Abstract
Objectives
To examine the confluence of depression, cognitive impairment, and vascular risk factors in older individuals.
Methods
The study uses baseline data from the National Alzheimer’s Coordinating Center. Data was collected across Alzheimer’s Disease Centers in the US. The sample included 12,634 individuals (Cognitive Intact = 8022; amnestic Mild Cognitive Impairment [aMCI] = 3652; nonamnestic MCI [nonaMCI] = 960). The Geriatric Depression Scale assessed depression; Trail Making Test assessed executive function.
Results
The proportion of participants with depression was higher in the aMCI (18%) and nonaMCI group (21%) as compared to the cognitively intact group (8%); there was no difference in rates of depression between aMCI and nonaMCI groups. The proportion of participants with executive dysfunction differed between nondepressed and depressed individuals for the cognitively intact (8% vs. 12%) and aMCI groups (28% vs. 35%) but not for the nonaMCI group (37% vs. 41%). 9% of the cognitively intact group had executive dysfunction compared to 31% of the aMCI and 40% of the nonaMCI groups. The proportion of participants with hypertension was greater in individuals with executive dysfunction compared to those with no executive deficits; the presence of hypertension was not associated with depression severity.
Conclusions
The confluence of vascular risk factors, episodic memory impairment, depression and executive dysfunction highlight the need for comprehensive assessment of depressed older adults that can aid clinicians in the formulation of treatment planning and inform clinicians and researchers about long-term prognosis.
Keywords: geriatrics, depression, mild cognitive impairment, vascular risk factors
Introduction
The confluence of depression and cognitive impairment in late life can occur as a result of different underlying pathologies, with different clinical courses, treatment responses, and outcomes. Decades ago, the prevailing notion was that cognitive impairment in the context of late life depression was reversible with remission of the depressive disorder.(Emery, 1988, Kiloh, 1981) It has since been shown, however, that even with cognitive improvement following treatment, depressed patients with comorbid cognitive impairment at baseline were still at higher risk for the development of dementia at follow-up compared to those patients who had depression and no baseline cognitive impairment.(Alexopoulos et al., 1993, Butters et al., 2000, Nebes et al., 2003) More recently, researchers studying the association between late life depression and cognitive impairment have focused specifically on two patient groups: older adults with depression and mild cognitive impairment (MCI)(Lyketsos et al., 2002, Rosenberg et al., 2011, Devanand et al., 2003, Reynolds et al., 2011, Barnes et al., 2006, Lopez et al., 2005) and older adults with comorbid executive dysfunction (vascular depression subtype).(Alexopoulos et al., 1997, Sneed et al., 2008, Sneed et al., 2007)
Patients with depression and cognitive impairment have a 2–3 fold increased risk of transition to Alzheimer’s disease (AD) compared to nondepressed elders with cognitive impairment.(Alexopoulos et al., 1993, Devanand et al., 1996, Devanand et al., 2003) Treating depressed patients with MCI with antidepressant medication provides mixed results for disentangling the relationship between depression and cognitive impairment. In one study, responders to antidepressant medication did not have cognitive improvement at follow-up, suggesting that the cognitive impairment is not due to the depression per se.(Devanand et al., 2003) Similarly, a pilot study of depressed patients with cognitive impairment showed cognitive improvement only when antidepressant medication was augmented with the cognitive-enhancer, donepezil.(Pelton et al., 2008) Recently, in a larger randomized double blind placebo-controlled trial of donepezil augmentation to antidepressant medication, patients with depression and MCI in the donepezil + antidepressant group had a lower rate of conversion to dementia than patients in the placebo + antidepressant group; patients in the donepezil group also had greater improvement in global cognition and cognitively-mediated instrumental activities of daily living compared to patients in the placebo + antidepressant group; patients in the donepezil group, however, also had a greater recurrence of the major depressive illness at follow-up.(Reynolds et al., 2011)
The vascular depression subtype, which is characterized by patients with depression and comorbid executive dysfunction, is another clinical manifestation of co-occurring depression and cognitive impairment. The depressive symptoms and executive dysfunction in patients with the vascular depression subtype(Taylor et al., 2013) are believed to be related to cerebral white matter hyperintensities,(de Groot et al., 2000, Taylor et al., 2003) and associated with vascular risk factors such as hypertension, diabetes, obesity, and coronary disease.(Sheline et al., 2010, Brickman et al., 2010) Patients with vascular depression are less likely to respond to antidepressant medication.(Alexopoulos et al., 2005, Sneed et al., 2007)
Older adults with late life depression and MCI and older adults with a vascular depression do not, however, represent mutually exclusive groups. In fact, older adults with depression and cognitive impairment may have multiple underlying pathologies that impact treatment response and dictate prognostic trajectory. Butters and colleagues(Butters et al., 2008) outlined five potential pathways in a review of the literature that explain the confluence of depression with cognitive impairment; these pathways include 1) depression with normal cognition, 2) depression with depression-associated neuropathology (hippocampal atrophy) that leads to cognitive impairment that is stable over time, 3) depression with Alzheimer’s neuropathology that leads to cognitive impairment and progression to dementia, 4) a combined cerebrovascular and Alzheimer’s pathology resulting in frontostriatal damage and hippocampal volume loss and leading to depression and/or cognitive impairment and progression to Alzheimer’s disease with cerebrovascular disease, and 5) cerebrovascular disease with frontostriatal damage that leads to depression and cognitive impairment and progresses to vascular dementia.
Thus both the MCI, and in particular the episodic memory dysfunction that denotes an amnestic MCI, and executive dysfunction status of the depressed patient independently communicate important information that should impact clinical management.(Royall et al., 2012, Royall and Palmer, 2013) Therefore, we sought to examine the confluence of depression, cognitive impairment, and vascular risk factors in a diverse group of cognitively intact and cognitively impaired older individuals. We hypothesized that the prevalence of depression would not differ between older adults with aMCI and those with nonaMCI(Lockwood et al., 2000, Lopez et al., 2005). Additionally, we hypothesized that depression would be more prevalent in both cognitively impaired groups compared with the cognitively intact group(Richard et al., 2013). We also hypothesized that depressed patients in both the aMCI and nonaMCI groups will have greater rates of executive dysfunction(Rosenberg et al., 2011) and vascular risk factors than nondepressed patients (Valkanova and Ebmeier, 2013).
