Abstract
Context:
The mechanical property of stiffness may be important to investigating how lateral ankle ligament injury affects the behavior of the viscoelastic properties of the ankle complex. A better understanding of injury effects on tissue elastic characteristics in relation to joint laxity could be obtained from cadaveric study.
Objective:
To biomechanically determine the laxity and stiffness characteristics of the cadaver ankle complex before and after simulated injury to the anterior talofibular ligament (ATFL) and calcaneofibular ligament (CFL) during anterior drawer and inversion loading.
Design:
Cross-sectional study.
Setting:
University research laboratory.
Patients or Other Participants:
Seven fresh-frozen cadaver ankle specimens.
Intervention(s):
All ankles underwent loading before and after simulated lateral ankle injury using an ankle arthrometer.
Main Outcome Measure(s):
The dependent variables were anterior displacement, anterior end-range stiffness, inversion rotation, and inversion end-range stiffness.
Results:
Isolated ATFL and combined ATFL and CFL sectioning resulted in increased anterior displacement but not end-range stiffness when compared with the intact ankle. With inversion loading, combined ATFL and CFL sectioning resulted in increased range of motion and decreased end-range stiffness when compared with the intact and ATFL-sectioned ankles.
Conclusions:
The absence of change in anterior end-range stiffness between the intact and ligament-deficient ankles indicated bony and other soft tissues functioned to maintain stiffness after pathologic joint displacement, whereas inversion loading of the CFL-deficient ankle after pathologic joint displacement indicated the ankle complex was less stiff when supported only by the secondary joint structures.
Key Words: ankle instability, joint laxity measurement, ankle sprains
Key Points
The injury mechanism consisted of serial sectioning of the major anatomic support structures of the lateral ankle complex.
Anterior displacement was greater in the ankles with isolated anterior talofibular ligament (ATFL) sectioning and combined ATFL and calcaneofibular ligament (CFL) sectioning than in the intact ankles, but end-range stiffness did not increase after lateral ligament sectioning, indicating that bony and other soft tissues functioned to maintain anterior stiffness after pathologic joint displacement.
With inversion loading, ankle-complex rotation increased and end-range stiffness decreased after CFL sectioning, indicating that the ankle complex was less stiff when supported only by the secondary joint structures.
Lateral ligament stress testing after an inversion ankle sprain is used to identify the presence of increased laxity within the talocrural and subtalar joints (ankle complex) when compared with the contralateral ankle.1 This assessment commonly involves the anterior drawer and inversion tests2–4 and is performed by applying an anteriorly directed force or inversion torque to the foot.5 In the biomechanical literature, researchers6–8 have shown the anterior talofibular ligament (ATFL) is the major ligamentous structure preventing forward subluxation of the talus and the calcaneofibular ligament (CFL) is the primary restraint of talar inversion. Thus, a positive anterior drawer sign indicates ATFL injury, and a positive inversion test indicates CFL injury.8
Laxity of a joint is measured as the motions of translation and rotation at a given force or torque.9,10 Increases in ankle-complex motion with isolated and combined sectioning of the ATFL and CFL have been reported extensively.2,8,10–12 General consensus in the literature is that measuring the relationship between ligament damage and joint laxity by simulating ligamentous injury in the cadaver specimen improves our understanding of joint motion and the effect ligament damage has on producing instability in the ankle-subtalar complex. Thus, objective documentation that describes change in the passive mechanical properties of the ankle complex with lateral ligament injury could be important in the differential diagnosis of these injuries.
An associated physical examination variable that evaluates the integrity of the ankle complex after injury is joint end feel.3,4 The clinician uses end feel at the end range of joint movement to identify the nature of the resistance and the pathologic limits to the joint's end range of passive motion. End feel acts as a subjective measure of the elasticity of tissue and can be quantified as the mechanical property of stiffness calculated as the change in the applied force divided by the resulting change in position length. Calculation of stiffness provides a measure of the elasticity of the affected capsuloligamentous structures and surrounding intact tissues. Given that soft tissue is more compliant at low loads, higher-force loads increase tissue stiffness as unit increases in force are produced. Lack of a solid end point implies that the ligamentous structures are injured, and the resulting end feel likely is produced by remaining intact soft and bony tissues that support the ankle complex.4
Limited biomechanical information is available regarding passive end-range stiffness characteristics of the ankle complex with anterior loading after ankle injury.2,3,13,14 With inversion loading, no authors of biomechanical studies have reported end-range stiffness characteristics for the intact ankle or for the ankle with combined lesions of the ATFL and CFL despite reporting increased instability.14–19 Therefore, the mechanical property of stiffness may be important to understanding how injury affects joint stability. Given the lack of information on the behavior of the viscoelastic properties of the ankle complex after ankle ligament injury, our understanding of the characteristics of the passive connective tissues before and after injury could be enhanced by examining these effects. Therefore, the purpose of our study was to biomechanically assess the effects of lateral ankle ligament sectioning on the load-displacement response of the ankle complex during anterior drawer and inversion loading. We hypothesized that sectioning the ATFL and CFL would (1) increase anterior and inversion ankle-complex motion and (2) decrease anterior and inversion end-range stiffness of the ankle complex.
