Abstract
Foam cell formation from macrophage is a major cause of atherosclerosis. An efficient macrophage-specific promoter is required for the targeting to macrophages. In this study, we develop a macrophage-specific synthetic promoter for the therapeutic application of adiponectin (APN), an antiatherogenic gene. Synthetic promoter-146 (SP146), registered on the NCBI website (http://www.ncbi.nlm.nih.gov/nuccore/DQ107383), was tested for promoter activities in two non-macrophage cell lines (293 T, HeLa) and a macrophage cell line (RAW264.7, bone marrow-derived macrophages). To enforce macrophage specificity, partial elements of p47phox including the PU.1 site with various lengths (-C1, -C2 and -C3) were inserted next to the synthetic promoters. SP146-C1 showed the highest specificity and efficacy in RAW264.7 cells and was selected for development of an APN-carrying macrophage-specific promoter. Green fluorescent protein (GFP)- or APN-expressing lentivirus under SP146-C1 (Lenti-SP-GFP or Lenti-SP-APN, respectively) showed the highest expression efficacy in RAW264.7 cells compared with the non-macrophage cell lines. APN overexpression in RAW264.7 cells successfully inhibited intracellular lipid accumulation, and atherosclerotic lesions and lipid accumulation were significantly reduced by Lenti-SP-APN in ApoE−/− atherosclerosis mice. In conclusion, the synthetic promoter SP146-C1, combined with a p47phox promoter element, was successfully developed to target macrophage, and macrophage-specific introduction of APN under SP146-C1 was shown to ameliorate the atherosclerotic pathology.
Keywords: macrophage-specific promoter, foam cell, atherosclerosis
Introduction
In the early progression of atherosclerosis, endothelial cells are activated by oxidatively modified lipoproteins at the site of intimal lesions to secrete chemokines and to recruit circulating monocytes.1 Increased circulating monocytes have a positive correlation with atherosclerosis.2, 3 Infiltrated monocytes differentiate to macrophages, take up oxidized low-density lipoprotein (LDL) and finally differentiate to foam cells.4, 5 The accumulated foam cells develop into atherosclerotic plaque. Therefore, inhibiting the differentiation of macrophages into foam cells could be an important therapeutic strategy for preventing atherosclerosis.
Some studies have shown that inhibiting the chemokines that act to recruit monocytes from blood can effectively inhibit the development of atherosclerosis.6, 7, 8 However, applying this to humans is not realistic because the therapy would have to take place in the early stages of atherogenesis. Other studies have investigated the cell transplantation of macrophages infected with retroviral-apolipoprotein AI (ApoAI) or the direct injection of retroviral-ApoAI into ApoE knockout mice.9, 10 Although these therapeutic applications successfully reduced the lesion area, nonspecific expression of a therapeutic gene can result in unexpected side effects. Furthermore, a nonspecific promoter integrated into a chromosome can increase the chance of tumorigenesis.11 Thus, a macrophage-specific promoter is needed to deliver therapeutic genes to macrophages with high specificity. Some studies have reported that the CD68 promoter and scavenger receptor-A (SA) promoter are macrophage-specific,12, 13 but these promoters had low specificity, low expression efficiency or inadequately long sequences. Another study reported a synthetic promoter (SP) made up of a mix of six elements from known macrophage-specific promoters such as CD68 promoter. When hematopoietic stem cells infected with SP-ApoE-expressing lentivirus were transplanted to ApoE knockout mice, atherosclerotic lesions were reduced.14
Adiponectin (APN), an anti-inflammatory protein released from adipocytes, inhibits the formation of foam cells from macrophages.15, 16 Decreased blood APN by dysfunction of adipose tissue may increase the rate of vascular diseases.17 Overexpression of APN in ApoE knockout mice results in significantly reduced atherosclerosis lesions.18
In this study, we selected APN as a therapeutic gene to be delivered in a macrophage-specific vector for the treatment of atherosclerosis. We confirmed the macrophage-specific expression of APN and demonstrated the inhibition of in vitro foam cell formation.
Results
SP146-C1 showed the highest specificity and expression efficiency among the SPs tested
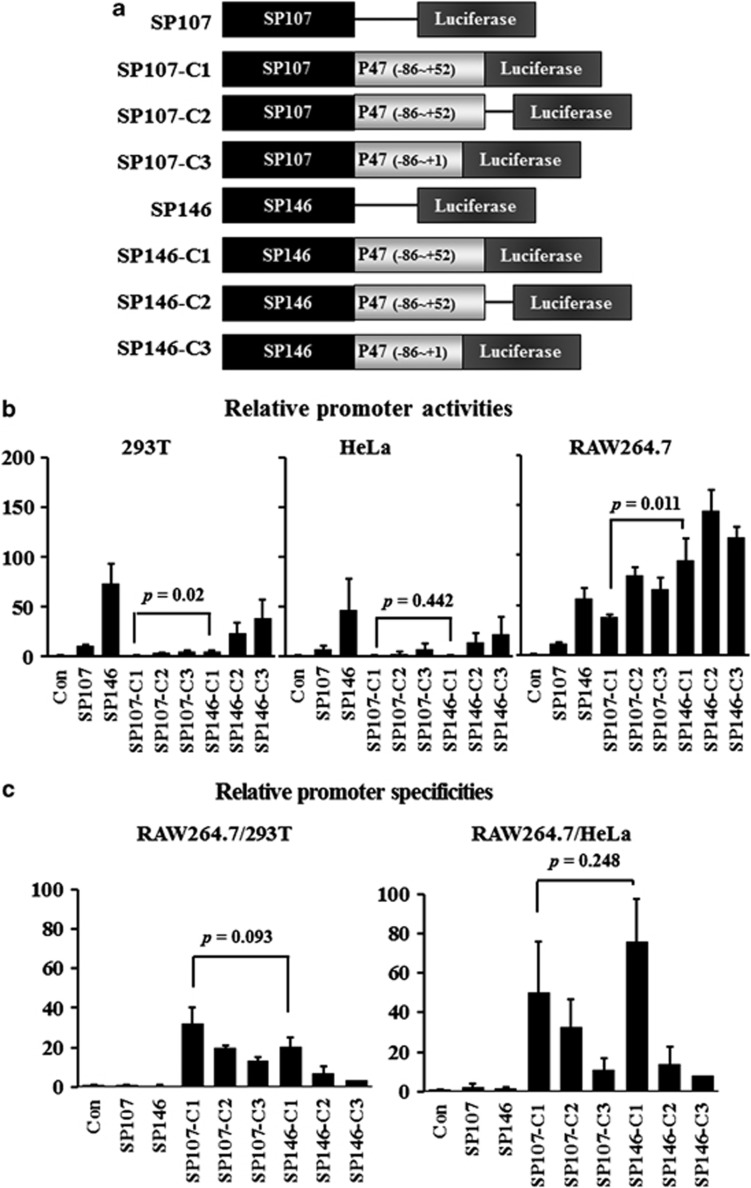
SPs reported in the literature14 were combined with the p47phox promoter element and inserted into pGL3-basic vector (Figure 1a). Our approach for evaluating promoter activities was first to test the transient transfection of all the SPs in two non-macrophage cell lines (293 T and HeLa) and a macrophage cell line (RAW264.7) (Figure 1b and Table 1). To avoid the influence of different transfection efficiencies with each cell line, the ratio of promoter activity in the macrophage cell line to that in the non-macrophage cell lines was calculated to express the macrophage specificity. SP107 and SP146 did not show macrophage specificity, whereas the promoters with the p47phox promoter element (SP107-C1, -C2 and -C3 and SP146-C1, -C2 and -C3) did (Figure 1c and Table 2). Thus, the macrophage-specific activity was dependent on the p47phox promoter element.