To address these hypotheses, we used data from the National Alzheimer’s Coordinating Center (NACC) which runs a consortium of Alzheimer’s Disease Centers (ADCs) studying a large cohort of cognitively intact and cognitively impaired older adults, a subset of whom report significant depressive symptoms. By using these data gathered from patients at 34 past and present ADCs, we can delineate these older adults by cognitive category (cognitively intact, amnestic MCI, nonamnestic MCI), depression status, comorbid executive dysfunction, and vascular risk factors. In doing so, we can improve the field’s understanding of the confluence of cognitive impairment and depression, thereby highlighting the need for comprehensive assessment of older depressed patients and resulting in more accurate diagnosis and treatment planning.
Methods
Participants
12,634 adults completed the initial NACC assessment between 2005 and 2009 at ADCs across the country and were categorized according to their memory (those classified as “probable or possible dementia” or as “impaired, not MCI” at the ADCs were excluded from this study and not included in the total of 12,634). Criteria for the classification of MCI were made across all ADCs(Beekly et al., 2007) using guidelines set forth by the International Working Group on Mild Cognitive Impairment.(Winblad et al., 2004) After it was determined that participants did not have normal memory or dementia, MCI classification was made. If memory impairment was present based on clinical judgment and/or neuropsychological tests, the person was categorized as aMCI; a similar approach was used to determine whether other cognitive domains are impaired. If memory problems did not exist but other cognitive domains were impaired, the patient was categorized as nonaMCI. Classification was made based on a consensus conference or a single clinician decision using clinical judgment and/or neuropsychological tests; single- and multiple-domain aMCI and nonaMCI were included.
Measures
Geriatric Depression Scale
Depressive symptoms were assessed using the 15-item Geriatric Depression Scale (GDS).(Yesavage et al., 1982) The 15-item GDS(Sheikh and Yesavage, 1986, Brown et al., 2007) utilizes a yes/no answer format. Scores range from 0 to 15, with a cut-off of 5 or greater on this short-form 15-item GDS shown to be highly sensitive to the identification of major depression.(Brown et al., 2007, Lesher and Berryhill, 1994) This cut-off was used to denote the depressed group in this study. In the regression analysis, raw scores on the 15-item GDS were used to denote depression severity.
Hypertension
Patients were classified as having a history or presence of hypertension or not based on clinician ratings to the hypertension item on the modified Hachinski ischemic scale,(Hachinski et al., 1975) a clinician-rated scale used to assess hypertension, stroke, and/or neurologic signs/symptoms.
Diabetes Mellitus
Patients were coded as being diabetic if ADC clinicians originally classified them as having “recent/active” diabetes. Patients whose diabetes were classified as “absent”, “remote/inactive”, or “unknown” were recoded as not having diabetes.
Body Mass Index
Body Mass Index (BMI) was calculated using weight and height data from the NACC database. The equation used to calculate BMI was: BMI = [Weight in lbs / (Height in inches)2] × 703. Using the Centers for Disease Control and Prevention criteria for classification of BMI groups (http://www.cdc.gov/healthyweight/index.html), patients with BMI’s less than 25 were classified as Normal; greater than or equal to 25 and less than 30 were classified as Overweight; those patients with BMI’s greater than or equal to 30 were classified as Obese. For the purposes of this paper, we grouped overweight and obese patients together. In the regression analysis, the continuous variable BMI was input into the model.
Executive dysfunction
Executive dysfunction was measured by Trail-Making Test A and B (Trails A and B).(Reitan, 1958) We calculated executive scores by subtracting Trails A from Trails B, a standard way of scoring the Trail-Making test as a measure of executive dysfunction (accounting for processing/motor speed). Using the cognitively intact depressed group as our reference group (Trails B – Trails A: M = 65.98 s, SD = 48.01), we classified individuals as having “executive dysfunction” if they had executive scores greater than or equal to 1 SD above the mean (Trails B – Trails A ≥ 114). In the regression analysis, raw scores were input for Trails B (independent variable) and Trails A (covariate) to account for processing/motor speed. These methods or similar methods have been used in prior research on executive function(Snitz et al., 2013, Brown et al., 2013).
Statistical Analysis
Analysis of variance or Chi square tests were used to detect group differences for continuous and categorical variables. In the post hoc group comparisons, Bonferroni correction on false positive error rate was used to account for multiple comparisons. Multinomial logistic regression models for the three groups (cognitively intact, aMCI, and nonaMCI) were used to assess the simultaneous effect of depression severity, executive dysfunction severity, and vascular risk factors including hypertension, body mass (BMI), and diabetes. Covariates for the multinomial models included age, gender, education, and Trails A. Missing data was noted where applicable.
Results
Of the total 12,634 patients who completed an initial NACC assessment, 8022 were classified as cognitively intact (71% by consensus diagnosis), 3652 were classified as aMCI (83% by consensus diagnosis), and 960 were classified as nonaMCI (90% by consensus diagnosis). Characteristics for these three samples are listed in Table 1. Patients in the aMCI group were significantly older and better educated with greater deficits on MMSE, Logical Memory Delayed and greater informant reported functional impairment than patients in the nonaMCI group. Depressive symptoms on the GDS were greater (F2, 12,631 = 355.52, p < .001) in cognitively impaired elders, with significant differences observed in the aMCI and nonaMCI groups as compared to the cognitively intact group (18% of aMCI, 21% of nonaMCI had 15-item GDS scores > 5 compared with only 8% of cognitively intact); there was no difference in depressive symptoms between the aMCI and nonaMCI groups. The proportion of participants with executive dysfunction differed significantly across groups (χ24 = 1525.86, P < .001), with a larger proportion of the nonaMCI group (38%) showing executive dysfunction compared to the aMCI (29%) and cognitively intact groups (9%).
Table 1.
