Cholangiocarcinomas are usually characterized by a prominent desmoplastic and hypovascularized stroma. However, the biological significance and clinical impact of the desmoplastic stroma in cholangiocarcinoma is only just beginning to be addressed at the cellular and molecular levels. Nevertheless, there is now increasing evidence to suggest that the desmoplastic reaction, marked by a dramatic accumulation of α-smooth muscle actin positive cancer-associated fibroblasts (α-SMA+CAFs) with increased production of extracellular matrix proteins, pro-invasive growth factors and cytokines, anti-angiogenic factors, and matrix modifying enzymes may be playing a crucial role in promoting enhanced malignant behavior and therapeutic resistance in cholangiocarcinoma (1). In this review, we will highlight current findings on the potential prognostic value of α-SMA+CAFs, tumor-associated macrophages, and select related blood and serum markers associated with the desmoplastic reaction in predicting cholangiocarcinoma progression and patient survival rates. We will also describe recent experimental approaches to achieve cholangiocarcinoma prevention by reducing hepatic fibrosis. Last, we will discuss the potential merits and challenges of α-SMA+CAF depletion as a novel strategy for cholangiocarcinoma therapy.
Prognostic Implications of Desmoplastic Stromal Components in Cholangiocarcinoma
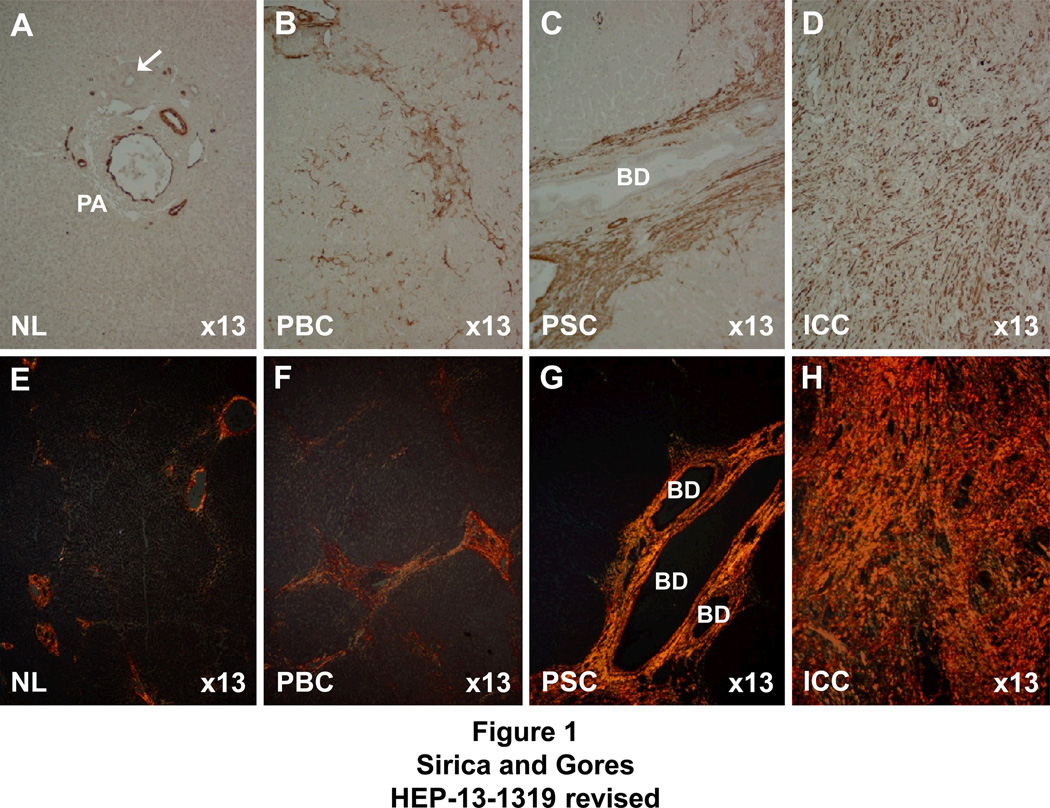
Kajiyama et al. (2) were the first to discuss the significance of stromal desmoplasia in intrahepatic cholangiocarcinoma (ICC) in relation to pathologic prognostic factors, noting that patients with tumors that exhibited an abundant fibrous stroma had significantly lower 1 and 3 year survival rates than those with ICC characterized by scanty fibrous stroma. As exemplified in Figure 1, the amount of type I collagen-enriched fibrosis formed in liver malignancies and in chronic inflammatory and fibrosing diseases of liver predisposing for these tumors, is closely related to the extent to which activated myofibroblastic cells positive for α-SMA accumulate in the stroma, being most abundant in desmoplastic cholangiocarcinoma and sparse in (non-scirrhous-type) trabecular hepatocellular carcinoma (HCC). In the case of ICC, it is also particularly noteworthy that patients with resected tumor whose stroma had high α-SMA expression (indicating increased numbers of α-SMA+CAFs) were found to have significantly shorter survival times and larger tumor sizes than those whose ICC stroma exhibited low levels of α-SMA expression (3, 4).
