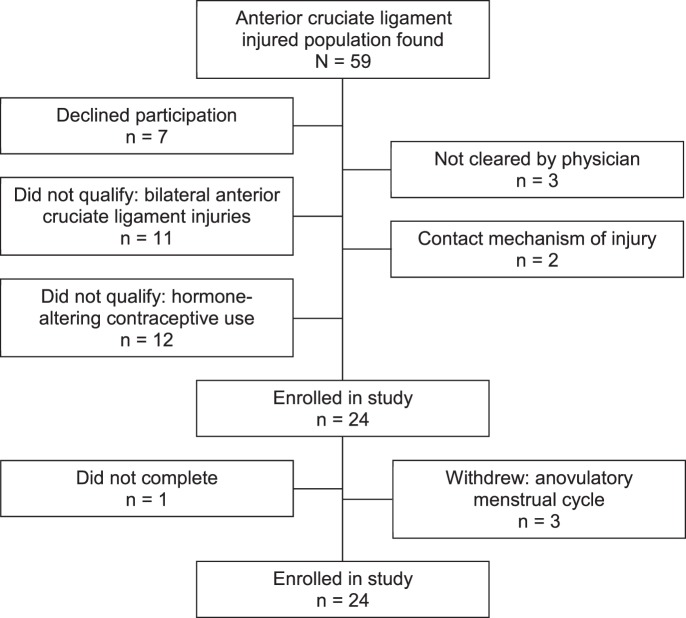
Based on previously collected data from our laboratory, we performed an a priori power analysis. This analysis revealed that, assuming a moderate to large effect size, a sample size of 20 was needed to achieve a power greater than 0.80 to determine differences among menstrual cycle phases for most variables of interest. Additionally, the inclusion criteria for this study were stringent, and we anticipated difficulty in finding participants who met all criteria. Therefore, we attempted to enroll 25 participants to properly power the study and to allow for withdrawal. The Figure depicts the total number of participants with ACL injuries who were contacted and how the final numbers were achieved.
Figure. .
Flow chart depicting the total number of participants with anterior cruciate ligament injury contacted and how the final study enrollment was achieved.
Procedures
Participants were tested at 2 time points during their menstrual cycles, which corresponded with low levels of estrogen and progesterone (menses) and high levels of estrogen and low levels of progesterone (ovulation). The 2 time points were 3 to 5 days after the onset of menses and within 3 days after a positive test using a urine-based ovulation prediction test (Earth's Magic, Cary, NC).46,47 Test phases were counterbalanced to avoid an order effect, with the first test session occurring at menses in 11 participants and at ovulation in 9 participants. We tested participants during the same time of day for each session and instructed them not to eat 2 hours before testing and not to exercise the day of testing. The primary investigator was blinded to the menstrual cycle phase of each participant. Each participant had a venous blood specimen analyzed for select reproductive hormone levels (estradiol-β-17, progesterone, and free testosterone) using previously reported methods.46,47 Participants completed all testing wearing the same pair of their personal athletic shoes for each session.
Kinematic and Kinetic Assessment
Lower extremity kinematics and kinetics were collected using an electromagnetic tracking system (Ascension Technologies, Inc, Burlington, VT) with a nonconductive force plate (Bertec Corporation, Columbus, OH) synchronized with the kinematic data. The MotionMonitor software system (Innovative Sports Training, Inc, Chicago, IL) was used to record kinematics at 144 Hz and kinetics at 1440 Hz. Electromagnetic tracking sensors were placed on each participant over the apex of the sacrum, midpoint of the lateral thigh, and medial tibia. The biomechanical model used in this study has been shown to provide a valid assessment of peak lower extremity kinetics even with the foot segment omitted.48 The following bony landmarks defined the segment endpoints and joint centers of the lower extremity segments: medial and lateral femoral condyles, medial and lateral malleoli, and left and right anterior-superior iliac spines. The malleoli defined the ankle joint center, and the femoral condyles defined the knee joint center. The hip joint center was determined by the Bell et al method.49 A static trial was recorded from a neutral position.
Participants completed 5 trials of a jump-landing task on a box that was 30 cm high and positioned at 50% of their heights from the edge of a force plate. They jumped forward and landed on both feet with the test limb on the force plate. Immediately after landing, participants jumped as high as possible. The trials were averaged and used for analysis. All kinematic and kinetic variables had good intrasession reliability (ICC[2,k] > 0.80).
Knee-Laxity Assessment
Knee laxity was defined as the amount of anterior tibial displacement resulting from an anterior drawer force of 133 N using a KT-1000 knee arthrometer (MEDmetric Corp, San Diego, CA).5 We positioned participants supine with the knee in 25° of flexion. Their ankles were placed in a cradle, and a thigh strap was added to control rotation of the thighs. The KT-1000 was placed on the anterior shank, aligned with the joint line, and secured to the lower limb using hook-and-loop straps. Two practice trials were used to ensure that the participant was relaxed and the KT-1000 was secured properly. Five trials were recorded and averaged. All laxity measurements were completed by the primary investigator, who was blinded, and excellent intrarater reliability was established before data collection (intrasession: ICC[2,k] = 0.98, standard error of the mean = 0.43 mm; intersession: ICC[2,k] = 0.88, standard error of the mean = 0.40 mm).