Baseline characteristics in cognitively intact, aMCI, and nonaMCI groups.
| Variable | Cognitively Intact N = 8022 |
aMCI n = 3652 |
nonaMCI n = 960 |
|---|---|---|---|
| Demographic | Mean (SD) | Mean (SD) | Mean (SD) |
| Age | 71.59 (10.67) | 74.50 (9.40)a | 72.21 (9.69)c |
| Education | 15.44 (3.08) | 14.85 (3.52)a | 14.44 (3.73)b,c |
| Gender M/F (% F) | 2761/5261 (66%) |
1798/1854 (51%)a |
433/527 (55%)b |
| Race | |||
| Caucasian | 6488 (81%) | 2925 (80%) | 702 (73%)b,c |
| African American | 1201 (15%) | 495 (14%) | 189 (20%) |
| Other | 312 (4%) | 226 (6%) | 61 (6%) |
| Missing | 21 (<1%) | 6 (<1%) | 8 (1%) |
| Neuropsychological | |||
| MMSE | 28.85 (1.46) | 26.90 (2.52)a | 27.55 (2.34)b,c |
| Logical Mem Delayed | 12.10 (4.26) | 6.01 (4.51)a | 9.73 (4.26)b,c |
| Trail-making Test A (seconds) | 35.43 (17.04) | 46.98 (25.42)a | 48.43 (24.39)b |
| Trail-making Test B (seconds) | 93.27 (53.00) | 142.89 (78.24)a | 160.28 (83.67)b,c |
| Executive Dysfunction Groups No/Yes (% Yes in group) | 7085/685 (9%) | 2377/1069 (29%)a | 541/360 (38%)b,c |
| Digit Symbol Substitution Task | 46.83 (13.01) | 36.36 (12.70)a | 35.44 (12.50)b |
| Clinical Dementia Rating | |||
| CDR Global 0/0.5 (% 0.5) | 7154/868 (11%) | 270/3310 (91%) | 251/686 (72%) |
| Diagnosis: Clinician/Consensus (% Consensus) | 2328/5694 (71%) | 627/3025 (83%)a | 96/864 (90%)b,c |
| Physical Health | |||
| Hypertension No/Yes (% Yes) | 4067/3768 (47%) | 1641/1991 (55%)a | 380/557 (58%)b |
| Diabetes No/Yes (% Yes) | 7161/861 (11%) | 3160/492 (14%)a | 795/165 (17%)b,c |
| Body Mass Index | 26.96 (5.91) | 26.29 (5.88)a | 26.85 (6.43)c |
| Normal/Overweight Obese (% Overweight/Obese) | 2923/4678 (62%) |
1413/1980 (58%)a |
336/539 (62%) |
| Psychiatric | |||
| Geriatric Depression Scale | 1.43 (2.12) | 2.55 (2.70)a | 2.76 (2.95)b |
| Geriatric Depression Scale ≥ 5, % (n) | 8% (619) | 18% (667)a | 21% (197)b |
| Function | |||
| Functional Activities Questionnaire | 0.39 (1.73) | 3.73 (5.25)a | 3.01 (4.91)b,c |
Note.
Abbreviations: MCI, mild cognitive impairment; aMCI, amnestic MCI; nonaMCI, nonamnestic MCI; MMSE, Mini Mental State Exam; Logical Mem Delayed, Logical Memory II (delayed recall); CDR, Clinical Dementia Rating Scale; Executive Dysfunction groups based on Trail-making Test B – Trail-making Test A ≥ 114 (1 SD worse than the cognitively intact depressed sample). Race, Other = this category combined rates from the American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander, Asian, or Other categories as rates within each of these categories were low in all three groups (those classified in the NACC data as Missing remained in this category). Percentages are based on total sample. Data presented as mean (SD) unless otherwise indicated. Neuropsychological scores are raw scores. Post hoc comparisons were conducted only when the omnibus effect in the Analysis of variance or Chi square tests was significant.
Significant difference in post hoc comparisons between the cognitively intact and aMCI groups (p<.01).
Significant difference in post hoc comparisons between cognitively intact and nonaMCI groups (p<.01).
Significant difference in post hoc comparisons between aMCI and nonaMCI groups (p<.01).
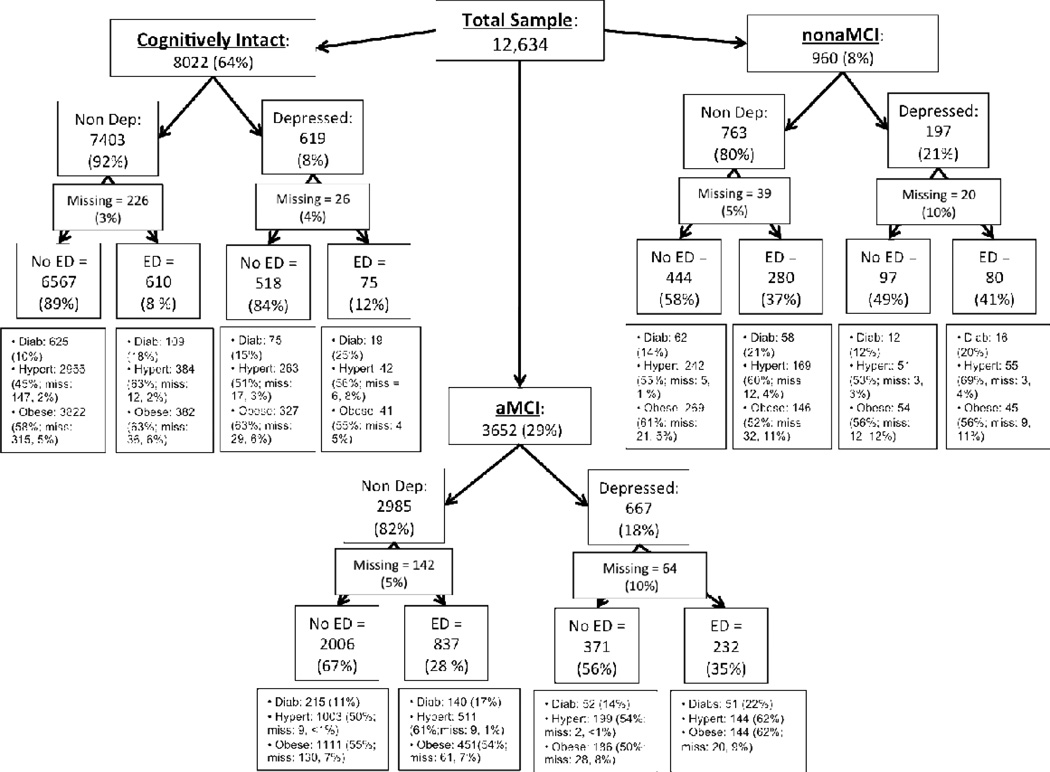
Figure 1 depicts the prevalence of depression, executive dysfunction, hypertension, overweight/obesity, and diabetes across cognitively intact, aMCI, and nonaMCI groups. Multinomial logistic regression analyses assessed the simultaneous effect of depression severity (total score on the 15-item GDS), executive dysfunction (total score on Trails B), hypertension, body mass (total BMI), and diabetes on group membership, with age, sex, educational level, and Trails A entered as covariates into each model. Table 2 lists the covariate-adjusted odds ratios for the group comparisons in the models. The cognitively impaired groups were associated with greater depressive symptomatology (aMCI: Wald χ21=286.52, p < .001; nonaMCI: Wald χ21 = 128.88, p < .001) and levels of executive dysfunction (aMCI: Wald χ21 = 489.95, p < .001; nonaMCI: Wald χ21 = 420.43, p < .001), and lower body mass (aMCI: Wald χ21 = 36.10, p < .001; nonaMCI: Wald χ21 = 8.66, p = .003) compared with the cognitively intact group. A larger proportion of the nonaMCI group had hypertension compared with both the cognitively intact (Wald χ21= 6.16, p = .013) and aMCI groups (Wald χ21 = 5.86, p = .016), as well as marginally greater levels of executive dysfunction compared with the aMCI group. Of note was whether or not there was a significant 2-way interaction (depression severity × executive dysfunction, depression severity × hypertension, depression severity × body mass, depression severity × diabetes, hypertension × diabetes, hypertension × executive dysfunction, hypertension × body mass, diabetes × executive dysfunction, diabetes × body mass, body mass × executive dysfunction) that differentiated the nonaMCI and aMCI groups. None of these interactions however were significant.
Figure 1.
The frequency of depression, executive dysfunction, and vascular risk factors in cognitively intact, amnestic mild cognitively impaired and nonamnestic mild cognitively impaired older adults from the National Alzheimer’s Coordinating Center.
Note. Abbreviations: Cognitively intact, Individuals classified as cognitively intact; aMCI, individuals classified with amnestic mild cognitive impairment; nonaMCI, Individuals classified with nonamnestic mild cognitive impairment; Non Dep, Nondepressed (Individuals with total scores on the Geriatric Depression Scale < 5); Depressed, Individuals with total scores on the Geriatric Depression Scale > 5; No ED, Individuals with scores on Trails B – Trails A ≤ 114; ED, Individuals with scores on Trails B – Trails A ≥ 114. Hypert, Individuals with clinician-rated hypertension on the modified Hachinski ischemic scale; Diab, Individuals with clinician-rated diabetes; Obese, Individuals with Body Mass Indexes ≥ 25 (categorizes overweight and obesity together). Missing data noted where applicable.
Table 2.
Covariate-adjusted odds ratios (95% confidence intervals) for comparing cognitively intact, aMCI, and nonaMCI groups.
| Group | |||
|---|---|---|---|
| Predictors | aMCI vs. Cognitively Intact OR (95% CI) |
nonaMCI vs. Cognitively Intact OR (95% CI) |
aMCI vs. nonaMCI OR (95% CI) |
| Geriatric Depression Scale | 1.179 (1.157, 1.202)c | 1.176 (1.143, 1.209)c | 1.003 (0.976, 1.030) |
| Trailmaking Test B | 1.011 (1.010, 1.012)c | 1.014 (1.013, 1.015)c | 0.997 (0.996, 0.998)c |
| Hypertension | 1.001 (0.908, 1.102) | 1.231 (1.045, 1.451)a | 0.813 (0.687, 0.961)b |
| Diabetes | 0.958 (0.829, 1.108) | 1.049 (0.840, 1.310) | 0.913 (0.730, 1.142) |
| Body Mass Index | 0.975 (0.967, 0.983)c | 0.981 (0.968, 0.993)b | 0.994 (0.981, 1.007) |
Abbreviations: aMCI, amnestic mild cognitive impairment; nonaMCI, nonamnestic mild cognitive impairment. Lower scores on Trailmaking Test B denote better performance. Covariates adjusted for age, sex, educational level, and Trailmaking Test A.
P < .05.
P < .01.
P < .001.
We examined the covariate-adjusted main effect of hypertension on group more closely, as hypertension and executive dysfunction were the only independent variables to significantly differentiate the aMCI and nonaMCI groups in the multinomial regression model. 58% of older adults with nonaMCI had clinician-reported hypertension compared to 55% of older adults with aMCI. Although this difference was not significant in the preliminary chi-square analysis, after covarying for age, education, gender, Trails A, Trails B, diabetes, body mass, and total GDS score, the difference was significant (Table 2). It appears however that this difference may be due more to the proportion of executive dysfunction than the aMCI/nonaMCI classification system. Although the executive dysfunction × hypertension interaction in the multinomial model was not significant, an exploration of the data show that in the total sample, 62% of older adults with executive dysfunction had hypertension compared to 47% of older adults without executive dysfunction, a nontrivial difference. It may be that although not statistically detected the higher preponderance of executive dysfunction in nonaMCI compared with aMCI groups (38% vs. 29%) may be responsible for the effect of hypertension observed in the multinomial model.