Figure 1.
Designs of the promoter constructs and comparisons of luciferase activity among macrophage-specific SPs. (a) Constructs of SPs. SP107 and SP146 were inserted in front of p47phox elements containing the PU.1 site (C1, C2 and C3). All promoters were constructed on the pGL3-basic plasmid. (b) The non-macrophage cell lines 293 T and HeLa and the macrophage cell line RAW264.7 were transfected, and promoter activities were measured 48 h later by dual-luciferase assay. Renilla luciferase vector was co-transfected with SPs for control of the transfection efficiency. Luciferase activity was further normalized to that obtained with the pGL3-basic vector. (c) To evaluate macrophage specificity, promoter activities were represented as the ratios of RAW264.7/293 T and RAW264.7/HeLa. Data shown are means±s.d.'s.
Table 1. Relative promoter activities of synthetic promoters.
| 293 T | HeLa | RAW264.7 | |
|---|---|---|---|
| Control | 1.0±0 | 1.0±0 | 1.0±0 |
| SP107 | 10.5±1.5 | 6.7±3.5 | 11.5±1.5 |
| SP146 | 73.2±19.1 | 46.5±30.8 | 56.9±10.2 |
| SP107-C1 | 1.2±0.2 | 0.9±0.6 | 38.4±2.6 |
| SP107-C2 | 4.0±0.2 | 3.0±2.0 | 79.3±8.0 |
| SP107-C3 | 4.9±0.8 | 7.5±4.9 | 66.2±10.8 |
| SP146-C1 | 4.8±0.8 | 1.3±0.6 | 94.6±21.7 |
| SP146-C2 | 23.9±10.0 | 13.9±9.3 | 143.9±20.9 |
| SP146-C3 | 39.0±17.9 | 22.7±16.9 | 117.4±10.6 |
Luciferase activities were used to represent promoter activities and were further normalized to that obtained with the pGL3-basic vector. Data shown are means±s.d.'s.
Table 2. Macrophage specificities of synthetic promoters.
| RAW264.7/293 T | RAW264.7/HeLa | |
|---|---|---|
| Control | 1.0±0 | 1.0±0 |
| SP107 | 1.1±0.1 | 2.3±1.7 |
| SP146 | 0.8±0.3 | 1.5±0.7 |
| SP107-C1 | 32.3±8.2 | 50.1±25.6 |
| SP107-C2 | 19.6±1.67 | 32.5±14.2 |
| SP107-C3 | 13.6±1.5 | 11.2±5.8 |
| SP146-C1 | 20.1±5.1 | 76.1±21.2 |
| SP146-C2 | 6.9±3.3 | 14.0±8.6 |
| SP146-C3 | 3.5±1.6 | 8.0±6.1 |
Promoter activities were used to represent macrophage specificity as the ratio of RAW264.7/293 T and RAW264.7/HeLa. Data shown are means±s.d.'s.
For gene therapy, a promoter should have strength as well as specificity. Thus, we next compared the promoter activities of SP107-C1 and SP146-C1. SP107-C1 did not show any promoter activity in 293 T or HeLa cells (1.1- and 0.9-fold) but showed high promoter activity in RAW264.7 cells (38.4-fold; Figure 1b and Table 1) similar to a previous report.14 SP146-C1 also showed low promoter activity in 293 T and HeLa cells (4.8- and 1.3-fold). In RAW264.7 cells, however, the promoter activity of SP146-C1 was much higher than that of SP107-C1 (94.6- vs 38.4-fold, P<0.05). To compare the macrophage specificities, the relative promoter activities were calculated and showed (Figure 1c and Table 2). Both the promoter activity and specificity were important factors. Therefore, we selected SP146-C1 to develop a macrophage-specific therapeutic vector.