Data Reduction
Kinematic data were filtered using a fourth-order, zero-phase-lag Butterworth low-pass filter at 14.5 Hz.50 We used Euler angles to calculate joint angles with flexion-extension about the y axis, valgus-varus about the x axis, and internal and external rotation about the z axis. Kinematic and kinetic data were exported into customized MATLAB software programs (version 7.0; The MathWorks, Inc, Natick, MA) for data reduction. The kinematic neutral stance was subtracted from each trial to account for sensor placement between phases.51 Ground reaction forces were normalized to body weight (N), and moment data were normalized to the product of body weight and height (BW·BH). Ground reaction forces defined the landing phase of the jump-landing task. Initial contact was defined as the point at which the vertical ground reaction forces (VGRFs) exceeded 10 N, and toe-off was defined as the point at which VGRFs dropped below 10 N. Three-dimensional hip and knee kinematics were assessed at initial contact for each trial. Additionally, peak hip and knee kinematics and kinetics were calculated during the landing phase of the jump landing, which occurred from initial contact to peak knee flexion. Peak VGRF also was examined during the jump landing. We calculated joint moments, which are internal, using standard inverse dynamic procedures.52
All variables were inspected to ensure that they met the statistical assumptions before analysis. Special attention was given to the ACL-deficient participant to ensure she was not identified as an outlier and to verify that she did not have undue influence on the data. The participant was not identified as an outlier and fit all other inclusion criteria; therefore, we had no compelling reason to exclude her from the analysis. Individual paired t tests or the Wilcoxon signed rank tests were used to compare each dependent variable between menstrual cycle phases. Effect sizes were calculated using the Cohen d statistic. We used SPSS (version 20.0; IBM Corporation, Armonk, NY) to perform all data analyses and set the α level a priori at .05.
RESULTS
Descriptive statistics for select reproductive hormones and knee laxity are presented in Table 2. Estradiol-β-17 (Cohen d = 1.2) and progesterone (Cohen d = 1.5) levels and anterior knee laxity (Cohen d = 0.5) increased at ovulation, but no changes were observed for testosterone. Descriptive values and statistics for joint kinematics and kinetics are presented in Tables 3 and 4. Tibial rotation was the only kinematic variable that changed at initial contact, and participants were in a more internally rotated position at menses than at ovulation (Cohen d = 0.6). Peak differences during the loading phase included increased knee-valgus moment at ovulation (Cohen d = 0.9), increased hip internal-rotation position at ovulation (Cohen d = 0.4), and greater hip internal rotation moment at menses (Cohen d = 0.3). No other kinematic and kinetic findings were different; however, a statistical trend was identified for peak tibial internal rotation (P ≤ .10, Cohen d = 0.6) at menses. Finally, participants had increased peak VGRF during the menses test session (Cohen d = 0.30).
Table 2. .
Reproductive Hormone Concentrations of and Anterior Knee Laxity at the Selected Time Points (Mean ± SD)
| Menses |
Ovulation |
Menses, 95% Confidence Interval |
Ovulation, 95% Confidence Interval |
t |
P |
|||
| Lower |
Upper |
Lower |
Upper |
|||||
| Estradiol-β-17, pg/mL | 31.1 ± 13.7 | 70.4 ± 54.7 | 24.5 | 37.7 | 44.0 | 96.7 | −2.9 | .009a |
| Progesterone, ng/mL | 0.5 ± 0.3 | 3.9 ± 4.2 | 0.4 | 0.6 | 1.9 | 5.9 | −3.4 | .003a |
| Free testosterone, pg/mL | 0.8 ± 0.3 | 0.9 ± 0.2 | 0.7 | 0.9 | 0.8 | 0.9 | −0.8 | .41 |
| Laxity, mm | 6.6 ± 1.4 | 7.3 ± 1.3 | 5.9 | 7.2 | 6.7 | 7.9 | −2.3 | .03a |
Indicates difference between menstrual cycle phases.
Table 3. .
Kinematic Variables at Initial Contact, ° (Mean ± SD)
| Kinematic Variable |
Menses |
Ovulation |
Menses, 95% Confidence Interval |
Ovulation, 95% Confidence Interval |
t |
P |
||
| Lower |
Upper |
Lower |
Upper |
|||||
| Knee flexion (+) | 21.1 ± 5.4 | 21.0 ± 7.4 | 18.6 | 23.7 | 17.6 | 24.5 | −0.1 | .94 |
| Knee valgus (−) | −1.3 ± 3.9 | −0.9 ± 5.3 | −3.1 | 0.6 | −3.5 | 1.5 | −0.3 | .77 |
| Knee rotation (+ internaI rotation/− external rotation) | 1.6 ± 7.3 | −3.3 ± 8.1 | −1.8 | 4.9 | −7.1 | 0.5 | 2.8 | .01a |
| Hip flexion (−) | −41.4 ± 8.9 | −43.5 ± 6.6 | −41.6 | −37.2 | −46.6 | −40.4 | 0.9 | .34 |
| Hip abduction (−) | −3.4 ± 4.9 | −3.7 ± 5.5 | −5.7 | −1.1 | −6.3 | −1.2 | 0.3 | .79 |
| Hip rotation (+ internal rotation/− external rotation) | −2.2 ± 8.6 | −0.6 ± 7.8 | −6.2 | 1.9 | −4.2 | 3.1 | 0.9 | .33 |
Indicates difference between phases.