Discussion
The findings from this study highlight the complexity of the confluence between depression and cognitive impairment in older adults. The data from this study show that: 1) depression is common in older adults with both aMCI and nonaMCI, 2) executive dysfunction is common not only in older adults with nonaMCI but in older adults with aMCI, and 3) vascular risk factors, specifically hypertension, are associated with comorbid executive dysfunction but not depression severity as originally hypothesized. The latter finding provides evidence for the independence of vascular risk factors and depression in older adults. As Barnes and colleagues previously reported,(Barnes et al., 2006) depressive symptoms were associated with increased risk of MCI, independent of vascular risk factors. Hypertension however was the only vascular risk factor to demonstrate such an effect in this study. Body mass was greater in the cognitively intact vs. cognitively impaired groups, counterintuitive to what one would expect, and was not associated with depression (57% of both the nondepressed and depressed elders in this study were overweight/obese) or executive dysfunction (57% of older adults with executive dysfunction in this study were overweight/obese compared with 58% without executive dysfunction). And although diabetes was more commonly found in both older adults with executive dysfunction (19% compared to 10% of the older adults with no executive dysfunction) and depression (16% of depressed elders had clinician-rated history of diabetes compared with 12% of the nondepressed elders), this effect was not significant in the multinomial models suggesting that it may be due to the covariates or other independent variables such as hypertension.
These data highlight a basic but significant message to geriatric psychiatrists: the high frequency of the confluence between hypertension, episodic memory impairment, and executive dysfunction should result in systematic, thorough assessments in geriatric clinics. Although research suggests that it is the presence of white matter hyperintensities and executive dysfunction that predict response to antidepressant treatment in late life depression, antidepressant response may also be impacted by underlying Alzheimer’s pathology, or the interaction between cerebrovascular disease and Alzheimer’s pathology. Without thorough assessment of our patients at baseline, however, it becomes difficult to disentangle these issues as well as enact an adequate treatment plan.
The results reinforce the multiple pathways outlined by Butters and her colleagues (2008) about the progression of depressive illness and cognitive impairment in late life. In particular, the data in this study reflect how common the occurrence may be for patients to present with combined cerebrovascular and Alzheimer’s pathology resulting in depression and cognitive impairment from both frontostriatal damage and hippocampal volume loss (Pathway IV in Butters and colleagues model(Butters et al., 2008)), leading to Alzheimer’s disease with comorbid cerebrovascular disease. Twenty-nine percent of older adults with aMCI had comorbid executive dysfunction (31% if you exclude missing data), and this proportion is greater (39%) in the aMCI subgroup with significant depressive symptoms, a rate of executive dysfunction not dissimilar to the 45% observed in the nonaMCI subgroup with significant depressive symptoms.
Although methods such as imaging and histopathology are necessary to document the underlying pathology of illness, the clinical and neuropsychological assessments that we present in this paper have been strongly associated with pathology in prior research. For instance, Monsell and her colleagues(Monsell et al., 2013) illustrate how the items on the modified Hachinski ischemic scale used in this study are associated with both clinical presentation and neuropathology. Patients who were asymptomatic (Clinical Dementia Rating Scale score of 0) had lower Hachinski scores, were less likely to have an expression of the ε4 allele of apolipoprotein E, and had lower neurofirillary tangle scores at autopsy. Similarly, a recent investigation(Gustafson et al., 2010) showed that clinical scales including items similar to the ones utilized on the modified Hachinski ischemic scale create a symptom pattern picture that matches up well with neuropathological diagnosis of different types of dementia. Recently, findings from Snitz and colleagues(Snitz et al., 2013) showed a greater decline over time in neuropsychological testing including Trails A and B for patients who were Aβ-positive as compared to patients who were Aβ-negative. Relatedly, it has been shown that executive function as measured by amongst other neuropsychological tests, Trails B, was associated with frontal and parietal periventricular white matter changes(Chen et al., 2009). Episodic memory dysfunction and the classification of aMCI are strongly associated with both increased likelihood of conversion to dementia and atrophy of the medial temporal lobe, a neurodegenerative feature of Alzheimer’s disease.(Brown et al., 2011, Shen et al., 2011, Sarazin et al., 2010, Wolk and Dickerson, 2011) Similarly, the presence of executive dysfunction and the association in this sample between executive dysfunction and vascular risk factors such as hypertension strongly suggest the presence of or increased risk for cerebrovascular disease.(Culang-Reinlieb et al., 2010, Jokinen et al., 2009, Gunning-Dixon and Raz, 2003, Sheline et al., 2008, Taylor et al., 2013)
These data have important clinical and research implications. Clinically, comprehensive assessment results in the formulation of more accurate treatment planning and can inform clinicians about the patient’s long-term trajectory. A comprehensive assessment should provide 1) the information necessary to make a DSM V diagnosis of a mood disorder and a measurement of severity, 2) review of vascular risk factors that let the clinician estimate the probability that the patient has ischemic cerebrovascular disease even in the absence of neurological findings, and 3) testing of cognitive function so that the patient can be categorized as having normal cognitive status, nonaMCI, aMCI and/or executive dysfunction. There are a number of instruments that can accomplish each task and each clinician can develop the systematic comprehensive assessment procedure that they prefer. If a 72-year-old depressed male presents at a physician’s office with memory complaints, only a comprehensive medical, clinical, and neuropsychological assessment will lead to the implementation of the best treatment plan. Does this patient have executive dysfunction? Episodic memory dysfunction? History of hypertension or diabetes? There are treatment implications based on the findings from each of these assessments. It allows for the differentiation between depression without cognitive impairment, depression with episodic memory dysfunction and no executive dysfunction (either depression with depression-associated neuropathology or depression with Alzheimer’s neuropathology), depression with executive dysfunction (vascular depression), and depression with both executive and episodic memory dysfunction (vascular depression with a high likelihood of underlying cerebrovascular disease and AD pathology). The latter two scenarios require multifaceted treatment implementation: 1) the treatment of untreated vascular risk factors, 2) the treatment of depression (pharmacotherapy, therapy, or both), and 3) the presence of episodic memory dysfunction may warrant the initiation of cholinesterase inhibitors.(Pelton et al., 2008, Reynolds et al., 2011) Thorough assessment will also inform doctors of long-term prognosis. Early identification of incipient Alzheimer’s disease can lead to earlier enrollment of patients in clinical trials for treatment of cognitive impairment, earlier financial and estate planning, the designation of health care proxies, and the preparation of families for the future responsibility and cost of providing care for the patient.(Brown et al., 2011)
For researchers, these data highlight a schism that has developed between geriatric depression research and MCI/dementia research. Although these are highly comorbid disorders,(Nebes et al., 2000, Bhalla et al., 2006) the rigorous exclusion criteria driven by internal validity concerns and the divide between the National Institute of Aging and National Institute of Mental Health have left the field segmented. Large studies such as the Alzheimer’s Disease Neuroimaging Initiative (ADNI) have excluded Axis 1 disorders such as major depression, and limited inclusion of comorbid vascular risk factors (http://www.adni.info.org). These choices minimize the generalizability of the findings, thereby limiting the effectiveness of any treatment paradigms that develop from the research. A recent study has shown the value of the information obtained through comprehensive psychiatric and neuropsychological assessment of older depressed patients(Reynolds et al., 2011) and provides a model for future interventions work to follow.