Figure 1.
Photomicrographs of representative chronic fibrosing diseases of human liver and classical intrahepatic cholangiocarcinoma (ICC) demonstrating a positive correlation between levels of immunoreactivity for α-smooth muscle actin (α-SMA), reflective of numbers of stromal myofibroblastic cells, and degrees of hepatic fibrosis or tumor desmoplasia. Note that the uniformly strong staining reaction for α-SMA-positive stromal cells and corresponding dense diffuse histochemical staining for fibrous type I collagen (orange staining) is most intense in the desmoplastic ICC stroma, followed by that of primary sclerosing cholangitis (PSC), a high risk condition for ICC with marked periductular fibrosis. Note, however, that the degree of α-SMA-positive cells and corresponding degree of collagen type 1 staining are less prominently expressed in the histological specimen of primary biliary cirrhosis (PBC), a lower risk condition for ICC with less developed fibrosis, and not expressed in normal adult (donor transplant) liver (NL) without fibrosis. A–D, immunohistochemically stained for α-SMA. E–H, histochemically stained for type I collagen using the picrosirius red staining method. BD = intrahepatic bile ducts, PA = portal area. α-SMA immunostaining in PA of NL is confined to vascular walls. Arrow points to normal interlobular bile duct.
The relationship between increasing numbers of α-SMA+CAFs and enhanced malignant behavior of cholangiocarcinoma cells is further supported by our recent results demonstrating in a novel organotypic culture model of rat cholangiocarcinoma that cholangiocarcinoma cell growth and invasiveness, as well as stiffening (matrix contraction), is significantly enhanced as a function of increasing numbers of plated α-SMA+CAFs (derived from an orthotopic rat ICC) when co-cultured with a fixed number of plated cholangiocarcinoma cells from the same rat tumor type in a 3-dimensional type I collagen gel matrix (5). Similar findings showing increasing proportions of desmoplastic stromal cells (pancreatic stellate cells) as driving aggressive cancer processes were also recently described for organotypic cultures of pancreatic, esophageal, and squamous skin cancer (6).
The possibility of phenotypically distinct populations of CAFs in desmoplastic stroma contributing to cholangiocarcinoma progression is suggested by the following, albeit limited, clinical observations demonstrating: (1) survival rates of ICC resected patients with stromal myofibroblasts imunoreactive for the transmembrane glycoprotein podoplanin (D2-40-positive myofibroblasts) to be worse than those of patients without D2-40-positive stromal cells (7) and (2) fibroblastic cells positive for neutral endopeptidase (CD10+ fibroblasts) to be more frequent in the stroma of hilar cholangiocarcinoma and in less differentiated cases of extrahepatic cholangiocarcinomas than in peripheral ICC, suggesting that CD10+ fibroblasts may be more involved with the progression of hilar and extrahepatic cholangiocarcinomas than of peripheral ICC (8). However, before the clinical significance of these limited findings can be fully realized, a much better understanding of how CAF heterogeneity and functional diversity affect cholangiocarcinoma progression within different regions of the biliary tract is needed.