Table 4. .
Peak Kinematic and Kinetic Variables During the Loading Phase of the Jump Landing (Mean ± SD)
| Variable |
Menses |
Ovulation |
Menses, 95% Confidence Interval |
Ovulation, 95% Confidence Interval |
t or Z |
P |
||
| Lower |
Upper |
Lower |
Upper |
|||||
| Kinematics, ° | ||||||||
| Knee flexion | 93.7 ± 8.9 | 92.7 ± 12.4 | 89.5 | 97.8 | 86.9 | 98.5 | 0.7 | .49 |
| Knee valgus | −13.2 ± 10.6 | −12.4 ± 7.9 | −18.2 | 8.3 | −16.1 | −8.7 | 0.4 | .67 |
| Knee internal rotation | 9.2 ± 7.5 | 5.3 ± 8.7 | 5.7 | 12.7 | 1.2 | 9.4 | 1.9 | .07 |
| Hip flexion | −77.7 ± 12.3 | −80.8 ± 16.8 | −83.5 | −71.9 | −88.6 | −72.9 | 1.2 | .25 |
| Hip adduction | 0.9 ± 5.9 | 0.9 ± 6.3 | −1.9 | 3.6 | −2.0 | 3.8 | −0.024 | .98 |
| Hip internal rotation | 2.9 ± 8.7 | 6.2 ± 6.8 | −1.2 | 6.9 | 3.3 | 9.4 | −2.1 | .047b |
| Kinetics, internal moment normalized to body mass height | ||||||||
| Knee extension | −0.21 ± 0.06 | −0.21 ± 0.07 | −0.24 | −0.18 | −0.24 | −0.18 | 0.4 | .68 |
| Knee valgus | −0.02 ± 0.03 | −0.06 ± 0.06 | −0.04 | −0.002 | −0.08 | −0.03 | −2.6a | .01b |
| Knee internal rotation | 0.03 ± 0.03 | 0.03 ± 0.03 | 0.02 | 0.04 | 0.02 | 0.05 | −0.5a | .62 |
| Hip extension | −0.31 ± 0.23 | −0.35 ± 0.40 | −0.42 | −0.21 | −0.54 | −0.16 | −0.8 | .41 |
| Hip adduction | 0.12 ± 0.10 | 0.12 ± 0.13 | 0.07 | 0.16 | 0.06 | 0.18 | −1.0a | .63 |
| Hip internal rotation | 0.17 ± 0.18 | 0.12 ± 0.15 | 0.09 | 0.26 | 0.06 | 0.19 | −2.4a | .02b |
| Forces | ||||||||
| Vertical ground reaction force, Nb | 1968.3 ± 623.8 | 1775.8 ± 642.9 | 1676.4 | 2260.3 | 1474.9 | 2076.7 | 2.2 | .04b |
Wilcoxon signed rank test.
Indicates difference between phases.
DISCUSSION
The goal of this study was to evaluate jump-landing mechanics and anterior knee laxity across the menstrual cycle in the uninjured limb of women with histories of unilateral ACL injury. We concluded that anterior knee laxity and jump-landing biomechanics changed across the menstrual cycle in this population. Both menstrual cycle phases had biomechanical variables associated with ACL loading. That is, the positions and moments observed during each session have been associated with increased ACL loading or increased risk of ACL injury. At menses, participants had greater peak VGRF and tibial internal rotation at initial contact and tended to have greater peak tibial internal rotation and hip internal-rotation moment. However, variables associated with ACL loading at ovulation included peak knee-valgus moment, hip internal rotation, and increased anterior knee laxity.
These findings tend to agree with our hypothesis that ovulation would be associated with a biomechanical profile that favors increased ACL loading and injury mechanisms. Our hypothesis was based on previous findings that indicated greater amounts of estradiol-β-17 and progesterone would negatively influence ligamentous laxity24 and muscle stiffness9 and these factors would alter biomechanical profiles at ovulation. However, we are not the first group to observe altered performance at menses. Friden et al53 noted better performance on a hop test (greatest number of jumps) at ovulation than at menses. The hop test challenges neuromuscular coordination and postural control, both of which may be impaired in females with a history of ACL injury.44,54,55 The idea of improved performance at ovulation also was supported by Dedrick et al,16 who showed better cocontraction between the gluteus maximus and semitendinosus at ovulation than at the late luteal phase of the menstrual cycle. Yet a limitation in comparing these studies with our study is that these authors examined uninjured populations rather than populations with a history of ACL injury. It is unclear how these variables would be influenced after reconstruction, rehabilitation, and return to sport participation.