There are limitations however that need to be taken into account when interpreting the results of this study. This is a sample presenting for evaluation at specialized memory disorder centers, making it difficult to generalize these relationships to a community sample or a purely depressed geriatric sample. Also, although 76% of the total sample was classified cognitively by a consensus conference, some participants were classified based on a single clinician. As such, the cognitive classification made across the ADCs utilized in this study is not an entirely uniform process. One standard deviation (SD) was used to denote impairment in executive dysfunction on the Trail-making Test B-A, a technique that has been utilized elsewhere(Brown et al., 2013). Although the criterion of 1 SD differs from the original neuropsychological criterion for MCI (≥ 1.5 SD’s below the norm)(Petersen et al., 1999), it is in keeping with the more recent movement towards identifying impairment sooner (early mild cognitive impairment).(Aisen et al., 2010) Also, the use of 1 SD was only used to classify the level of impairment in executive function in the categorical analysis. It was not used to classify amnestic or nonamnestic MCI. Additionally, raw scores for Trails B with Trails A used as a covariate were utilized in the regression analyses. Also, the use of the 15-item GDS as a diagnostic tool for depressive illness is suboptimal. The GDS is a screening tool, although a cut-off of 5 or greater on this short-form 15-item GDS is highly sensitive to the identification of major depression(Brown et al., 2007, Lesher and Berryhill, 1994). Finally, no data from imaging modalities was used in this study, instead relying on clinical and neuropsychological measurements as proxy for underlying disease pathology. Although this is commonly done in clinical research, imaging would ideally have been conducted in conjunction with the clinical and neuropsychological data to confirm the presence of underlying disease pathology and the association between the imaging and the clinical and neuropsychological measures. That was not the case however with this dataset.
Conclusion
In conclusion, this study showed that both depression and executive dysfunction are common in older adults with aMCI and nonaMCI. Patients with executive dysfunction were also more likely to be depressed and have hypertension. The latter however was not associated with depression severity, but rather with comorbid executive dysfunction. The high frequency of the confluence between vascular risk factors, episodic memory impairment, depression and executive dysfunction highlight the need for comprehensive assessment of depressed older adults that can aid clinicians in the formulation of more accurate treatment planning and inform clinicians and researchers about long-term prognosis.
Acknowledgements
This research was supported by grants from the National Alzheimer’s Coordinating Center (grant numbers U01 AG016976 and 2010-JI-01), and a National Institute of Mental Health (grant number T32 MH20004).
Dr. Devanand has received research support from Novartis AG and Eli Lilly, and has served as a consultant to Bristol-Myers Squibb. Dr. Roose has served as a consultant for Medtronics. These sponsors had no role in this current manuscript.
Footnotes
Disclosure: Drs. Brown, Rutherford, and Sneed have nothing to disclose.
References
- Aisen PS, Petersen RC, Donohue MC, Gamst A, Raman R, Thomas RG, Walter S, Trojanowski JQ, Shaw LM, Beckett LA, Jack CR, Jr, Jagust W, Toga AW, Saykin AJ, Morris JC, Green RC, Weiner MW. Clinical Core of the Alzheimer's Disease Neuroimaging Initiative: progress and plans. Alzheimers Dement. 2010;6:239–246. doi: 10.1016/j.jalz.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Kiosses DN, Heo M, Murphy CF, Shanmugham B, Gunning-Dixon F. Executive dysfunction and the course of geriatric depression. Biol Psychiatry. 2005;58:204–210. doi: 10.1016/j.biopsych.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. 'Vascular depression' hypothesis. Arch Gen Psychiatry. 1997;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Meyers BS, Young RC, Mattis S, Kakuma T. The course of geriatric depression with "reversible dementia": a controlled study. Am J Psychiatry. 1993;150:1693–1699. doi: 10.1176/ajp.150.11.1693. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Alexopoulos GS, Lopez OL, Williamson JD, Yaffe K. Depressive symptoms, vascular disease, and mild cognitive impairment: findings from the Cardiovascular Health Study. Arch Gen Psychiatry. 2006;63:273–279. doi: 10.1001/archpsyc.63.3.273. [DOI] [PubMed] [Google Scholar]
- Beekly DL, Ramos EM, Lee WW, Deitrich WD, Jacka ME, Wu J, Hubbard JL, Koepsell TD, Morris JC, Kukull WA. The National Alzheimer's Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord. 2007;21:249–258. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- Bhalla RK, Butters MA, Mulsant BH, Begley AE, Zmuda MD, Schoderbek B, Pollock BG, Reynolds CF, 3rd, Becker JT. Persistence of neuropsychologic deficits in the remitted state of late-life depression. Am J Geriatr Psychiatry. 2006;14:419–427. doi: 10.1097/01.JGP.0000203130.45421.69. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Reitz C, Luchsinger JA, Manly JJ, Schupf N, Muraskin J, Decarli C, Brown TR, Mayeux R. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch Neurol. 2010;67:564–569. doi: 10.1001/archneurol.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PJ, Devanand DP, Liu X, Caccappolo E. Functional impairment in elderly patients with mild cognitive impairment and mild Alzheimer disease. Arch Gen Psychiatry. 2011;68:617–626. doi: 10.1001/archgenpsychiatry.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PJ, Liu X, Sneed JR, Pimontel MA, Devanand DP, Roose SP. Speed of processing and depression affect function in older adults with mild cognitive impairment. Am J Geriatr Psychiatry. 2013;21:675–684. doi: 10.1016/j.jagp.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PJ, Woods CM, Storandt M. Model stability of the 15-item Geriatric Depression Scale across cognitive impairment and severe depression. Psychol Aging. 2007;22:372–379. doi: 10.1037/0882-7974.22.2.372. [DOI] [PubMed] [Google Scholar]