Extracellular matrix proteins specifically produced by CAFs may also have clinical significance as poor prognostic markers of cholangiocarcinoma. This possibility is particularly nicely exemplified by the matricellular protein periostin (POSTN), which has been shown to be solely expressed in α-SMA+CAFs in both human (9) and rat cholangiocarcinomas (5, 10), to correlate with shorter survival times in post-resected cholangiocarcinoma patients (9), and to associate with increased malignancy in our “human-like” orthotopic ICC syngeneic rat model (10). In vitro studies have further shown POSTN to be an inducer of cholangiocarcinoma cell invasion (9, 11), likely through paracrine activation of integrin signaling pathways (11). In addition, POSTN has been reported to be detected at significantly higher levels in the serum of patients with cholangiocarcinoma compared to those with normal liver, liver cirrhosis, HCC, and other hepatic malignancies (12), suggesting its potential as a serodiagnostic (or prognostic) biomarker for cholangiocarcinoma. While the development of a serodiagnostic bioassay based on a CAF secreted protein is novel and potentially important, it was also recognized that a prospective study with a much larger patient sampling from multiple centers will now be needed to confirm and validate POSTN usefulness as a measurable serum biomarker for cholangiocarcinoma development and/or progression.
Although α-SMA+CAFs are recognized as a major cellular component of the desmoplastic stroma associated with cholangiocarcinoma progression, there is increasing evidence to also implicate tumor-associated macrophages in cholangiocarcinoma progression and as having prognostic significance for predicting poor clinical outcome in cholangiocarcinoma patients following tumor resection. High infiltrates of CD163(+) macrophages (M2 macrophages) into ICC stroma were found to correlate with poor disease-free survival in surgically resected ICC patients compared with those whose tumors had low CD163(+) counts (13). A high density of MAC387-positive macrophages at the leading edge of the invasive tumor was also reported to be associated with more aggressive non-papillary type cholangiocarcinoma and further shown that cholangiocarcinoma patients with high tumor tissue density of MAC387 macrophages had a significantly worse overall post-resectional survival than those with a low tissue density of MAC387 cells (14). In addition, elevated levels of circulating CD14+CD16+ monocytes in cholangiocarcinoma patient blood were found to correlate with the degree of MAC387-positive tumor-associated macrophage infiltration and to be associated with a poorer post-resectional survival, but not overall survival, compared to those with low levels of CD14+CD16+ monocytes (15).
The clinical observations described above suggest the potential usefulness of desmoplastic stromal components as prognostic biomarkers for cholangiocarcinoma progression. Monitoring α-SMA+CAF levels and secreted extracellular matrix proteins, such as POSTN, also are likely to provide an effective approach to assessing therapeutic strategies for cholangiocarcinoma based on stromal depletion (see below). Furthermore, the interrelationships between activated myofibroblastic-like cells, such as α-SMA+CAFs, and tumor-associated macrophages should be much more fully investigated, particularly in lieu of recent findings demonstrating that specific signaling molecules from activated hepatic stellate cells can mediate the differentiation of macrophages that are characterized by a high production of both pro-inflammatory and profibrotic signals (16).
Antifibrotic Treatments for Cholangiocarcinoma Prevention
It is well recognized that chronic bile duct inflammation and progressive fibrosis within the biliary tract leading to bile stasis, as especially exemplified by primary sclerosing cholangitis, predispose to cholangiocarcinoma development. Liver cirrhosis of various etiologies is also now recognized as a risk factor for ICC. In addition, chronic liver injury and inflammation, periductular fibrosis, cholangiofibrosis and cirrhosis, and development of intense tumoral desmoplastic stroma are all characteristic features of several rodent models of cholangiocarcinogenesis, including the furan and thioacetamide (TAA) rat models, as well as the liver fluke-infected hamster model. CCl4-induced liver cirrhosis associated with increased immunoreactivity for α-SMA+ myofibroblastic cells has further been shown to provoke desmoplastic cholangiocarcinoma in p53-deficient mice (17). These findings support the notion that progressively increased fibrogenic matrix as that seen preceding cholangiocarcinoma actually favors malignancy, and that the desmoplastic reaction may not just be a response to invasive tumor cells, but a niche for cancer cells to develop and progress (18).