Knee rotation was altered in the contralateral limb in women with unilateral ACL injuries during the jump landing. One potential explanation for this landing position is that hormonal fluctuations may influence knee laxity, which can influence tibial rotational position during landing.8,56 At menses, the tibia was internally rotated at initial contact (Cohen d = 0.6). Tibial internal rotation is a provocative ACL-loading mechanism, especially when associated with low knee-flexion angles.57,58 Additionally, researchers59 have demonstrated that the contralateral limb in individuals with ACL injuries has greater internal-rotation motion than in uninjured control participants. This supports the idea that rotational motion could play a role in ACL injury. Our findings disagree with those of authors8,40,41 who have investigated similar variables across the menstrual cycle, specifically regarding rotational kinematics. We observed no changes in sagittal-plane or frontal-plane kinematics across the menstrual cycle, which agrees with the findings reported for populations with no history of ACL reconstruction.8,40,41
During the ovulation test session, participants landed with increased knee-valgus moment and greater hip internal rotation. This disagrees with findings of researchers8,40,41 studying participants with no histories of ACL reconstruction. Peak knee-valgus moment was 67% greater (Cohen d = 0.9) at ovulation during the jump landing. Knee-valgus moment has been identified as a prospective risk factor for noncontact ACL injury60 and is associated with ACL loading.61 Frontal-plane loading is vital to ACL injury, as researchers62 have demonstrated that sagittal-plane knee-joint moments alone are not sufficient to rupture the ACL. McLean et al62 used forward dynamic musculoskeletal modeling simulations to conclude that sagittal-plane moments cannot injure the ACL and that valgus loading is an important mechanism of injury, specifically in females. Collectively, these results demonstrate the importance of knee-valgus loading in ACL injury and point to the ovulatory phase of the menstrual cycle, when this variable is greatest. During the loading phase of landing, the hip was internally rotated at ovulation. This motion is associated with dynamic knee valgus and valgus collapse and is associated with the “position of no return,” which has been described as a common mechanism of injury associated with noncontact ACL injury.63 Our inclusion criterion of previous unilateral ACL reconstruction is perhaps one of the most important reasons we were able to demonstrate observable changes in these variables across the menstrual cycle.
Our knee-laxity values were approximately 1.5 mm greater than previously reported values.11 This is an interesting finding considering the study population had a history of ACL injury. Greater generalized joint laxity has been noted in a population with ACL injuries.64 Uhorchak et al65 found that 24 military cadets with ACL injuries had greater generalized joint laxity and also had knee-laxity measures more than 1 standard deviation greater than cadets without ACL injuries. Increased joint laxity may be one of the potential explanations for the high rate of injury in this population. This evidence suggests that increased joint laxity is present in our population and is exacerbated at ovulation.
The average magnitude of change in knee laxity across the menstrual cycle was 0.7 mm of displacement, which is a 10% increase in anterior knee laxity. This change in knee laxity is similar to values reported by Shultz et al,5 who found the average magnitude of knee laxity change across the menstrual cycle was 3.2 mm. Our values are less than this reported range but are associated with a moderate effect size (Cohen d = 0.5). The primary difference between these studies is that Shultz et al5 measured knee laxity for multiple days around ovulation to ensure they could capture the peak laxity assessment, whereas we were limited to 1 time point around ovulation. However, we removed a threat of internal validity by blinding the primary investigator to the menstrual cycle phase of each participant during testing.
We observed appreciable increases in estradiol-β-17 and progesterone levels at ovulation. Minimal concentrations of estrogen and progesterone have been found to be important predictors of the response of knee laxity to hormonal fluctuations across the menstrual cycle.10 Specifically, low levels of estrogen and higher minimal levels of progesterone during the early follicular phase of the menstrual cycle influenced knee laxity.10 The relationship between minimal levels of estrogen and progesterone may be similar for muscle, as well. However, the roles of estrogen and progesterone in ligament are better understood66–68 than the way in which these hormones influence muscle.46,47 More research is needed to better understand the role these hormones play in muscle and how they influence landing mechanics.
To our knowledge, we are the first to examine jump-landing biomechanics across the menstrual cycle in women with a history of ACL reconstruction. The findings of our research demonstrate that the biomechanical profiles change across the menstrual cycle in a complex manner and, depending on the variable of interest, exhibit high risk at both menses and ovulation. Clinicians working with individuals who have had ACL reconstruction should be aware of the implications of returning this population to sport, and a clinical assessment of high-risk motions should occur as part of the return-to-sport criteria. Whereas hormonal changes will not directly change biomechanics, these individuals might be more sensitive to hormonal fluctuations causing altered tissue mechanics, which could influence landing mechanics.
Our participants had histories of unilateral, noncontact ACL injury and represent a population at increased risk of second ACL injury.42,44 However, we cannot extrapolate these findings to the original mechanism of noncontact ACL injury. Additionally, we could not control for factors related to differences in rehabilitation and surgery, including graft type. The number of months from ACL injury to testing ranged from 7 to 52 months (average = 25.7 ± 12.6 months), but all participants were cleared to return to sport by their physicians. To our knowledge, no evidence exists to suggest that graft type influences the kinematics or kinetics of the contralateral limb, but researchers should consider these factors. Another limitation is that we did not include a control group, which would allow for increased confidence in our results. However, our study has several strengths, such as randomization of testing sessions and blinding of the primary investigator, which is a limitation of previous investigations in this area. Finally, the biomechanical model that we used omitted the foot segment that may have produced kinetic values slightly higher than a model in which the foot segment is included.48 However, this omission most likely had a negligible effect on the results of our study given the repeated-measures design.