METHODS
Design
A cross-sectional design used a within-subject, repeated-measures laboratory experiment on cadaver ankles. The independent variable (ankle condition) consisted of 3 levels: intact ankle (no injury), isolated ATFL-sectioned ankle, and combined ATFL- and CFL- (ATFL + CFL–) sectioned ankle. The dependent variables consisted of anterior and inversion motion and stiffness of the ankle complex.
Specimens
Seven fresh-frozen ankle specimens (mean donor age = 62 years; range = 53–71 years) without evidence of degenerative disease or ligamentous injury were obtained from 4 male and 3 female cadavers. The leg was separated from the rest of the limb approximately 25 cm above the ankle joint, frozen at −20°C, and thawed at room temperature before testing. The Institutional Review Board of the University of South Alabama approved the study.
Ankle Arthrometer
Ankle-complex loading was performed using the Hollis instrumented ankle arthrometer (Blue Bay Research Inc, Navarre, FL), consisting of a spatial kinematic linkage, an adjustable plate fixed to the foot, a load-measuring handle attached to the footplate through which the load was applied, and a reference pad attached to the tibia.9,10 The spatial kinematic linkage is a 6-degrees-of-freedom electrogoniometer used for measurements of applied forces and moments and the resultant translations and rotations of the ankle complex.9,10,17 The arthrometer spatial kinematic linkage connects the tibial pad to the footplate and measures the motion of the footplate relative to the tibial pad.9,10 Ankle-flexion angle is measured from the plantar surface of the foot relative to the anterior tibia and determined by the 6-degrees-of-freedom electrogoniometer. An Inspiron 1525 computer (Dell Inc, Round Rock, TX) with an analog-to-digital converter (National Instruments Corp, Austin, TX) was used to simultaneously record and calculate the data. We used a custom software program written in LabVIEW (National Instruments Corp) for data collection and reduction.
This device has been shown to be valid and reliable in the measurement of ankle-complex laxity.9,10,20 High validity of measurement has been derived by comparison with concurrent measurement of tibial-calcaneal bone motion in cadaver specimens for sagittal-plane translation (r = 0.88) and frontal-plane rotation (r = 0.86).10 High intratester and intertester reliability coefficients and measurement precision have been reported using the ankle arthrometer for anteroposterior (AP) displacement (intraclass correlation coefficient [ICC] [2,1] = 0.80–0.91, SEM = 0.58–1.02 mm) and inversion-eversion (I-E) rotation (ICC [2,1] = 0.97–0.99, SEM = 2.37°–2.4°).9,10,20
Experimental Setup
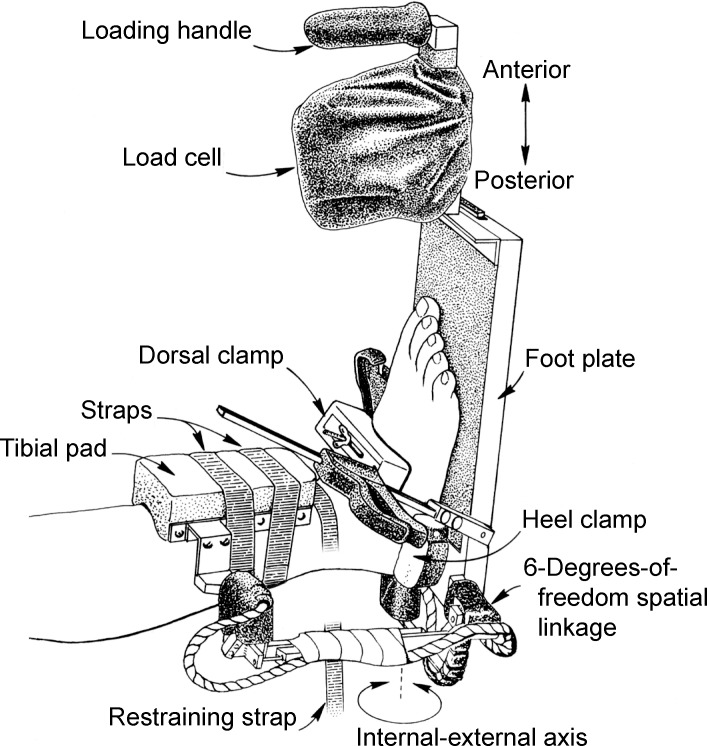
A tibial rod was screwed and cemented into the medullary cavity of the tibia down to approximately 3 cm above the ankle joint of the thawed specimen. We mounted each specimen at the proximal end of the tibial rod in a table vise with the leg positioned parallel to the floor and the foot positioned so it extended over the edge of the table to provide full freedom of motion of the ankle and subtalar joints during testing (Figure).