APN under the SP146-C1 promoter was expressed only in macrophages
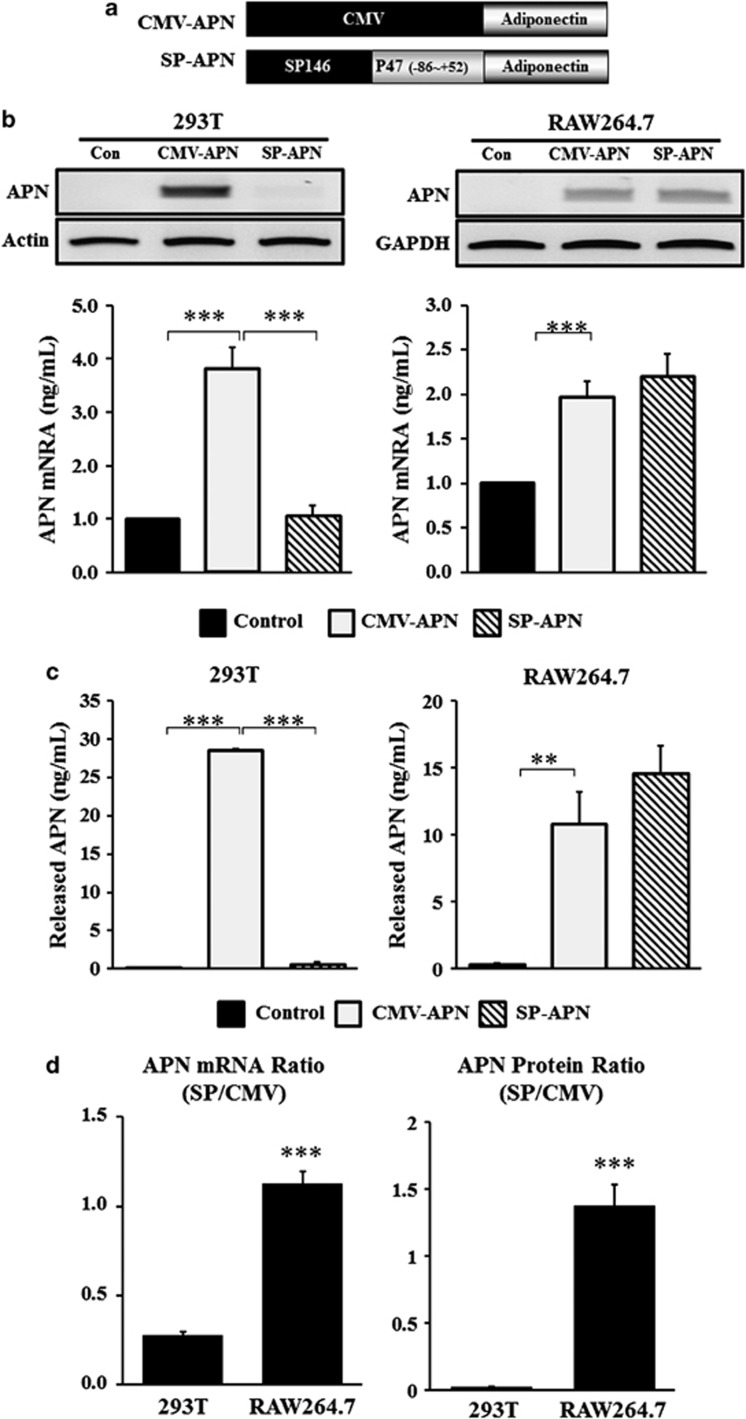
APN was selected as a therapeutic gene and a vector was constructed with APN under the SP146-C1 promoter (SP-APN) (Figure 2a). A vector with APN under the cytomegalovirus (CMV) promoter (CMV-APN) was also constructed as a positive control. SP146-C1 was established on the basis of the pGL3-basic plasmid; thus, the original luciferase gene in the plasmid was exchanged with the APN gene. Macrophage-specific expression of APN was determined by reverse transcriptase-polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay.
Figure 2.
Constructs of plasmid vectors for APN expression. (a) SP146-C1 was selected to develop a therapeutic vector. The original luciferase gene in the pGL3-basic plasmid was replaced by the APN gene. (b) Cells were transfected and mRNA expression of APN with CMV-APN and SP146-C1-APN (SP-APN) was detected 24 h later by RT-PCR in 293 T and RAW264.7 cells. CMV-APN was used as a positive control. Densities of bands were measured using Alpha easy FC software and normalized to that obtained with actin or glyceraldehyde 3-phosphate dehydrogenase (GAPDH). (c) Concentrations of APN in the media were measured 48 h later using a human APN ELISA Kit in 293 T and RAW264.7 cells. Data shown are means±s.d.'s. (d) The ratio of expressions of SP-APN and CMV-APN was calculated and expressed in graphs. Both mRNA level of APN and released APN showed high ratio in RAW264.7 cells. **P<0.01 and ***P<0.001. APN, adiponectin; ELISA, enzyme-linked immunosorbent assay; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
As shown by RT-PCR, 293 T cells transfected with SP-APN did not express adiponecitn, whereas RAW264.7 cells transfected with SP-APN expressed high levels of adiponecitn (Figure 2b). This result was comparable with CMV-APN in RAW264.7 cells.
Concentrations of APN in media were determined in 293 T and RAW264.7 cells. Transfection with CMV-APN showed high expression of APN in 293 T (28.5±0.2 ng ml−1) and RAW264.7 cells (10.8±2.4 ng ml−1). However, 293 T cells transfected with SP-APN did not express APN, whereas RAW264.7 cells transfected with SP-APN showed higher expression (14.6±2.0 ng ml−1) (Figure 2c). The ratio of APN mRNA expression was threefold higher (P<0.01), and the ratio of APN protein expression was 69-fold higher in RAW264.7 cells (P<0.01) than in 293 T cells.
The expression ratio of SP-APN to CMV-APN confirmed the macrophage-specific expression of SP-APN in RAW264.7 cells (Figure 2d).