CONCLUSIONS
Our study highlighted the importance of biomechanical profiles in individuals with ACL reconstruction. Whereas this population often returns to sport participation, clinicians should be aware of the high rate of second injury and biomechanical consequences of multiple factors related to return to sport participation, including sensitivity to hormonal fluctuations and asymmetrical limb loading. Biomechanical profiles of these individuals change during the preovulatory phase of the menstrual cycle, and these changes may increase the risk of second ACL injury. We recommend that individuals with ACL reconstruction have landing mechanics evaluated as part of return-to-sport criteria. Low-cost clinical tools, such as the Landing Error Scoring System, are available to evaluate jump-landing mechanics using a standardized method.
ACKNOWLEDGMENTS
This project was funded in part by the National Academy of Sports Medicine. We thank Paige Wall, Jessica Hannigan, Courtney Ross, and Adam Schessel for their assistance with this project.
REFERENCES
- 1.Arendt EA, Agel J, Dick R. Anterior cruciate ligament injury patterns among collegiate men and women. J Athl Train. 1999;34(2):86–92. [PMC free article] [PubMed] [Google Scholar]
- 2.Arendt EA, Bershadsky B, Agel J. Periodicity of noncontact anterior cruciate ligament injuries during the menstrual cycle. J Gend Specif Med. 2002;5(2):19–26. [PubMed] [Google Scholar]
- 3.Beynnon BD, Johnson RJ, Braun S, et al. The relationship between menstrual cycle phase and anterior cruciate ligament injury: a case-control study of recreational alpine skiers. Am J Sports Med. 2006;34(5):757–764. doi: 10.1177/0363546505282624. [DOI] [PubMed] [Google Scholar]
- 4.Ruedl G, Ploner P, Linortner I, et al. Are oral contraceptive use and menstrual cycle phase related to anterior cruciate ligament injury risk in female recreational skiers? Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1065–1069. doi: 10.1007/s00167-009-0786-0. [DOI] [PubMed] [Google Scholar]
- 5.Shultz SJ, Kirk SE, Johnson ML, Sander TC, Perrin DH. Relationship between sex hormones and anterior knee laxity across the menstrual cycle. Med Sci Sports Exerc. 2004;36(7):1165–1174. doi: 10.1249/01.MSS.0000132270.43579.1A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hewett TE, Zazulak BT, Myer GD. Effects of the menstrual cycle on anterior cruciate ligament injury risk: a systematic review. Am J Sports Med. 2007;35(4):659–668. doi: 10.1177/0363546506295699. [DOI] [PubMed] [Google Scholar]
- 7.Shultz SJ, Schmitz RJ, Nguyen AD, et al. ACL Research Retreat V: an update on ACL injury risk and prevention, March 25–27, 2010, Greensboro, NC. J Athl Train. 2010;45(5):499–508. doi: 10.4085/1062-6050-45.5.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park SK, Stefanyshyn DJ, Ramage B, Hart DA, Ronsky JL. Alterations in knee joint laxity during the menstrual cycle in healthy women leads to increases in joint loads during selected athletic movements. Am J Sports Med. 2009;37(6):1169–1177. doi: 10.1177/0363546508330146. [DOI] [PubMed] [Google Scholar]
- 9.Eiling E, Bryant AL, Petersen W, Murphy A, Hohmann E. Effects of menstrual-cycle hormone fluctuations on musculotendinous stiffness and knee joint laxity. Knee Surg Sports Traumatol Arthrosc. 2007;15(2):126–132. doi: 10.1007/s00167-006-0143-5. [DOI] [PubMed] [Google Scholar]
- 10.Shultz SJ, Gansneder BM, Sander TC, Kirk SE, Perrin DH. Absolute serum hormone levels predict the magnitude of change in anterior knee laxity across the menstrual cycle. J Orthop Res. 2006;24(2):124–131. doi: 10.1002/jor.20021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park SK, Stefanyshyn DJ, Loitz-Ramage B, Hart DA, Ronsky JL. Changing hormone levels during the menstrual cycle affect knee laxity and stiffness in healthy female subjects. Am J Sports Med. 2009;37(3):588–598. doi: 10.1177/0363546508326713. [DOI] [PubMed] [Google Scholar]
- 12.Bambaeichi E, Reilly T, Cable NT, Giacomoni M. The isolated and combined effects of menstrual cycle phase and time-of-day on muscle strength of eumenorrheic females. Chronobiol Int. 2004;21(4–5):645–660. doi: 10.1081/cbi-120039206. [DOI] [PubMed] [Google Scholar]