The possibility of achieving cholangiocarcinoma prevention by inducing regression of pre-existing hepatic fibrosis is only just beginning to be investigated. Nevertheless, the preclinical efforts to date have been somewhat encouraging. Notably, dosing of TAA-treated rats with the transforming growth factor-β (TGF-β) antagonist 1D11 was recently shown to reverse pre-existing hepatic fibrosis, which was accompanied by a marked reduction in developed cholangiocarcinomas (19). Long-term treatment with the nutraceutical agent curcumin, derived from turmeric, has also been reported to significantly reduce inflammatory changes and periductal fibrosis in liver-fluke infected hamsters and to significantly reduce the incidence of cholangiocarcinoma and increase the survival of infected animals (20, 21). The clinically active Hedgehog (Hh) signaling antagonist vismodegib (GDC-0449) was also recently shown to promote the regression of hepatic fibrosis and HCC in a murine model of primary liver cancer (22), as well as to attenuate cholestatic liver injury and fibrosis and down-regulate the expression of α-SMA in the livers of common bile duct-ligated rats (23). Imatinib mesylate has also been shown to exhibit anti-fibrotic activity in the TAA rat model of liver injury and fibrosis, leading to reduced collagen content, with significant down-regulation of fibrogenic genes, including α-SMA, platelet derived growth factor (PDGF) receptor-β,and collagen α1 type I (24). Unexpectedly, however, interleukin-6 (IL-6), a major mitogenic cytokine for cholangiocytes and hepatocytes, was found to be significantly increased in the livers of TAA rats treated with imatinib, raising the concern that induction of IL-6 in liver by imatinib could potentially promote cholangiocarcinogenesis (or hepatocarcinogenesis) under known risk conditions.
Therapeutic Targeting of α-SMA+CAFs in Cholangiocarcinoma
The potential clinical implications of mechanism-based targeting of α-SMA+CAFs as an innovative approach to cholangiocarcinoma therapy were recently demonstrated by Mertens et al. (25), demonstrating (1) α-SMA+CAFs to be significantly more sensitive than cholangiocarcinoma cells to apoptotic cell death triggered by the BH3-mimetic navitoclax (ABT-263) and (2) when tested in vivo in syngeneic rats orthotopically transplanted with our highly malignant rat BDEneu cholangiocarcinoma cell line, navitoclax treatment elicited significantly increased CAF apoptosis, concomitant reduction in quantitative α-SMA immunostaining and decreased expression of the proinvasive extracellular matrix protein tenascin C, a marked reduction in tumor burden and metastasis, and significantly improved animal survival. Together, these findings provide a “proof-of-principle” for CAF deletion as a novel anticancer strategy for cholangiocarcinoma, which is also germane to other desmoplastic tumor types.
PDGF cross-talk between α-SMA+CAFs and cholangiocarcinoma cells represents another growing topic of potential clinical interest. It is now well established that paracrine PDGF signaling between cholangiocytes and myofibroblasts occurs in rodent models of biliary tract inflammation and fibrogenesis, and further shown that PDGF-BB generated by reactive cholangiocytes causes both myofibroblasts and cholangiocytes to produce Hh ligands (26). Moreover, PDGF-BB generated by human LX-2 immortalized myofibroblastic cells and human primary myofibroblastic hepatic stellate cells has been demonstrated in vitro to protect human cholangiocarcinoma cells immunoreactive for PDGFR-β from TRAIL-induced apoptosis by a Hh-dependent mechanism (27). Targeting human KMCH-1cholangiocarcinoma cells, expressing PDGFR-β receptor, but not c-Kit, with imatinib mesylate or linifanib, as well as by PDGFR-β shRNA silencing has also been found to significantly decrease TRAIL-induced cholangiocarcinoma cell apoptosis in vitro. Furthermore, imatinib mesylate was determined to be therapeutic in vivo in our orthotopic rat BDEneu cholangiocarcinoma model, although the extent to which c-kit inhibition may have also contributed to reduced cholangiocarcinoma tumor size in the imatinib mesylate group is not yet known (28). Of further note are the recent findings of Cadamuro et al. (29) demonstrating PDGF-D secreted by cultured human cholangiocarcinoma cells significantly enhanced the migration of human myofibroblastic cells in Boyden chambers, which was suppressed by selective blockage of myofibroblast PDGFR-β by imatinib mesylate, as well as by siRNA silencing of PDGF-D in cholangiocarcinoma cells. Overall, these data suggest that targeting PDGF cross-talk may offer a novel approach to cholangiocarcinoma therapy, although preliminary, clinical experience with imatinib mesyalate as a monotherapy for biliary tract cancers has to date been disappointing (30).