Figure.
Specimen testing position and arthrometric instrumentation setup used to measure ankle-complex motion.
Arthrometric Procedure
Testing replicated previous studies9,10 in which researchers reported using the ankle arthrometer. We secured the arthrometer to the foot by placing the bottom of the foot onto the footplate and adjusting the heel and dorsal clamps. The heel clamp prevented the device from rotating on the calcaneus, whereas the dorsal clamp secured the foot to the footplate. A tibial reference pad was positioned 3 to 5 cm above the ankle malleoli and secured to the leg. To minimize variation in positioning and loading with the arthrometer, 1 examiner (J.E.K.) performed all arthrometric testing.
Anterior-loading, inversion-torque, and flexion-angle positioning were applied manually through the load handle. The ankle was positioned at zero AP load and zero I-E moment at 10° of plantar flexion, which was defined as the measurement reference position.8–10 The other degrees of freedom (internal-external, medial-lateral, and proximal-distal) also were maintained at their zero-load neutral positions before loading. Starting at the neutral position, a 125-N anterior load was applied. For inversion rotation, the ankle was loaded to 4 N·m of inversion torque.9,10 The rate of loading was consistent with the rate used in previous studies involving the same instrument. Each ankle was tested by applying the AP and I-E loads over approximately a 1- to 2-second interval.8–10 The slow loading rate was intended to minimize the viscoelastic response of the soft tissues so the effects of our experimental variables could be determined.
The examiner viewed the computer monitor to control and maintain ankle-flexion angle and the amount of anterior and inversion loading. The resulting anterior-load displacement (millimeters) and inversion torque rotation (degrees of range of motion [ROM]) were recorded.
Simulated Lateral Ankle Injury
Each trial consisted of initially testing the intact ankle in anterior drawer, then in inversion.8,10 After testing the intact ankle, we sectioned the ATFL and repeated the testing. The ATFL was visualized along its length from the anterior edge of the lateral malleolus to the lateral aspect of the talar neck. Next, we sectioned the CFL and repeated the testing. The CFL was visualized along its length from the anterior edge of the fibular malleolus obliquely distal, posterior, and medial to the midlateral surface of the calcaneus. Minimal soft tissue dissection was performed to expose both ligaments, with each ligament sectioned between the midsubstance and proximal attachment.21 One examiner (J.M.H.) performed all ligament sectioning.