- 13.Sarwar R, Niclos BB, Rutherford OM. Changes in muscle strength, relaxation rate and fatiguability during the human menstrual cycle. J Physiol. 1996;493(pt 1):267–272. doi: 10.1113/jphysiol.1996.sp021381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips SK, Sanderson AG, Birch K, Bruce SA, Woledge RC. Changes in maximal voluntary force of human adductor pollicis muscle during the menstrual cycle. J Physiol. 1996;496(pt 2):551–557. doi: 10.1113/jphysiol.1996.sp021706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friden C, Ramsey DK, Backstrom T, Benoit DL, Saartok T. Lindén Hirschberg A. Altered postural control during the luteal phase in women with premenstrual symptoms. Neuroendocrinology. 2005;81(3):150–157. doi: 10.1159/000086592. [DOI] [PubMed] [Google Scholar]
- 16.Dedrick GS, Sizer PS, Merkle JN, et al. Effect of sex hormones on neuromuscular control patterns during landing. J Electromyogr Kinesiol. 2008;18(1):68–78. doi: 10.1016/j.jelekin.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Bell DR, Myrick MP, Blackburn JT, Shultz SJ, Guskiewicz KM, Padua DA. The effect of menstrual-cycle phase on hamstring extensibility and muscle stiffness. J Sport Rehabil. 2009;18(4):553–563. doi: 10.1123/jsr.18.4.553. [DOI] [PubMed] [Google Scholar]
- 18.Van Lunen BL, Roberts J, Branch JD, Dowling EA. Association of menstrual-cycle hormone changes with anterior cruciate ligament laxity measurements. J Athl Train. 2003;38(4):298–303. [PMC free article] [PubMed] [Google Scholar]
- 19.Friden C, Hirschberg AL, Saartok T. Muscle strength and endurance do not significantly vary across 3 phases of the menstrual cycle in moderately active premenopausal women. Clin J Sport Med. 2003;13(4):238–241. doi: 10.1097/00042752-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Hertel J, Williams NI, Olmsted-Kramer LC, Leidy HJ, Putukian M. Neuromuscular performance and knee laxity do not change across the menstrual cycle in female athletes. Knee Surg Sports Traumatol Arthrosc. 2006;14(9):817–822. doi: 10.1007/s00167-006-0047-4. [DOI] [PubMed] [Google Scholar]
- 21.Romani W, Patrie J, Curl LA, Flaws JA. The correlations between estradiol, estrone, estriol, progesterone, and sex hormone-binding globulin and anterior cruciate ligament stiffness in healthy, active females. J Womens Health (Larchmt) 2003;12(3):287–298. doi: 10.1089/154099903321667627. [DOI] [PubMed] [Google Scholar]
- 22.Hicks-Little CA, Thatcher JR, Hauth JM, Goldfuss AJ, Cordova ML. Menstrual cycle stage and oral contraceptive effects on anterior tibial displacement in collegiate female athletes. J Sports Med Phys Fitness. 2007;47(2):255–260. [PubMed] [Google Scholar]
- 23.Heitz NA, Eisenman PA, Beck CL, Walker JA. Hormonal changes throughout the menstrual cycle and increased anterior cruciate ligament laxity in females. J Athl Train. 1999;34(2):144–149. [PMC free article] [PubMed] [Google Scholar]
- 24.Deie M, Sakamaki Y, Sumen Y, Urabe Y, Ikuta Y. Anterior knee laxity in young women varies with their menstrual cycle. Int Orthop. 2002;26(3):154–156. doi: 10.1007/s00264-001-0326-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shultz SJ, Sander TC, Kirk SE, Perrin DH. Sex differences in knee joint laxity change across the female menstrual cycle. J Sports Med Phys Fitness. 2005;45(4):594–603. [PMC free article] [PubMed] [Google Scholar]
- 26.Huston LJ, Wojtys EM. Neuromuscular performance characteristics in elite female athletes. Am J Sports Med. 1996;24(4):427–436. doi: 10.1177/036354659602400405. [DOI] [PubMed] [Google Scholar]
- 27.Rosene JM, Fogarty TD. Anterior tibial translation in collegiate athletes with normal anterior cruciate ligament integrity. J Athl Train. 1999;34(2):93–98. [PMC free article] [PubMed] [Google Scholar]
- 28.Rozzi SL, Lephart SM, Gear WS, Fu FH. Knee joint laxity and neuromuscular characteristics of male and female soccer and basketball players. Am J Sports Med. 1999;27(3):312–319. doi: 10.1177/03635465990270030801. [DOI] [PubMed] [Google Scholar]
- 29.Trimble MH, Bishop MD, Buckley BD, Fields LC, Rozea GD. The relationship between clinical measurements of lower extremity posture and tibial translation. Clin Biomech (Bristol, Avon) 2002;17(4):286–290. doi: 10.1016/s0268-0033(02)00010-4. [DOI] [PubMed] [Google Scholar]
- 30.Loudon JK, Jenkins W, Loudon KL. The relationship between static posture and ACL injury in female athletes. J Orthop Sports Phys Ther. 1996;24(2):91–97. doi: 10.2519/jospt.1996.24.2.91. [DOI] [PubMed] [Google Scholar]
- 31.Pollard CD, Braun B, Hamill J. Influence of gender, estrogen and exercise on anterior knee laxity. Clin Biomech (Bristol, Avon) 2006;21(10):1060–1066. doi: 10.1016/j.clinbiomech.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Beynnon BD, Bernstein IM, Belisle A, et al. The effect of estradiol and progesterone on knee and ankle joint laxity. Am J Sports Med. 2005;33(9):1298–1304. doi: 10.1177/0363546505275149. [DOI] [PubMed] [Google Scholar]