Myofibroblasts have also been recently demonstated to contribute to increased cholangiocarcinoma tumor growth and progression in vivo in mouse xenografting experiments, as well as to enhance cholangiocarcinoma cell migration and invasion in vitro though a paracrine mechanism involving activation of epidermal growth factor (EGF) receptor signaling in cholangiocarcinoma cells by myofibroblast-derived heparin-binding (HB)-EGF (31). That these effects were shown to be abolished by the EGF receptor inhibitor gefitinib or HB-EGF neutralizing antibody suggests that targeting the EGFR pathway can have potential value as part of a therapeutic strategy for cholangicarcinoma, although like imatinib mesylate, EGFreceptor antagonists have at present exhibited only very limited effectiveness in human cholangiocarcinoma clinical trials.
The Hh signaling pathway has also been implicated as a potentially important therapeutic target in cholangiocarcinoma (27, 32) and our preliminary findings (5, 33) have suggested that paracrine sonic hedgehog signaling is likely playing a role in promoting the desmoplastic reaction in cholangiocarcinoma, comparable to what has been clearly demonstrated for pancreatic ductal adenocarcinoma (34). Most notable are the paradigm-shifting experiments of Tuveson and his colleagues (35), in which IPI-926, a semisynthetic derivative of the Smoothened antagonist cyclopamine, was shown to significantly reduce collagen type I deposition and α-SMA+CAF levels in the tumor microenvironment in the KPC mouse model of desmoplastic pancreatic adenocarcinoma. This stromal depleting effect of the Hh inhibitor treatment further correlated with a transient increase in intratumoral vascular density and enhanced delivery of the anti-cancer drug gemcitabine to the cancer, leading to a significant, but transient therapeutic response. Paracrine Hh signaling has also recently been demonstrated in a murine HCC model to stimulate glycolysis in stromal myofibroblasts, which could be depleted from the tumor stroma by treatment with GDC-0449 (36).
Several combination trials, including the use of Hh antagonists, are currently in progress (37), which portends an innovative approach to desmoplastic cancer therapy. That the Hh pathway is active in cholangiocarcinoma cells as well (27, 32), also makes it likely that both paracrine and autocrine Hh signaling cooperate to promote apoptosis resistance and malignant progression, although the extent to which paracrine versus autocrine Hh signaling affects therapeutic responses of desmoplastic cholangiocarcinoma to Hh antagonists remains an open question.
Challenges and Future Direction
Most efforts over the past several years to achieve improved therapeutic responses in patients with advanced biliary tract cancer have focused on the development of agents targeting cholangiocarcinoma cells, with only limited consideration to targeting the tumor stroma. The inability for cancer chemotherapeutic drugs, including targeted agents, to penetrate this dense collagenous and hypovascularized stroma, together with the generation of intratumoral interstitial pressure represent critical factors that severely limit therapeutic efficacy. Thus, devising and testing new approaches to more efficiently deliver drug combinations to desmoplastic cholangiocarcinoma, such as the use of nanotechnology-based combinational drug delivery systems constructed to home to the cancer or stromal cell receptors, or the recently described strategy to enhance the tumor penetration of chemotherapeutic drugs through use of iRGD peptide homing to tumor vessel endothelium (38) are clearly warranted. Moreover, new and currently existing animal models that recapitulate key pathological, cellular and molecular, and clinical features of the human desmoplastic cholangiocarcinoma need to be rigorously explored for their usefulness as preclinical platforms to identify and accurately predict clinical outcomes of new combinational therapies based on stromal depletion and cholangiocarcinoma cell targeting.
In an effort to develop new effective combinational treatments to manage aggressive desmoplastic cholangiocarcinoma and/or prevent cholangiocarcinoma development or recurrent cancer, mechanistic insight is also needed concerning evolving changes in the cellular and extracellular matrix composition and remodeling of the tumor microenvironment that would favor malignant progression over host protection in which desmoplasia may act as a barrier to cancer diffusion and vascular invasion.
Such concerns emphasize the need for the development of new drug designs (i.e., anti-fibrotic drugs) to enhance stromal depletion and minimize undue side effects, and a critical need to expand preclinical studies with appropriate animal models more closely mimicking human desmoplastic cholangiocarcinoma that would accurately predict stromal depletion combined with cholangiocarcinoma cell targeting as an effective strategy for desmoplastic cholangiocarcinoma therapy in patients.