SP146-C1-APN (SP-APN) inhibited in vitro foam cell formation
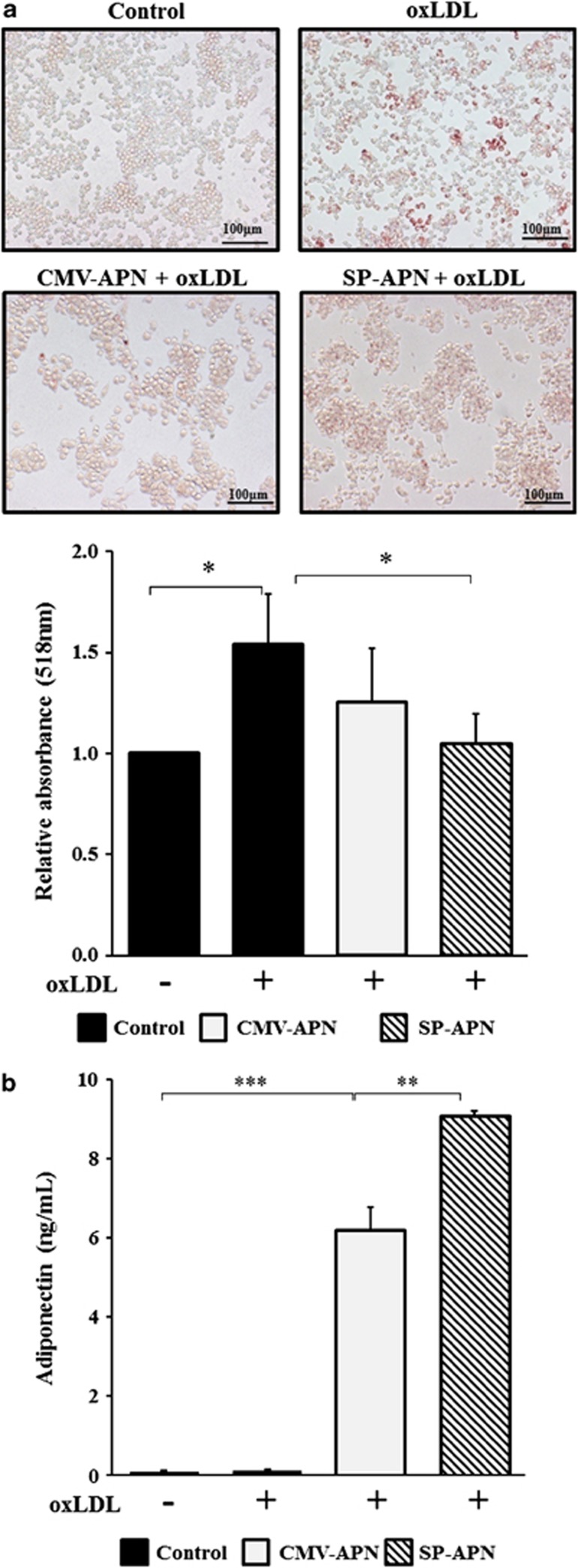
Macrophage-derived foam cells have been recognized as a characteristic feature of atherosclerosis.19 The foam cell formation assay is used as a biological indicator of the therapeutic effect of an antiatherogenic treatment.20 To confirm the antiatherogenic effect of SP-APN, an in vitro foam cell formation assay was performed.
Incubation of RAW264.7 cells with oxidized LDL (oxLDL) for 24 h led to increased lipid accumulation in cells that was detected by oil red O staining (Figure 3a). After transfection with CMV-APN (P=0.243) or SP-APN (P=0.041), RAW264.7 cells showed decreased lipid accumulation when treated with oxLDL in SP-APN-transfected cells. To evaluate lipid deposition in macrophages, oil red O was redissolved from stained cells and absorbance was detected at 518 nm in a spectrophotometer. As expected, the absorbance of oil red O was significantly decreased by SP-APN. These data demonstrated that application of SP-APN inhibited foam cell formation. In oxLDL-treated RAW264.7 cells, the level of released APN was higher in SP-APN-transfected cells than in CMV-APN-transfected cells (Figure 3b).
Figure 3.
Foam cell formation assay using macrophage-specific plasmid vector. (a) RAW264.7 cells were transfected with a plasmid vector and treated with oxLDL (100 μg ml−1) for 24 h. Lipid depositions of control, oxLDL alone, CMV-APN+oxLDL and SP-APN+oxLDL were detected 24 h later by oil red O staining and observed under an optical microscope at × 200 magnification. Absorbance of oil red O was detected by spectrophotometer at 518 nm and normalized to that obtained with the control group. (b) Released APN protein in the cultured media was higher in SP-APN-transfected cells than in CMV-APN-transfected cells. Data shown are means±s.d.'s. *P<0.05, **P<0.01 and ***P<0.001. APN, adiponectin.
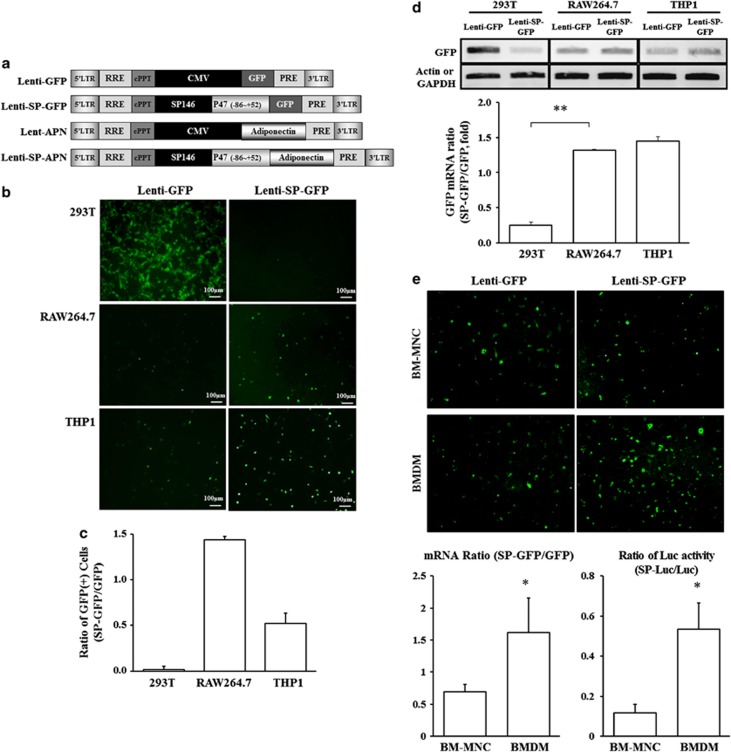
To enforce the transfection efficacy, lentiviral vectors were constructed as shown in Figure 4a. Lentiviral green fluorescent protein (GFP) under the SP146-C1 promoter showed high expression in myeloid/macrophage cell lines. To confirm whether the SP146-C1 promoter would work in myeloid/macrophage cell lines when applied as a virus, GFP-expressing lentivirus under the CMV (Lenti-GFP) or SP146-C1 (Lenti-SP-GFP) promoter was generated. GFP-positive cells were observed and counted under a fluorescence microscope, and expression levels of GFP were determined by RT-PCR (Figures 4b and c).
Figure 4.