Data Analysis
Anterior displacement at the 125-N force load was recorded and defined as anterior motion. Inversion rotation at 4 N·m was recorded and defined as inversion motion. To quantify the elasticity of the ankle complex, secant stiffness was calculated as the change in applied force divided by the resulting change in anterior displacement and inversion rotation over the end range of loading. As the magnitude of the applied load increased beyond 50%, the ankle complex showed linear increases in stiffness.2,3,9 To measure stiffness in this range, the data were plotted as applied load versus anterior displacement (75–125 N) and inversion rotation (2–4 N·m per degree of ROM). Thus, anterior end-range stiffness was defined as force per displacement (N/mm) and was calculated by dividing 50 N (load difference between 75 and 125 N) by the anterior displacement between the 75- and 125-N loads. Inversion end-range stiffness (N·m per degree ROM) was defined as torque (N·m) per degree of ROM and was calculated by dividing 2 N·m of torque (torque difference between 2 and 4 N·m) by the inversion rotation between the 2- and 4-N·m torque loads.3,9,10
Using SPSS statistical software (version 18.0; SPSS Inc, Chicago, IL), we examined each dependent variable (anterior displacement, anterior end-range stiffness, inversion rotation, and inversion end-range stiffness) with 1-way repeated-measures analyses of variance with the within-subjects factor of ankle condition (intact, ATFL-sectioned, and ATFL + CFL–sectioned ankle). The α level was set a priori at .05. When we found a difference, we performed a post hoc paired t test corrected for α inflation by the Bonferroni procedure, which established .02 as the adjusted α level for determination of differences. We also calculated within-ankles condition effect sizes (η2 values) for all main effects. These values were interpreted as small (0.01–0.059), medium (0.06–0.139), or large (>0.14).22
RESULTS
For anterior displacement, we observed a main effect for ankle condition (F2,12 = 12.77, P = .001, η2 = 0.28). Displacement increased across sequential sectioning of the ligaments (ATFL condition, P = .006; ATFL + CFL condition, P = .007) when compared with the intact ankle condition but not between the isolated ATFL- and ATFL + CFL–sectioned ankles (P > .99). For anterior end-range stiffness, we found no main effect for ankle condition (F2,12 = 0.637, P = .55, η2 = 0.04; Table).
Table.
Summary of Ankle-Complex Motion and End-Range Stiffness Characteristics by Ankle Condition (Mean ± SD)
| Anterior |
Inversion |
|||
| Ankle Condition |
Displacement, mm |
End-Range Stiffness, N/mm |
Rotation, ° Range of Motion |
End-Range Stiffness, N·m/° Range of Motion |
| Intact | 7.63 ± 2.6a | 19.10 ± 4.8 | 17.51 ± 5.6 | 0.2775 ± 0.11 |
| Anterior talofibular ligament sectioned | 10.39 ± 3.1 | 17.34 ± 5.8 | 17.85 ± 5.8 | 0.2816 ± 0.12 |
| Anterior talofibular and calcaneofibular ligaments sectioned | 11.14 ± 2.1 | 19.69 ± 4.9 | 24.79 ± 9.2b | 0.2323 ± 0.09c |
Indicates less anterior displacement than the ankles with the anterior talofibular ligament sectioned (P = .006) and anterior talofibular and calcaneofibular ligaments sectioned (P = .007).
Indicates greater inversion rotation than the intact ankles (P = .004) and ankles with the anterior talofibular ligament sectioned (P = .008).
Indicates lower inversion end-range stiffness than the intact ankles (P = .01) and ankles with the anterior talofibular ligament sectioned (P = .02).
For inversion rotation, a main effect for ankle condition was present (F2,12 = 16.44, P < .001, η2 = 0.21). The CFL sectioning increased inversion rotation when compared with both the intact (P = .004) and the isolated ATFL-sectioned (P = .008) ankles. We did not find a difference between the intact and isolated ATFL-sectioned ankles (P = .60). For inversion end-range stiffness, we noted a main effect for ankle condition (F2,12 = 4.98, P = .02, η2 = 0.06). End-range stiffness in the ATFL + CFL–sectioned ankle was decreased when compared with both the intact (P = .01) and ATFL-sectioned (P = .02) ankles. No difference in inversion end-range stiffness was observed between the intact and isolated ATFL-sectioned ankles (P > .99; Table).
DISCUSSION
The passive response of the ankle complex to external loads is a complex combination of ankle and subtalar joint motions limited by osseous shapes and soft tissue interactions.1,8,10,11,15,23,24 Using the arthrometric method, forces and moments are controlled and applied across the ankle complex, and the resulting displacement and rotation are measured.9,10,17,19,25 These measurements reflect both the integrity and mechanical properties of the ligamentous, osseous, and cartilaginous structures of the ankle complex.10
The objective of testing after ankle-ligament sectioning was to assess and compare the passive mechanical stability characteristics of the ankle complex between the intact and ligament-deficient ankles. Numerous investigators2,5,8,10–12,15,18 have reported increased in vitro ankle-complex instability after isolated and sequential sectioning of the ATFL + CFL when loaded in AP drawer and I-E. Fewer researchers2,15,25 have reported the effects of ligament sectioning on stiffness characteristics of the ankle complex.