Acknowledgments
Financial Support: This work was supported by grants NIH R01 CA83650 & CA39225 (A.E.S) and DK59427 (G.J.G.)
Abbreviations
- α-SMA
alpha-smooth muscle actin
- CAFs
cancer-associated fibroblasts
- ICC
intrahepatic cholangiocarcinoma
- HCC
hepatocellular carcinoma
- POSTN
periostin
- TAA
thioacetamide
- PDGF
platelet-derived growth factor
- TGF-β
transforming growth factor-β
- Hh
Hedgehog
- IL-6
interleukin-6
- EGF
epidermal growth factor
- HB
heparin-binding
References
- 1.Sirica AE. The role of cancer-associated myofibroblasts in intrahepatic cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2012;9:44–54. doi: 10.1038/nrgastro.2011.222. [DOI] [PubMed] [Google Scholar]
- 2.Kajiyama K, Maeda T, Takenaka K, Sugimachi K, Tsuneyoshi M. The significance of stromal desmoplasia in intrahepatic cholangiocarcinoma: a special reference of “scirrhous-type” and “nonscirrhous-type” growth. Am J Surg Pathol. 1999;23:892–902. doi: 10.1097/00000478-199908000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Chuaysri C, Thuwajit P, Paupairoj A, Chau-In S, Suthiphongchai T, Thuwajit C. Alpha-smooth muscle actin-positive fibroblasts promote biliary cell proliferation and correlate with poor survival in cholangiocarcinoma. Oncol Rep. 2009;21:957–969. doi: 10.3892/or_00000309. [DOI] [PubMed] [Google Scholar]
- 4.Okabe H, Beppu T, Hayashi H, Horino K, Masuda T, Komori H, et al. Hepatic stellate cells may relate to progression of intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2009;16:2555–2564. doi: 10.1245/s10434-009-0568-4. [DOI] [PubMed] [Google Scholar]
- 5.Campbell DJW, Dumur CI, Lamour NF, DeWitt JL, Sirica AE. Novel organotypic culture model of cholangiocarcinoma progression. Hepatol Res. 2012;42:1119–1130. doi: 10.1111/j.1872-034X.2012.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kadaba R, Birke H, Wang J, Hooper S, Andl CD, Di Maggio F, et al. Imbalance of desmoplastic stromal cell numbers drives aggressive cancer processes. J Pathol. 2013;230:107–117. doi: 10.1002/path.4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aishima S, Nishihara Y, Iguchi T, Taguchi K, Taketomi A, Maehara Y, Tsuneyoshi M. Lymphatic spread is related to VEGF-C expression and D2-40-positive myofibroblasts in intrahepatic cholangiocarcinoma. Mod Pathol. 2008;21:256–264. doi: 10.1038/modpathol.3800985. [DOI] [PubMed] [Google Scholar]
- 8.Nishihara Y, Aishima S, Hayashi A, Iguchi T, Fujita N, Taketomi A, et al. CD10+ fibroblasts are more involved in the progression of hilar/extrahepatic cholangiocarcinoma than of peripheral intrahepatic cholangiocarcinoma. Histopathol. 2009;55:423–431. doi: 10.1111/j.1365-2559.2009.03398.x. [DOI] [PubMed] [Google Scholar]
- 9.Utispan K, Thuwajit P, Abiko Y, Charngkaew K, Paupairoj A, Chau-in S, Thuwajit C. Gene expression profiling of cholangiocarcinoma-derived fibroblast reveals alterations related to tumor progression and indicates periostin as a poor prognostic marker. Mol Cancer. 2010;9:13. doi: 10.1186/1476-4598-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumur CI, Campbell DJW, DeWitt JL, Oyesanya RA, Sirica AE. Differential gene expression profiling of cultured neu-transformed versus spontaneously-transformed rat cholangiocytes and of corresponding cholangiocarcinomas. Exp Mol Pathol. 2010;89:227–235. doi: 10.1016/j.yexmp.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Utispan K, Sonongbua J, Thuwajit P, Chau-In S, Pairojkul C, Wongkham S, Thuwajit C. Periostin activates integrin α5β1 through a PI3K/AKT-dependent pathway in invasion of cholangiocarcinoma. Int J Oncol. 2012;41:1110–1118. doi: 10.3892/ijo.2012.1530. [DOI] [PubMed] [Google Scholar]