- 33.Karageanes SJ, Blackburn K, Vangelos ZA. The association of the menstrual cycle with the laxity of the anterior cruciate ligament in adolescent female athletes. Clin J Sport Med. 2000;10(3):162–168. doi: 10.1097/00042752-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Arnold C, Van Bell C, Rogers V, Cooney T. The relationship between serum relaxin and knee joint laxity in female athletes. Orthopedics. 2002;25(6):669–673. doi: 10.3928/0147-7447-20020601-18. [DOI] [PubMed] [Google Scholar]
- 35.Belanger MJ, Moore DC, Crisco JJ, III, Fadale PD, Hulstyn MJ, Ehrlich MG. Knee laxity does not vary with the menstrual cycle, before or after exercise. Am J Sports Med. 2004;32(5):1150–1157. doi: 10.1177/0363546503261360. [DOI] [PubMed] [Google Scholar]
- 36.Lovering RM, Romani WA. Effect of testosterone on the female anterior cruciate ligament. Am J Physiol Regul Integr Comp Physiol. 2005;289(1):R15–R22. doi: 10.1152/ajpregu.00829.2004. [DOI] [PubMed] [Google Scholar]
- 37.Shultz SJ, Carcia CR, Perrin DH. Knee joint laxity affects muscle activation patterns in the healthy knee. J Electromyogr Kinesiol. 2004;14(4):475–483. doi: 10.1016/j.jelekin.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Park SK, Stefanyshyn DJ, Ramage B, Hart DA, Ronsky JL. Relationship between knee joint laxity and knee joint mechanics during the menstrual cycle. Br J Sports Med. 2009;43(3):174–179. doi: 10.1136/bjsm.2008.049270. [DOI] [PubMed] [Google Scholar]
- 39.Shimokochi Y, Shultz SJ. Mechanisms of noncontact anterior cruciate ligament injury. J Athl Train. 2008;43(4):396–408. doi: 10.4085/1062-6050-43.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abt JP, Sell TC, Laudner KG, et al. Neuromuscular and biomechanical characteristics do not vary across the menstrual cycle. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):901–907. doi: 10.1007/s00167-007-0302-3. [DOI] [PubMed] [Google Scholar]
- 41.Chaudhari AM, Lindenfeld TN, Andriacchi TP, et al. Knee and hip loading patterns at different phases in the menstrual cycle: implications for the gender difference in anterior cruciate ligament injury rates. Am J Sports Med. 2007;35(5):793–800. doi: 10.1177/0363546506297537. [DOI] [PubMed] [Google Scholar]
- 42.Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE. Incidence of contralateral and ipsilateral anterior cruciate ligament (ACL) injury after primary ACL reconstruction and return to sport. Clin J Sport Med. 2012;22(2):116–121. doi: 10.1097/JSM.0b013e318246ef9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sward P, Kostogiannis I, Roos H. Risk factors for a contralateral anterior cruciate ligament injury. Knee Surg Sports Traumatol Arthrosc. 2010;18(3):277–291. doi: 10.1007/s00167-009-1026-3. [DOI] [PubMed] [Google Scholar]
- 44.Paterno MV, Schmitt LC, Ford KR, et al. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Am J Sports Med. 2010;38(10):1968–1978. doi: 10.1177/0363546510376053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marshall SW, Padua DA, McGrath ML. Incidence of ACL injury. In: TE Hewett, Shultz SJ, Griffin LY., editors. Understanding and Preventing Noncontact ACL Injuries. Champaign, IL: Human Kinetics;; 2007. In. eds. [Google Scholar]
- 46.Bell DR, Blackburn JT, Norcorss MF, et al. Estrogen and muscle stiffness have a negative relationship in females. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):361–367. doi: 10.1007/s00167-011-1577-y. [DOI] [PubMed] [Google Scholar]
- 47.Bell DR. Blackburn J, Ondrak KS, et al., editors. The effects of oral contraceptive use on muscle stiffness across the menstrual cycle. Clin J Sport Med. 2011;21(6):467–473. doi: 10.1097/JSM.0b013e318230f50a. Troy. [DOI] [PubMed] [Google Scholar]
- 48.Blackburn JT, Norcross MF, Cannon LN, Zinder SM. Hamstrings stiffness and landing biomechanics linked to anterior cruciate ligament loading. J Athl Train. 2013;48(6):764–772. doi: 10.4085/1062-6050-48.4.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bell AL, Pedersen DR, Brand RA. A comparison of the accuracy of several hip center location prediction methods. J Biomech. 1990;23(6):617–621. doi: 10.1016/0021-9290(90)90054-7. [DOI] [PubMed] [Google Scholar]