Compared with the intact ankle, anterior displacement increased 27% after sectioning the ATFL and 32% after sequentially sectioning the CFL. Compared with the ATFL-sectioned ankle, sequentially sectioning the CFL increased anterior displacement only 6.7% (mean = 0.75 ± 1.0 mm). Given that the anterior-displacement changes between the intact ankle and the ATFL-sectioned (mean = 2.76 ± 0.5 mm) and ATFL + CFL–sectioned (mean = 3.5 ± 0.5 mm) ankles were large, this finding implies it may be difficult to clinically distinguish anterior-displacement differences between an isolated ATFL tear and an ATFL + CFL tear. Lapointe et al12 reported that an isolated rupture of the ATFL increased anterior displacement by almost 60% when compared with the intact ankle, whereas sequentially sectioning the CFL caused only a 9% increase in anterior displacement of the ankle complex. Hollis et al8 also reported that sequential sectioning of the CFL after sectioning the ATFL produced very little change in AP displacement of the ankle complex. Because the ATFL does not cross the subtalar joint, any increased motion observed in the ankle complex likely is due to instability of the ankle joint and not due to increased instability of the subtalar joint. The increase in AP displacement after CFL sectioning likely was caused by increased subtalar displacement, which indicates that the CFL helps restrain the talus in the ankle mortise during anterior drawer loading.8
With inversion loading, sectioning the ATFL increased rotation only 2% when compared with the intact ankle. After CFL sectioning, rotation increased 29% when compared with the intact or ATFL-sectioned ankle. Clinically, these findings indicate that when loading in inversion, isolated ATFL injury may be difficult to distinguish, as evidenced by the small change in inversion rotation when compared with the intact ankle. However, inversion rotation becomes more pronounced after CFL injury, which indicates it may be easier to distinguish injury between the intact ankle and the ankle with ATFL + CFL tears. In the ATFL + CFL–sectioned ankle, the talus can be observed tilting in the ankle mortise when an adduction force is applied, which implies that the increased rotation occurs in the ankle joint.8 Given that the CFL constrains the talus through the calcaneus, injury to this ligament allows both bones to move with inversion loading. Clinically, this indicates that if talar tilt is abnormal, the CFL likely is torn.8
Our analysis of the in vitro stiffness measurements showed that lateral ligament injury produced different results based on whether the loading was performed in the sagittal or frontal plane. Sectioning the ATFL or CFL caused no change in anterior stiffness when the ankle was loaded in anterior drawer. Taga et al13 reported that mean anterior stiffness of the uninjured ankle at 50 N of anterior drawer was 24 N/mm. They also reported no change in anterior-drawer stiffness after sectioning the ATFL,13 which supports our finding of no change in anterior-drawer end-range stiffness after sectioning the ATFL. We also found minimal change in inversion end-range stiffness with inversion loading after sectioning the ATFL. However, after sequentially sectioning the CFL, we found inversion end-range stiffness was decreased when compared with the intact and ATFL-sectioned ankles. This observation illustrates that the secondary constraints to inversion loading in the CFL-deficient ankle are not as stiff as the CFL.
Stiffness is defined as the slope of the load-displacement curve over a load interval and is measured as the ratio of the change in force to the change in length. Given that the load-displacement behavior of human joints is nonlinear, the slope of this relationship depends on the magnitude and rate of the applied load and is not a singular value.2 As the stiffness of a soft tissue increases, the force needed to produce a specific elongation increases. In turn, a tissue of low stiffness cannot resist stretching as well as a tissue of high stiffness, and the former will need a lesser force than the latter to produce the same degree of deformation. This implies that soft tissues with greater stiffness could be less susceptible to injury or reinjury, such as a sprain. When soft tissues are stretched beyond their capacities to resist permanent lengthening and are torn, they cannot return to their original lengths after the stretching or injurious force is removed.26 Any difference between the original length and the new length represents plastic deformation, which is correlated with degree of tissue damage. In laboratory studies,27,28 researchers have demonstrated that some degree of mechanical weakening takes place in permanently elongated connective tissue structures. Thus, when the ankle complex is injured, ligamentous tissues that are affected may not return to their original lengths, which could cause increased joint instability and decreased tissue stiffness.
Our study had limitations. Given that this was a cadaveric study, the dynamic effects of the stabilizing muscles could not be incorporated. Thus, we can report only the intrinsic passive stability that was provided by the joint, osseous, and soft tissues of the ankle complex. When partial tears or ruptures of the lateral ligaments are encountered in the clinical setting, the influence of the neuromuscular component on joint stability also will be present, at least to some extent. The in vivo laxity values, therefore, should be expected to be similar to or lower than those found in our study.