- 12.Fujimoto K, Kawaguchi T, Nakashima O, Ono J, Ohta S, Kawaguchi A, et al. Periostin, a matrix protein, has potential as a novel serodiagnostic marker for cholangiocarcinoma. Oncol Rep. 2011;25:1211–1216. doi: 10.3892/or.2011.1194. [DOI] [PubMed] [Google Scholar]
- 13.Hasita H, Komohara Y, Okabe H, Masuda T, Ohnishi K, Lei XF, et al. Significance of alternatively activated macrophages in patients with intrahepatic cholangiocarcinoma. Cancer Sci. 2010;101:1913–1919. doi: 10.1111/j.1349-7006.2010.01614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subimerb C, Pinlaor S, Khuntikeo N, Leelayuwat C, Morris A, McGrath MS, Wongkham S. Tissue invasive macrophage density is correlated with prognosis in cholangiocarcinoma. Mol Med Rep. 2010;3:597–605. doi: 10.3892/mmr_00000303. [DOI] [PubMed] [Google Scholar]
- 15.Subimerb C, Pinlaor S, Lulitanond V, Khuntikeo N, Okada S, McGrath MS, Wongkham S. Circulating CD14+CD16+ monocyte levels predict tissue invasive character of cholangiocarcinoma. Clin Exp Immunol. 2010;161:471–479. doi: 10.1111/j.1365-2249.2010.04200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang J, Hisamatsu T, Shimamura K, Yoneno K, Adachi M, Naruse H, et al. Activated hepatic stellate cells mediate the differentiation of macrophages. Hepatol Res. 2012 doi: 10.1111/j.1872-034X.2012.01111.x. epub ahead of print. PMID: 23107150. [DOI] [PubMed] [Google Scholar]
- 17.Farazi PA, Zeisberg M, Glickman J, Zhang Y, Kalluri R, DePinho RA. Chronic bile duct injury associated with fibrotic matrix microenvironment provokes cholangiocarcinoma in p53-deficient mice. Cancer Res. 2006;66:6622–6627. doi: 10.1158/0008-5472.CAN-05-4609. [DOI] [PubMed] [Google Scholar]
- 18.DeClerck YA. Desmoplasia: a response or a niche? Cancer Discov. 2012;2:772–774. doi: 10.1158/2159-8290.CD-12-0348. [DOI] [PubMed] [Google Scholar]
- 19.Ling H, Roux E, Hempel D, Tao J, Smith M, Lonning S, et al. Transforming growth factor β neutralization ameliorates pre-existing hepatic fibrosis and reduces cholangiocarcinoma in thioacetamide-treated rats. PLOS One. 2013;8:e54499. doi: 10.1371/journal.pone.0054499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinlaor S, Prakobwong S, Hiraku Y, Pinlaor P, Laothong U, Yongvanit P. Reduction of periductual fibrosis in liver fluke-infected hamsters after long-term curcumin treatment. Eur J Pharmacol. 2010;638:134–141. doi: 10.1016/j.ejphar.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 21.Prakobwong S, Khoontawad J, Yongvanit P, Pairojkul C, Hiraku Y, Sithithaworn P, et al. Curcumin decreases cholangiocarcinogenesis in hamsters by suppressing inflammation-mediated molecular events related to multistep carcinogenesis. Int J Cancer. 2011;129:88–100. doi: 10.1002/ijc.25656. [DOI] [PubMed] [Google Scholar]
- 22.Philips GM, Chan IS, Swiderska M, Schroder VT, Guy C, Karaca GF, et al. Hedgehog signaling antagonist promotes regression of both liver fibrosis and hepatocellular carcinoma in a murine model of primary liver cancer. PLOS One. 2011;6:e23943. doi: 10.1371/journal.pone.0023943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pratap A, Singh S, Mundra V, Yang N, Panakanti R, Eason JD, Mahato RI. Attenuation of early liver fibrosis by pharmacological inhibition of smoothened receptor signaling. J Drug Target. 2012;20:770–782. doi: 10.3109/1061186X.2012.719900. [DOI] [PubMed] [Google Scholar]