- Butters MA, Becker JT, Nebes RD, Zmuda MD, Mulsant BH, Pollock BG, Reynolds CF., 3rd Changes in cognitive functioning following treatment of late-life depression. Am J Psychiatry. 2000;157:1949–1954. doi: 10.1176/appi.ajp.157.12.1949. [DOI] [PubMed] [Google Scholar]
- Butters MA, Young JB, Lopez O, Aizenstein HJ, Mulsant BH, Reynolds CF, 3rd, Dekosky ST, Becker JT. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin Neurosci. 2008;10:345–357. doi: 10.31887/DCNS.2008.10.3/mabutters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TF, Chen YF, Cheng TW, Hua MS, Liu HM, Chiu MJ. Executive dysfunction and periventricular diffusion tensor changes in amnesic mild cognitive impairment and early Alzheimer's disease. Hum Brain Mapp. 2009;30:3826–3836. doi: 10.1002/hbm.20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culang-Reinlieb ME, Johnert LC, Brickman AM, Steffens DC, Garcon E, Sneed JR. MRI-defined vascular depression: a review of the construct. Int J Geriatr Psychiatry. 2010 doi: 10.1002/gps.2668. [DOI] [PubMed] [Google Scholar]
- De Groot JC, De Leeuw FE, Oudkerk M, Hofman A, Jolles J, Breteler MM. Cerebral white matter lesions and depressive symptoms in elderly adults. Arch Gen Psychiatry. 2000;57:1071–1076. doi: 10.1001/archpsyc.57.11.1071. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Pelton GH, Marston K, Camacho Y, Roose SP, Stern Y, Sackeim HA. Sertraline treatment of elderly patients with depression and cognitive impairment. Int J Geriatr Psychiatry. 2003;18:123–130. doi: 10.1002/gps.802. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Sano M, Tang MX, Taylor S, Gurland BJ, Wilder D, Stern Y, Mayeux R. Depressed mood and the incidence of Alzheimer's disease in the elderly living in the community. Arch Gen Psychiatry. 1996;53:175–182. doi: 10.1001/archpsyc.1996.01830020093011. [DOI] [PubMed] [Google Scholar]
- Emery O. Pseudodementia: A Theoretical and Empirical Discussion: Interdisciplinary Monograph Series. Cleveland: Western Reserve Geriatric Education Center, Case Western Reserve University School of Medicine; 1988. [Google Scholar]
- Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: a prospective MRI study. Neuropsychologia. 2003;41:1929–1941. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Gustafson L, Erikson C, Warkentin S, Brun A, Englund E, Passant U. A factor analytic approach to symptom patterns in dementia. Int J Alzheimers Dis. 2010;2011:632604. doi: 10.4061/2011/632604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachinski VC, Iliff LD, Zilhka E, Du Boulay GH, Mcallister VL, Marshall J, Russell RW, Symon L. Cerebral blood flow in dementia. Arch Neurol. 1975;32:632–637. doi: 10.1001/archneur.1975.00490510088009. [DOI] [PubMed] [Google Scholar]
- Jokinen H, Kalska H, Ylikoski R, Madureira S, Verdelho A, Gouw A, Scheltens P, Barkhof F, Visser MC, Fazekas F, Schmidt R, O'Brien J, Hennerici M, Baezner H, Waldemar G, Wallin A, Chabriat H, Pantoni L, Inzitari D, Erkinjuntti T. MRI-defined subcortical ischemic vascular disease: baseline clinical and neuropsychological findings. The LADIS Study. Cerebrovasc Dis. 2009;27:336–344. doi: 10.1159/000202010. [DOI] [PubMed] [Google Scholar]
- Kiloh LG. Depressive illness masquerading as dementia in the elderly. Med J Aust. 1981;2:550–553. doi: 10.5694/j.1326-5377.1981.tb112983.x. [DOI] [PubMed] [Google Scholar]
- Lesher EL, Berryhill JS. Validation of the Geriatric Depression Scale--Short Form among inpatients. J Clin Psychol. 1994;50:256–260. doi: 10.1002/1097-4679(199403)50:2<256::aid-jclp2270500218>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Lockwood KA, Alexopoulos GS, Kakuma T, Van Gorp WG. Subtypes of cognitive impairment in depressed older adults. Am J Geriatr Psychiatry. 2000;8:201–208. [PubMed] [Google Scholar]
- Lopez OL, Becker JT, Sweet RA. Non-cognitive symptoms in mild cognitive impairment subjects. Neurocase. 2005;11:65–71. doi: 10.1080/13554790490896893. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, Dekosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288:1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- Monsell SE, Mock C, Roe CM, Ghoshal N, Morris JC, Cairns NJ, Kukull W. Comparison of symptomatic and asymptomatic persons with Alzheimer disease neuropathology. Neurology. 2013;80:2121–2129. doi: 10.1212/WNL.0b013e318295d7a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebes RD, Butters MA, Mulsant BH, Pollock BG, Zmuda MD, Houck PR, Reynolds CF., 3rd Decreased working memory and processing speed mediate cognitive impairment in geriatric depression. Psychol Med. 2000;30:679–691. doi: 10.1017/s0033291799001968. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Pollock BG, Houck PR, Butters MA, Mulsant BH, Zmuda MD, Reynolds CF., 3rd Persistence of cognitive impairment in geriatric patients following antidepressant treatment: a randomized, double-blind clinical trial with nortriptyline and paroxetine. J Psychiatr Res. 2003;37:99–108. doi: 10.1016/s0022-3956(02)00085-7. [DOI] [PubMed] [Google Scholar]