CONCLUSIONS
Cadaver ankle-complex loading showed that anterior displacement increased but anterior end-range stiffness did not change after sectioning of the lateral ligaments. The absence of change in anterior stiffness between the intact and ligament-deficient ankles indicates that bony and other soft tissues functioned to maintain stiffness after pathologic joint displacement. After the CFL was sectioned, inversion loading produced an increase in rotation along with a decrease in end-range stiffness. These results indicate that the ankle complex is less stiff when supported only by the secondary joint structures with inversion loading.
REFERENCES
- 1.Bahr R, Pena F, Shine J, et al. Mechanics of the anterior drawer and talar tilt tests: a cadaveric study of lateral ligament injuries of the ankle. Acta Orthop Scand. 1997;68(5):435–441. doi: 10.3109/17453679708996258. [DOI] [PubMed] [Google Scholar]
- 2.Tohyama H, Beynnon BD, Renstrom PA, Theis MJ, Fleming BC, Pope MH. Biomechanical analysis of the ankle anterior drawer test for anterior talofibular ligament injuries. J Orthop Res. 1995;13(4):609–614. doi: 10.1002/jor.1100130417. [DOI] [PubMed] [Google Scholar]
- 3.Kovaleski JE, Norrell PM, Heitman RJ, Hollis JM, Pearsall AW IV. Knee and ankle position, anterior drawer laxity, and stiffness of the ankle complex. J Athl Train. 2008;43(3):242–248. doi: 10.4085/1062-6050-43.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starkey C, Brown SD, Ryan J. Examination of Orthopedic & Athletic Injuries. 3rd ed. Philadelphia, PA: FA Davis Company;; 2010. [Google Scholar]
- 5.Kjaersgaard-Andersen P, Frich LH, Madsen F, Helmig P, Søgård P, Søjbjerg JO. Instability of the hindfoot after lesion of the lateral ankle ligaments: investigations of the anterior drawer and adduction maneuvers in autopsy specimens. Clin Orthop Relat Res. 1991;266:170–179. [PubMed] [Google Scholar]
- 6.Hintermann B. Biomechanics of ligaments in ankle instability. In: Nyska M, Mann G, editors. The Unstable Ankle. Champaign, IL: Human Kinetics;; 2002. pp. 27–35. In. eds. [Google Scholar]
- 7.Colville MR, Marder RA, Boyle JJ, Zarins B. Strain measurement in lateral ankle ligaments. Am J Sports Med. 1990;18(2):196–200. doi: 10.1177/036354659001800214. [DOI] [PubMed] [Google Scholar]
- 8.Hollis JM, Blasier RD, Flahiff CM. Simulated lateral ankle ligamentous injury. Am J Sports Med. 1995;23(6):672–677. doi: 10.1177/036354659502300606. [DOI] [PubMed] [Google Scholar]
- 9.Kovaleski JE, Gurchiek LR, Heitman RJ, Hollis JM, Pearsall AW., IV Instrumented measurement of anteroposterior and inversion-eversion laxity of the normal ankle joint complex. Foot Ankle Int. 1999;20(12):808–814. doi: 10.1177/107110079902001210. [DOI] [PubMed] [Google Scholar]
- 10.Kovaleski JE, Hollis JM, Heitman RJ, Gurchiek LR, Pearsall AW., IV Assessment of ankle-subtalar joint complex laxity using an instrumented ankle arthrometer: an experimental cadaveric investigation. J Athl Train. 2002;37(4):467–474. [PMC free article] [PubMed] [Google Scholar]
- 11.Kerkhoffs G, Blankevoort L, van Poll D, Marti RK, van Dijk CN. Anterior lateral ankle ligament damage and anterior talocrural-joint laxity: an overview of the in vitro reports in literature. Clin Biomech (Bristol, Avon) 2001;16(8):635–643. doi: 10.1016/s0268-0033(01)00054-7. [DOI] [PubMed] [Google Scholar]
- 12.Lapointe SJ, Siegler S, Hillstrom H, Nobilini RR, Mlodzienski A, Techner L. Changes in the flexibility characteristics of the ankle complex due to damage to the lateral ligaments: an in vitro and in vivo study. J Orthop Res. 1997;15(3):331–341. doi: 10.1002/jor.1100150304. [DOI] [PubMed] [Google Scholar]