- 50.Yu B, Gabriel D, Nobel L, An KN. Estimate of the optimum cutoff frequency for the butterworth low-pass digital filter. J Appl Biomech. 1999;15(3):318–329. [Google Scholar]
- 51.Pappas E, Carpes FP. Lower extremity kinematic asymmetry in male and female athletes performing jump-landing tasks. J Sci Med Sport. 2012;15(1):87–92. doi: 10.1016/j.jsams.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 52.Gagnon D, Gagnon M. The influence of dynamic factors on triaxial net muscular moments at the L5/S1 joint during asymmetrical lifting and lowering. J Biomech. 1992;25(8):891–901. doi: 10.1016/0021-9290(92)90229-t. [DOI] [PubMed] [Google Scholar]
- 53.Friden C, Hirschberg AL, Saartok T, Renstrom P. Knee joint kinaesthesia and neuromuscular coordination during three phases of the menstrual cycle in moderately active women. Knee Surg Sports Traumatol Arthrosc. 2006;14(4):383–389. doi: 10.1007/s00167-005-0663-4. [DOI] [PubMed] [Google Scholar]
- 54.Hoffman M, Schrader J, Koceja D. An investigation of postural control in postoperative anterior cruciate ligament reconstruction patients. J Athl Train. 1999;34(2):130–136. [PMC free article] [PubMed] [Google Scholar]
- 55.Ortiz A, Olson S, Libby CL, et al. Landing mechanics between noninjured women and women with anterior cruciate ligament reconstruction during 2 jump tasks. Am J Sports Med. 2008;36(1):149–157. doi: 10.1177/0363546507307758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shultz SJ, Schmitz RJ. Effects of transverse and frontal plane knee laxity on hip and knee neuromechanics during drop landings. Am J Sports Med. 2009;37(9):1821–1830. doi: 10.1177/0363546509334225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Markolf KL, Burchfield DM, Shapiro MM, Shepard MF, Finerman GA, Slauterbeck JL. Combined knee loading states that generate high anterior cruciate ligament forces. J Orthop Res. 1995;13(6):930–935. doi: 10.1002/jor.1100130618. [DOI] [PubMed] [Google Scholar]
- 58.Miyasaka T, Matsumoto H, Suda Y, Otani T, Toyama Y. Coordination of the anterior and posterior cruciate ligaments in constraining the varus-valgus and internal-external rotatory instability of the knee. J Orthop Sci. 2002;7(3):348–353. doi: 10.1007/s007760200058. [DOI] [PubMed] [Google Scholar]
- 59.Branch TP, Browne JE, Campbell JD, et al. Rotational laxity greater in patients with contralateral anterior cruciate ligament injury than healthy volunteers. Knee Surg Sports Traumatol Arthrosc. 2010;18(10):1379–1384. doi: 10.1007/s00167-009-1010-y. [DOI] [PubMed] [Google Scholar]
- 60.Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33(4):492–501. doi: 10.1177/0363546504269591. [DOI] [PubMed] [Google Scholar]
- 61.Oh YK, Lipps DB, Ashton-Miller JA, Wojtys EM. What strains the anterior cruciate ligament during a pivot landing? Am J Sports Med. 2012;40(3):574–583. doi: 10.1177/0363546511432544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McLean SG, Huang X, Su A, Van Den Bogert AJ. Sagittal plane biomechanics cannot injure the ACL during sidestep cutting. Clin Biomech (Bristol, Avon) 2004;19(8):828–838. doi: 10.1016/j.clinbiomech.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 63.Ireland ML. Anterior cruciate ligament injury in female athletes: epidemiology. J Athl Train. 1999;34(2):150–154. [PMC free article] [PubMed] [Google Scholar]
- 64.Kramer LC, Denegar CR, Buckley WE, Hertel J. Factors associated with anterior cruciate ligament injury: history in female athletes. J Sports Med Phys Fitness. 2007;47(4):446–454. [PubMed] [Google Scholar]
- 65.Uhorchak JM, Scoville CR, Williams GN, Arciero RA, St Pierre P, Taylor DC. Risk factors associated with noncontact injury of the anterior cruciate ligament: a prospective four-year evaluation of 859 West Point cadets. Am J Sports Med. 2003;31(6):831–842. doi: 10.1177/03635465030310061801. [DOI] [PubMed] [Google Scholar]
- 66.Miller BF, Hansen M, Olesen JL, et al. Tendon collagen synthesis at rest and after exercise in women. J Appl Physiol. 2007;102(2):541–546. doi: 10.1152/japplphysiol.00797.2006. [DOI] [PubMed] [Google Scholar]
- 67.Slauterbeck J, Clevenger C, Lundberg W, Burchfield DM. Estrogen level alters the failure load of the rabbit anterior cruciate ligament. J Orthop Res. 1999;17(3):405–408. doi: 10.1002/jor.1100170316. [DOI] [PubMed] [Google Scholar]
- 68.Bryant AL, Clark RA, Bartold S, et al. Effects of estrogen on the mechanical behavior of the human Achilles tendon in vivo. J Appl Physiol. 2008;105(4):1035–1043. doi: 10.1152/japplphysiol.01281.2007. [DOI] [PubMed] [Google Scholar]