- Pelton GH, Harper OL, Tabert MH, Sackeim HA, Scarmeas N, Roose SP, Devanand DP. Randomized double-blind placebo-controlled donepezil augmentation in antidepressant-treated elderly patients with depression and cognitive impairment: a pilot study. Int J Geriatr Psychiatry. 2008;23:670–676. doi: 10.1002/gps.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Reitan R. Validity of the Trail-Making Test as an indication of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- Reynolds CF, 3rd, Butters MA, Lopez O, Pollock BG, Dew MA, Mulsant BH, Lenze EJ, Holm M, Rogers JC, Mazumdar S, Houck PR, Begley A, Anderson S, Karp JF, Miller MD, Whyte EM, Stack J, Gildengers A, Szanto K, Bensasi S, Kaufer DI, Kamboh MI, Dekosky ST. Maintenance Treatment of Depression in Old Age: A Randomized, Double-blind, Placebo-Controlled Evaluation of the Efficacy and Safety of Donepezil Combined With Antidepressant Pharmacotherapy. Arch Gen Psychiatry. 2011;68:51–60. doi: 10.1001/archgenpsychiatry.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard E, Reitz C, Honig LH, Schupf N, Tang MX, Manly JJ, Mayeux R, Devanand D, Luchsinger JA. Late-life depression, mild cognitive impairment, and dementia. JAMA Neurol. 2013;70:374–382. doi: 10.1001/jamaneurol.2013.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg PB, Mielke MM, Appleby B, Oh E, Leoutsakos JM, Lyketsos CG. Neuropsychiatric symptoms in MCI subtypes: the importance of executive dysfunction. Int J Geriatr Psychiatry. 2011;26:364–372. doi: 10.1002/gps.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royall DR, Palmer R, Chiodo LK, Polk MJ. Depressive symptoms predict longitudinal change in executive control but not memory. Int J Geriatr Psychiatry. 2012;27:89–96. doi: 10.1002/gps.2697. [DOI] [PubMed] [Google Scholar]
- Royall DR, Palmer RF. Alzheimer's disease pathology does not mediate the association between depressive symptoms and subsequent cognitive decline. Alzheimers Dement. 2013;9:318–325. doi: 10.1016/j.jalz.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarazin M, Chauvire V, Gerardin E, Colliot O, Kinkingnehun S, De Souza LC, Hugonot-Diener L, Garnero L, Lehericy S, Chupin M, Dubois B. The amnestic syndrome of hippocampal type in Alzheimer's disease: an MRI study. J Alzheimers Dis. 2010;22:285–294. doi: 10.3233/JAD-2010-091150. [DOI] [PubMed] [Google Scholar]
- Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clinical Gerontologist. 1986;5:165–172. [Google Scholar]
- Sheline YI, Pieper CF, Barch DM, Welsh-Bohmer K, Mckinstry RC, Macfall JR, D'Angelo G, Garcia KS, Gersing K, Wilkins C, Taylor W, Steffens DC, Krishnan RR, Doraiswamy PM. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010;67:277–285. doi: 10.1001/archgenpsychiatry.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Vaishnavi SN, Mintun MA, Barch DM, Epstein AA, Wilkins CH, Snyder AZ, Couture L, Schechtman K, Mckinstry RC. Regional white matter hyperintensity burden in automated segmentation distinguishes late-life depressed subjects from comparison subjects matched for vascular risk factors. Am J Psychiatry. 2008;165:524–532. doi: 10.1176/appi.ajp.2007.07010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Loewenstein DA, Potter E, Zhao W, Appel J, Greig MT, Raj A, Acevedo A, Schofield E, Barker W, Wu Y, Potter H, Duara R. Volumetric and visual rating of magnetic resonance imaging scans in the diagnosis of amnestic mild cognitive impairment and Alzheimer's disease. Alzheimers Dement. 2011;7:e101–e108. doi: 10.1016/j.jalz.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneed JR, Rindskopf D, Steffens DC, Krishnan KR, Roose SP. The vascular depression subtype: evidence of internal validity. Biol Psychiatry. 2008;64:491–497. doi: 10.1016/j.biopsych.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneed JR, Roose SP, Keilp JG, Krishnan KR, Alexopoulos GS, Sackeim HA. Response inhibition predicts poor antidepressant treatment response in very old depressed patients. Am J Geriatr Psychiatry. 2007;15:553–563. doi: 10.1097/JGP.0b013e3180302513. [DOI] [PubMed] [Google Scholar]
- Snitz BE, Weissfeld LA, Lopez OL, Kuller LH, Saxton J, Singhabahu DM, Klunk WE, Mathis CA, Price JC, Ives DG, Cohen AD, Mcdade E, Dekosky ST. Cognitive trajectories associated with beta-amyloid deposition in the oldest-old without dementia. Neurology. 2013;80:1378–1384. doi: 10.1212/WNL.0b013e31828c2fc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, Steffens DC, Macfall JR, Mcquoid DR, Payne ME, Provenzale JM, Krishnan KR. White matter hyperintensity progression and late-life depression outcomes. Arch Gen Psychiatry. 2003;60:1090–1096. doi: 10.1001/archpsyc.60.11.1090. [DOI] [PubMed] [Google Scholar]
- Valkanova V, Ebmeier KP. Vascular risk factors and depression in later life: a systematic review and meta-analysis. Biol Psychiatry. 2013;73:406–413. doi: 10.1016/j.biopsych.2012.10.028. [DOI] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, De Leon M, Decarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, Van Duijn C, Visser P, Petersen RC. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Dickerson BC. Fractionating verbal episodic memory in Alzheimer's disease. Neuroimage. 2011;54:1530–1539. doi: 10.1016/j.neuroimage.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]