- 13.Taga I, Shino K, Inoue M, Nakagawa S, Maeda A. In vivo measurement of instability of the ankle. Trans Orthop Res Soc. 1992;17:470. [Google Scholar]
- 14.Kerkhoffs GM, Blankevoort L, Schreurs AW, Jaspers JE, van Dijk CN. An instrumented, dynamic test for anterior laxity of the ankle joint complex. J Biomech. 2002;35(12):1665–1670. doi: 10.1016/s0021-9290(02)00189-6. [DOI] [PubMed] [Google Scholar]
- 15.Siegler S, Chen J, Schneck CD. The three-dimensional kinematics and flexibility characteristics of the human ankle and subtalar joints, part I: kinematics. J Biomech Eng. 1988;110(4):364–373. doi: 10.1115/1.3108455. [DOI] [PubMed] [Google Scholar]
- 16.Siegler S, Wang D, Plasha E, Berman AT. Technique for in vivo measurement of the three-dimensional kinematics and laxity characteristics of the ankle joint complex. J Orthop Res. 1994;12(3):421–431. doi: 10.1002/jor.1100120315. [DOI] [PubMed] [Google Scholar]
- 17.Siegler S, Lapointe S, Nobilini R, Berman AT. A six-degrees of freedom instrumented linkage for measuring the flexibility characteristics of the ankle joint complex. J Biomech. 1996;29(7):943–947. doi: 10.1016/0021-9290(95)00165-4. [DOI] [PubMed] [Google Scholar]
- 18.Takebayashi T, Yamashita T, Sakamoto N, Yamada Y, Minaki Y, Ishii S. Biomechanical characteristics of the lateral ligaments of the ankle joint. J Foot Ankle Surg. 2002;41(3):154–157. doi: 10.1016/s1067-2516(02)80064-3. [DOI] [PubMed] [Google Scholar]
- 19.Liu W, Siegler S, Techner L. Quantitative measurement of ankle passive flexibility using an arthrometer on sprained ankles. Clin Biomech (Bristol, Avon) 2001;16(3):237–244. doi: 10.1016/s0268-0033(00)00088-7. [DOI] [PubMed] [Google Scholar]
- 20.Hubbard TJ, Kovaleski JE, Kaminski TW. Reliability of intratester and intertester measurements derived from an instrumented ankle arthrometer. J Sport Rehabil. 2003;12(3):208–220. [Google Scholar]
- 21.Burks RT, Morgan J. Anatomy of the lateral ankle ligaments. Am J Sports Med. 1994;22(1):72–77. doi: 10.1177/036354659402200113. [DOI] [PubMed] [Google Scholar]
- 22.Leardini A, O'Connor JJ, Catani F, Giannini S. The role of the passive structures in the mobility and stability of the human ankle joint: a literature review. Foot Ankle Int. 2000;21(7):602–615. doi: 10.1177/107110070002100715. [DOI] [PubMed] [Google Scholar]
- 23.Green SB, Salkind NJ, Akey TM. Using SPSS for Windows: Analyzing and Understanding Data. 2nd ed. Upper Saddle River, NJ: Prentice-Hall Inc;; 2000. p. 159. [Google Scholar]
- 24.Chen J, Siegler S, Schneck CD. The three-dimensional kinematics and flexibility characteristics of the human ankle and subtalar joint, part II: flexibility characteristics. J Biomech Eng. 1988;110(4):374–385. doi: 10.1115/1.3108456. [DOI] [PubMed] [Google Scholar]
- 25.Siegler S, Block J, Schneck CD. The mechanical characteristics of the collateral ligaments of the human ankle joint. Foot Ankle. 1988;8(5):234–242. doi: 10.1177/107110078800800502. [DOI] [PubMed] [Google Scholar]
- 26.Hubbard TJ, Cordova M. Mechanical instability after an acute lateral ankle sprain. Arch Phys Med Rehabil. 2009;90(7):1142–1146. doi: 10.1016/j.apmr.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 27.Warren CG, Lehmann JF, Koblanski JN. Elongation of rat tail tendon: effect of load and temperature. Arch Phys Med Rehabil. 1971;52(10):465–474. [PubMed] [Google Scholar]
- 28.Warren CG, Lehmann JF, Koblanski JN. Heat and stretch procedures: an evaluation using rat tail tendon. Arch Phys Med Rehabil. 1976;57(3):122–126. [PubMed] [Google Scholar]