- 24.Kim Y, Fiel MI, Albanis E, Chou HI, Zhang W, Khitrov G, Friedman SL. Anti-fibrotic activity and enhanced interleukin-6 production by hepatic stellate cells in response to imatinib mesylate. Liver Int. 2012;32:1008–1017. doi: 10.1111/j.1478-3231.2012.02806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mertens JC, Fingas CD, Christensen JD, Smoot RL, Bronk SF, Werneburg NW, et al. Therapeutic effects of deleting cancer-associated fibroblasts in cholangiocarcinoma. Cancer Res. 2013;73:897–907. doi: 10.1158/0008-5472.CAN-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Omenetti A, Diehl AM. Hedgehog signaling in cholangiocytes. Curr Opin Gastroenterol. 2011;27:268–275. doi: 10.1097/MOG.0b013e32834550b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fingas CD, Bronk SF, Werneburg NW, Mott JL, Guicciardi ME, Cazanave SC, et al. Myofibroblast-derived PDGF-BB promotes hedgehog survival signaling in cholangiocarcinoma cells. Hepatology. 2011;54:2076–2088. doi: 10.1002/hep.24588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fingas CD, Mertens JC, Razumilava N, Bronk SF, Sirica AE, Gores GJ. Targeting PDGFR-β in cholangiocarcinoma. Liver Int. 2012;32:400–409. doi: 10.1111/j.1478-3231.2011.02687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cadamuro M, Nardo G, Indraccolo S, Dall’olmo L, Sambado L, Moserle L, et al. Platelet-derived growth factor-D and Rho GTPases regulate recruitment of cancer-associated fibroblasts in cholangiocarcinoma. Hepatology. 2013 doi: 10.1002/hep.26384. Epub ahead of print. PMID: 23505219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiedmann MW, Mössner J. Molecular targeted therapy of biliary tract cancer – results of the first clinical studies. Curr Drug Targets. 2010;11:834–850. doi: 10.2174/138945010791320818. [DOI] [PubMed] [Google Scholar]
- 31.Clapéron A, Mergey M, Aoudjehane L, Huong T, Ho-Bouldoires TH, Wendum D, et al. Hepatic myofibroblasts promote the progression of human cholangiocarcinoma through activation of epidermal growth factor receptor. Hepatology. 2013 doi: 10.1002/hep.26585. Epub ahead of print. PMID: 23787814. [DOI] [PubMed] [Google Scholar]
- 32.Khatib ME, Kalnytska A, Palagani V, Kossatz U, Manns MP, Malek NP, et al. Inhibition of hedgehog signaling attenuates carcinogenesis in vitro and increases necrosis of cholangiocellular carcinoma. Hepatology. 2013;57:1035–1045. doi: 10.1002/hep.26147. [DOI] [PubMed] [Google Scholar]
- 33.Sirica AE, Campbell DJ, DeWitt JL. Organotypic cell culture modeling of desmoplastic cholangiocarcinoma progression. Hepatology. 2012;56:616A. doi: 10.1111/j.1872-034X.2012.01026.x. (Abstract). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walter K, Omura N, Hong S-M, Griffith M, Vincent A, Borges M, Goggins M. Overexpression of smoothened activates the sonic hedgehog signaling pathway in pancreatic cancer-associated fibroblasts. Clin Cancer Res. 2010;16:1781–1789. doi: 10.1158/1078-0432.CCR-09-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan IS, Guy CD, Chen Y, Lu J, Swiderska-Syn M, Michelotti GA, et al. Paracrine hedgehog signaling drives metabolic changes in hepatocellular carcinoma. Cancer Res. 2012;72:6344–6350. doi: 10.1158/0008-5472.CAN-12-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garber K. Stromal depletion goes on trial in pancreatic cancer. JNCI. 2010;102:448–450. doi: 10.1093/jnci/djq113. [DOI] [PubMed] [Google Scholar]
- 38.Alberici L, Roth L, Sugahara KN, Agemy L, Kotamraju VR, Teesalu T, et al. De Novo design of a tumor-penetrating peptide. Cancer Res. 2013;73:804–812. doi: 10.1158/0008-5472.CAN-12-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]