Constructs of lentiviral vectors for APN expression. (a) Macrophage-specific lentiviral vectors were generated. (b) Cell-type-specific GFP expression in 293 T, RAW264.7 and THP1 cells infected by macrophage-specific lentivirus GFP (Lenti-GFP) and SP146-C1-GFP (Lenti-SP-GFP) lentivirus. GFP-expressing cells were distinguished from lentivirus-infected cells under fluorescence microscopy at × 200 magnification. (c) GFP-positive cells were counted and the ratio of the cell number in Lenti-SP-GFP-infected group to Lenti-GFP-infected group (SP-GFP/GFP) is shown. (d) The expressions of GFP mRNA were analyzed by RT-PCR, and the relative densities of the bands are shown. Higher expression ratio of Lenti-SP-GFP to Lenti-GFP was observed in RAW264.7 and THP1 cells than in 293 T cells. Data shown are means±s.d.'s. (e) Macrophage specificity of lentiviral-GFP was tested in bone marrow-derived mononuclear cells (BM-MNC) and bone marrow-derived macrophages (BMDM). Representative images were shown, and the ratio of mRNA and Luc activity were expressed in graphs. *P<0.05 and **P<0.01. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; Luc, luciferase.
HEK293 T cells infected with Lenti-GFP showed more GFP positivity than did 293 T cells infected with Lenti-SP-GFP (Figure 4b). On the other hand, GFP-positive cells were observed after infection of RAW264.7 (mouse macrophage cell line) cells and THP1 (human monocyte cell line) cells with Lenti-GFP and Lenti-SP-GFP (Figure 4c). The RT-PCR results showed that the Lenti-SP-GFP promoter significantly increased the expression of GFP in RAW264.7 and THP1 cells compared with the Lenti-GFP promoter, whereas 293 T cells showed reduced expression of GFP (Figure 4d). These data suggested that the lentiviral vector under the SP146-C1 promoter worked well in myeloid/macrophage cell lines. To prove the macrophage specificity of SP146-C1 in primary cells, bone marrow cells were tested for the expression of GFP. Representative images showed that more GFP-positive cells were observed in bone marrow-derived macrophages than in bone marrow-mononuclear cells (BM-MNC) by Lenti-SP-GFP infection. The macrophage specificity was confirmed by comparison of two reporters: GPF and luciferase under lentiviral vector containing CMV or SP promoter. Both mRNA ratio and luciferase activity ratio of Lenti-SP to Lenti were higher in bone marrow-derived macrophage than in BM-MNC (Figure 4e).
SP146-C1-APN lentivirus inhibited in vitro foam cell formation
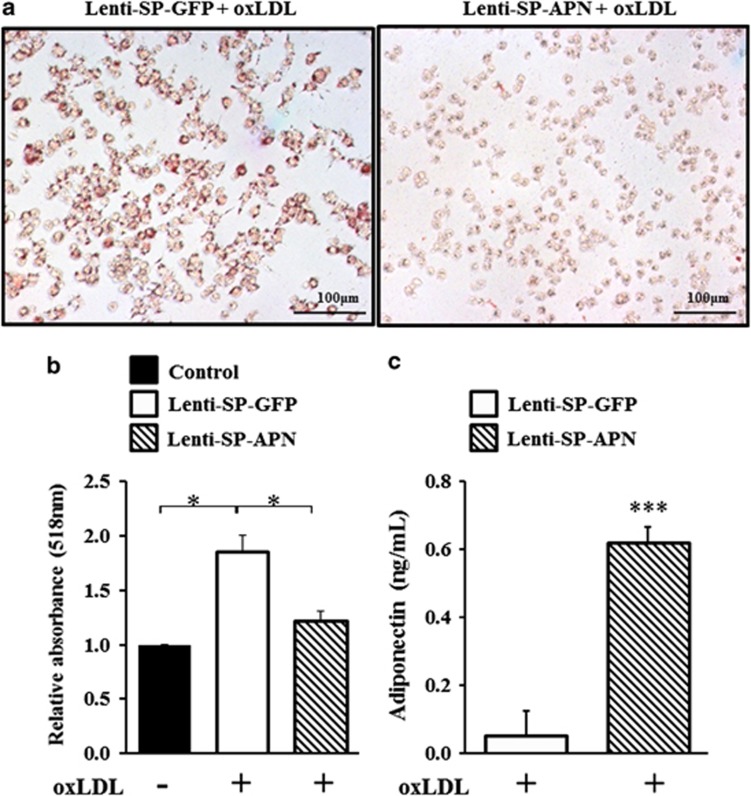
To apply to atherosclerosis, SP146-C1-APN lentivirus (Lenti-SP-APN) was generated and its antiatherogenic function was verified. The APN gene combined with SP146-C1 was extracted from SP-APN and was inserted into the Lenti-X2 vector in which the original CMV promoter was deleted. The foam cell formation assay was performed to determine whether the virus had the same effect as the plasmid. RAW264.7 cells infected with Lenti-SP-APN showed decreased lipid accumulation compared with cells infected with Lenti-SP-GFP (Figure 5a). The absorbance of oil red O was also decreased by Lenti-SP-APN (Figure 5b, P=0.0161). The level of released APN was markedly increased in Lenti-SP-APN-infected cells (Figure 5c). These results demonstrated that Lenti-SP-APN successfully inhibited foam cell formation.
Figure 5.
Foam cell formation assay using macrophage-specific lentiviral vector. (a) RAW264.7 cells were infected and treated with oxLDL (100 μg ml−1) 48 h later. Lipid depositions of Lenti-SP-GFP+oxLDL (a) and Lenti-SP-APN+oxLDL (b) were detected 24 h later by oil red O staining and observed under an optical microscope at × 200 magnification. (b) Absorbance of oil red O was detected by the spectrophotometer at 518 nm and normalized to that obtained in the control group. In Lenti-SP-APN-infected RAW264.7 cells, the foam cell formation was inhibited. (c) APN protein was highly expressed in Lenti-SP-APN-infected RAW264.7 cells in the presence of oxLDL treatment. Data shown are means±s.d.'s. *P<0.05 and ***P<0.001. APN, adiponectin; Lenti-GFP, lentivirus of GFP; Lenti-SP-APN, lentivirus of SP146-C1-APN.
Atherosclerotic lesion was reduced by Lenti-SP-APN
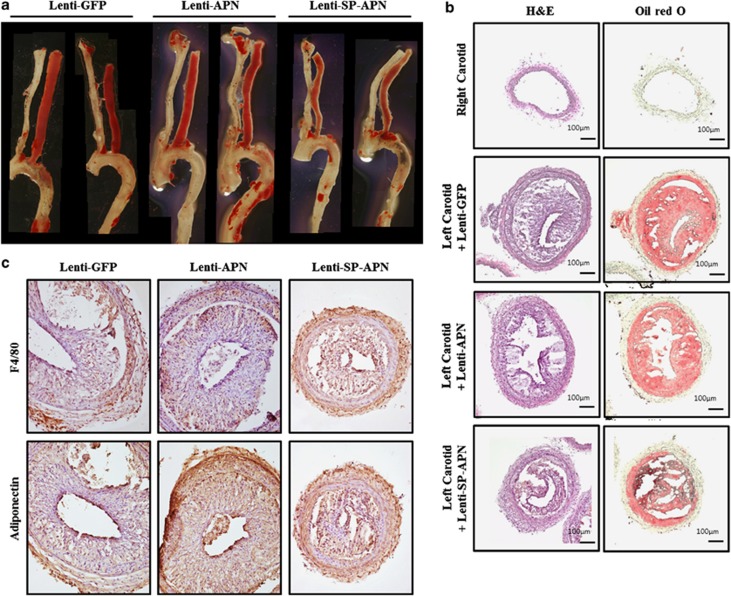
To examine the therapeutic effect of Lenti-SP-APN, atherosclerosis lesions were analyzed after its application to mouse atherosclerosis model. Representative images of Sudan-stained carotid artery showed that the atherosclerotic lesions were significantly reduced by Lenti-SP-APN infection compared with Lenti-GFP or Lenti-APN infection (Figure 6a). To examine the atherosclerotic lesions, hematoxylin and eosin staining and oil red O staining were performed (Figure 6b). Significant reductions of size and lipid deposit were observed in Lenti-SP-APN-infected atherosclerotic lesion. Next, the expression of APN in the lesions was examined by immunostaining. The infiltrated macrophages were detected in Lenti-GFP-, Lenti-APN- and Lenti-SP-APN-infected carotid arteries without significant differences (Figure 6c, F4/80). On the other hand, the expression patterns of APN were different. Although, the expression of APN was observed both in Lenti-APN group and Lenti-SP-APN group, lesion size was significantly smaller in Lenti-SP-APN group than in Lenti-APN group (Figure 6c).
Figure 6.
Macrophage-specific expression of APN reduced atherosclerotic lesions in ApoE knockout mice. (a) Representative images of Sudan-stained atherosclerotic lesions after application of lentiviral vectors for 2 weeks. Lenti-SP-APN-infected arteries showed a significant reduction of lipid deposit compared with that in Lenti-GFP- and Lenti-APN-infected arteries. (b) Hematoxylin and eosin (H&E) staining and oil red O staining were performed to characterize the lesions in lentiviral vector-infected lesions. (c) Macrophages were observed in all the lesions (F4/80). The expression of APN was detected in Lenti-APN- and Lenti-SP-APN-infected lesions. The size of the lesion was reduced by Lenti-SP-APN infection. APN, adiponectin.
Discussion
On the path from early preclinical research to final commercial products, gene therapy tolls and production methods have undergone tremendous changes to improve safety and efficacy.21 One way to increase safety is the use of a cell-specific promoter. Macrophage-specific promoters are being investigated in attempts to stave off the onset of atherosclerosis by inhibiting foam cell formation.
In this study, we constructed SPs and compared their macrophage-specific activity and then generated a therapeutic vector using the promoter that worked only in myeloid/macrophage cell lines. In the functional assessment, the antiatherogenic effect of APN in this macrophage-specific therapeutic vector was confirmed using the foam cell formation assay.
We performed luciferase assay to select the constructs with the macrophagespecificity. Our data showed that SP146 with C1 (SP146-C1), a p47phox promoter element, was superior to the other promoters tested and resulted in the highest macrophage specificity and protein expression efficiency.
Previous studies have used well-known macrophage-specific promoters such as CD68 promoter, SA promoter and SP.12, 13, 14 However, the CD68 promoter has long sequences and low specificity.14 To overcome these limitations, Levin et al.22 investigated the exclusive introduction of the 150-bp proximal part of the CD68 promoter, which was found to be more efficient and macrophage-specific than the full-length promoter. This promoter showed similar activity to SP in the macrophage.
Candidates of SP have been generated by random ligation of myeloid-specific elements such as PU.1, C/EBPα, AML1, sp1 and AP-1.14 Among them, SP146 showed high specificity and efficiency in 293, HeLa, RAW264.7 and THP1 cell lines.
In the present study, the SP107 and SP146 sequences from the NCBI website were synthesized and their promoter activities were tested in various cell lines. These sequences did not show macrophage-specific activity, however (Figure 1). The macrophage-specific activity of the SPs depended on the presence of the PU.1 element, including a part of the transcription start site of the p47phox promoter.14 Therefore, an additional six promoters were designed by incorporating the p47phox promoter region into the SP107 and SP146 promoters and their macrophage specificity was characterized (Figure 1 and Table 2). C1 has a partial region of the p47phox promoter (−86 to +52) immediately adjacent to the translation start site, whereas C2 has nonspecific sequences between C1 and the translation start site. C3 has a partial region of the p47phox promoter (−86 to +1) without a regulatory sequence of mRNA. Our results revealed that SP107-C1 showed the highest specificity for macrophages (Figure 2). SP107 and SP146 with C2 or C3 showed relatively high activities, but their macrophage specificities were lower.
Previously, it was reported that the PU.1 site is an essential element for p47phox promoter activity.23, 24 Because C1 had the PU.1 site in an accurate position on its promoter, it showed high activity only in a macrophage cell line. SP107 or SP146 may not have a critical role in macrophage specificity and may just support efficiency. According to our results, the macrophage specificity of SP was dependent on the PU.1 element being in an accurate position from the translation start site.
To apply gene therapy, it is important that the promoter has strength and specificity. SP107-C1 activity in RAW264.7 cells was similar to that in a previous study,14 whereas SP146-C1 showed three times higher promoter activity than SP107-C1. Therefore, we selected SP146-C1 for use as a therapeutic vector owing to its high specificity in a macrophage cell line.
To verify macrophage-specific gene introduction, GFP-expressing lentivirus under CMV (Lenti-GFP) or SP146-C1 (Lenti-SP-GFP) promoter was produced. GFP expression under the CMV promoter was detected in all cell lines tested, whereas GFP expression was not detected in 293 T cells under the SP146-C1 promoter (Figure 4). The GFP mRNA results were compatible with this result. Thus, cell-specific expression of SP146-C1 was achieved even when applied as a lentivirus.
APN is produced from adipocyte and its blood level is negatively correlated with adipose tissue mass.16 Most cytokines produced in adipose tissue are increased by increasing fat mass in situations such as obesity, insulin resistance and type 2 diabetes, but not in APN.17 APN has a wide range of effects with antiatherogenic, antidiabetic and anti-inflammatory activities.15, 17 In particular, APN effectively prevents vascular diseases by direct action on endothelial cells, smooth muscle cells, platelets and macrophages. Nitric oxide production is increased by APN in endothelial cells and APN has a protective effect on the vascular system through enhancing vasodilation, inhibiting monocyte adhesion and enhancing the proliferation of smooth muscle cell.25, 26
In atherosclerosis, foam cells develop through the uptake of oxidized LDLs from activated endothelium. APN inhibits foam cell formation by suppressing class A scavenger receptor and consequently leads to a decrease in the absorption of modified LDLs.27 LDL uptake is dose-dependently decreased by APN treatment. Therefore, APN is a suitable gene for use in a therapeutic vector for atherosclerosis.
HEK293T cells are well transfected and highly expressed proteins, whereas it is difficult to introduce plasmids into RAW264.7 cells. Despite the differences between the two cell lines, 293 T cells showed decreased expression of APN, and RAW264.7 cells showed higher expression under the SP146-C1 promoter than the CMV promoter (Figure 2). These results demonstrated that the macrophage-specific protein expression was mainly regulated at the transcriptional level.
In the foam cell formation assay, APN under the CMV and SP146-C1 promoters decreased lipid accumulation (Figure 3). Finally, SP146-C1-APN lentivirus (Lenti-SP-APN) resulted in clearly decreased lipid accumulation in macrophages (Figure 5). Therefore, APN under an SP containing the p47phox promoter element has a strong therapeutic potential when applied as either a plasmid or a lentivirus.
In summary, SPs containing the p47phox promoter element were highly active in macrophages. The specificity of the SP was determined by the position of the PU.1 site in relation to the translation start site. APN expression under the SP146-C1 promoter was higher than that under the CMV promoter in the macrophage cell line and resulted in a decreased lipid accumulation in macrophages. These results were the same in both the plasmid and virus applications. We found that the interaction of SP and p47 promoter is critical, and the p47 promoter is essential for gene regulation.
Intensive studies are needed to apply clinical settings, and the modification of macrophage-specific promoter cassette with conditional regulation system would be one of them. Development of safe carriers rather than lentivirus for the introduction of therapeutic genes is critical for clinical application.
Our data suggest that a macrophage-specific promoter can be a safe and useful tool in a therapeutic approach to atherosclerosis.
Materials and methods
Promoter constructs
All promoters were constructed on the basis of the pGL3-basic vector (Promega, Madison, WI, USA). The SP107 and SP146 sequences registered on the NCBI website (for SP107: http://www.ncbi.nlm.nih.gov/nuccore/DQ107382 (GenBank: DQ107382.1); for SP146: http://www.ncbi.nlm.nih.gov/nuccore/DQ107383 (GenBank: DQ107383.1)) were synthesized by including NheI restriction enzyme linker (Bioneer, Daejeon, Korea). In addition, a part of the p47phox promoter region from −250 to +100 bp was also synthesized to be used as a template. Three PU.1 site-containing elements, C1, C2 and C3, with different lengths of the p47phox promoter were synthesized to give macrophage specificity. These three elements were inserted into a pGL3-basic vector at two restriction enzyme sites of NheI and NcoI (Figure 1a). The SP107 or SP146 promoter was inserted in front of each element by NheI digestion. All construct sequences were confirmed by a sequencing service (Macrogen, Seoul, Korea). A total of eight constructs were generated and amplified using a Plasmid Maxi Amplification Kit (Qiagen, Hilden, Germany). The SP146-C1 promoter was selected to develop a therapeutic vector, and the original luciferase gene in the pGL3-basic plasmid was exchanged with the APN gene (Open Biosystem, Huntsville, AL, USA) using the restriction enzymes NcoI and XbaI (Figure 1b).
Cell culture and transient transfection
HEK293 T and HeLa cells were cultured in Dulbecco's modified Eagle's medium (Hyclone, Auckland, New Zealand) supplemented with 10% fetal bovine serum (Hyclone) and penicillin/streptomycin (Gibco, Grand Island, NY, USA). RAW264.7 cells were cultured in Dulbecco's modified Eagle's medium with 10% fetal bovine serum without penicillin/streptomycin. All cells were maintained at 37 °C in a 5% CO2 atmosphere. THP1 cells were maintained in RPMI1640 (Hyclone) supplemented with 10% fetal bovine serum and penicillin/streptomycin. For promoter study, THP-1 cells were differentiated into adherent macrophages by the addition of 0.1 mM phorbol myristate acetate for 4 h.
BM-MNC was isolated from tibia and femurs of mouse by flushing out and was differentiated into macrophages by treatment with 100 ng ml−1 macrophage-colony-stimulating factor (R&D Systems, Minneapolis, MN, USA) for 7 days. BM-MNC and bone marrow-derived macrophage were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. For transient transfection, cells were plated in 12-well plates. At 60–70% confluence, cells were transfected with pGL3-basic vector for control or promoter constructs with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Promoter activities were measured by luciferase assay.
Cells were transfected with APN-encoding plasmid vector (CMV-APN) or APN-encoding SP plasmid vector (SP-APN) and the concentration of APN in media was determined using a human APN ELISA Kit (AbFrontier, Seoul, Korea) 48 h later according to the manufacturer's manual. All experiments were repeated at least three times.
Luciferase assay
For luciferase assay, the Renilla luciferase vector was co-transfected with SPs for control of transfection efficiency. Promoter activities were measured by using the Dual-Glo Luciferase Assay kit (Promega, Madison, WI, USA) and a Centro XS3 LB 960 Microplate Luminometer (Berthold Technologies, Bad Wildbad, Germany) according to the manufacturer's instructions.
RT-PCR
Cells transfected with various vectors were harvested using the Trizol reagent (Ambion, Austin, TX, USA) 24 h later. cDNA was synthesized using M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA, USA). PCR for APN was performed using sense primer (5′-AATTCCACTGCAACATTCCTGGGC-3′) and antisense primer (5′-AGCCTGTGAAGGTGGAGTCATTGT-3′). PCR conditions were as follows: 20 cycles of 95 °C for 30 s, 60 °C for 30 s and 72 °C for 45 s. Cells infected by GFP-expressing lentivirus were harvested by the Trizol reagent 48 h later. After cDNA synthesis, PCR for GFP was performed using sense primer (5′-CATGAAGCAGCACGACTTCT-3′) and antisense primer (5′-CTGCTTGTCGGCCATGATATAG-3′). PCR conditions were as follows: 30 cycles of 95 °C for 30 s, 60 °C for 30 s and 72 °C for 45 s. All PCR products were analyzed by gel electrophoresis. Densities of bands were measured by Alpha easy FC software (Genetic Technologies, Miami, FL, USA) and normalized to actin or glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Construction of lentiviral vector
To produce APN-expressing lentiviral vector, SP-APN was cloned into Lenti-X2 (Addgene, Cambridge, MA, USA) and SP146-C1 was cloned into Lenti-X2-GFP lentiviral vector (Figure 1c). The ClaI and SmaI restriction enzyme sites were added in PCR primers to amplify SP146-C1 (SP) or SP-APN. Each PCR product was inserted into the viral vector to generate X2-SP-APN and X2-SP-GFP vectors. CMV promoter that originated from Lenti-X2 was deleted using the same restriction enzymes.
Two viral vectors were transfected into HEK293 T with three components of the viral vectors using a calcium-based transfection method overnight. Cells were changed to fresh media the next morning and incubated for an additional 2 days. Then, the media were centrifuged at 1500 r.p.m. for 3 min and the supernatant was mixed with Lenti-X Concentrator (Clontech, Mountain View, CA, USA) at 4 °C for 1 h and then centrifuged 3000 r.p.m. for 30 min at 4 °C. The pellet was diluted with phosphate-buffered saline and titrated by Lenti-X p24 Rapid Titer Kit (Clontech) as per the manufacturer's manual and stored at −80 °C. For lentiviral infection, cells at 60–70% confluence were treated with virus-containing solution at a concentration 105–106 infection-forming units (IFU) for 24 h and were replaced with fresh growth media. GFP-expressing cells were distinguished from lentivirus-infected cells under a fluorescence microscope and exposure time and brightness were not changed to compare with the Lenti-GFP and Lenti-SP-GFP groups at the same magnification.
Foam cell formation assay
An in vitro foam cell formation assay was performed using RAW264.7 cells plated in 12-well plate. After transfection or infection, cells were treated with 100 μg ml−1 oxidized LDL (Kalen Bio, Montgomery Village, MD, USA) for 24 h to induce foam cell formation. To detect lipid deposition, cells were fixed by 10% formalin solution for 1 h and washed two times using water. Then, cells were treated with 60% isopropanol for 5 min and exchanged to oil red O solution (diluted 2:3 of H2O:0.3% oil red O in isopropanol) for 5 min After washing two times with water, cells were observed using a microscope.
For quantitative analysis of lipid accumulation in cells, cells stained with oil red O were treated with 1 ml of 60% isopropanol for 1 h to redissolve the oil red O and absorbance was detected at 518 nm through a spectrophotometer.
Animals and atherosclerotic lesion analysis
Male ApoE knockout mice were obtained from Jung Ang Animals (Seoul, Korea). In total, nine mice were used in this study and all mice were fed a chow diet and water ad libitum until subtotal occlusion. Subtotal occlusion of left carotid artery was carried out as described previously28 and was divided into three groups: Lenti-GFP, Lenti-APN and Lenti-SP-APN. Briefly, anesthesia was induced by intramuscular injection of ketamine (50 mg kg−1) and xylazine (5 mg kg−1) mixture. Anterior area of the neck was epilated and disinfected with iodine. A ventral midline incision (1 cm) was made in the neck. Left carotid artery was exposed and three of four caudal branches of left carotid artery (left external carotid, internal carotid and occipital artery) were ligated with 7-0 silk suture under the surgical microscope, while the superior thyroid artery was left intact. Lentiviral solution (108 IFU) was injected into jugular vein near the left carotid artery directly. The incision was then closed with 5-0 silk suture and mice were monitored until recovery on a heating pad following surgery. After surgery, ApoE knockout mice were fed the Paigen's Atherogenic Rodent Diet (D12336; Research Diets, New Brunswick, NJ, USA) for 2 weeks until they were killed.
The atherosclerotic plaque burden was determined by oil red O staining. Mice were anesthetized, and the left ventricle of the heart was perfused and fixed with paraformaldehyde. Carotid and aorta were fixed by formalin for 24 h and was drained from formalin-fixed tissue and treated with Sudan IV staining solution in acetone/70% ethanol (1:1) for 15 min with periodical rocking. Tissues were then destained by 80% ethanol for 5 min Destained tissues were placed under running tap water for 1 h and observed on the light microscope.
For immunohistochemical analysis, the artery was embedded in OCT and sliced. For macrophage and APN staining, the sections were stained with antibodies against F4/80 (Abcam, Cambridge, MA, USA) and APN (Abcam). Subsequently, the sections were incubated with biotinylated antimouse or rat secondary antibodies (Vector Laboratories, Burlingame, CA, USA), ABC Kit, Peroxidase Substrate Kit (DAB; Vector Laboratories) and hematoxylin (Scytek Lab, Logan, UT, USA).
Images were obtained and digitized on a computer using an Olympus CX31 microscope (Olympus, Tokyo, Japan).
Statistical analysis
All values are expressed as means±s.d.. Student's t-test was performed with P<0.05 considered significant.
Acknowledgments
This work was supported by a grant of the National Research Foundation of Korea Grant funded by the Korean Government (MEST), Republic of Korea (2010-0020261 and 2011-0013889) and the Korea Healthcare Technology R&D Project, Ministry for Health and Welfare, Republic of Korea (HI12C0199).
The authors declare no conflict of interest.
References
- Mestas J, Ley K. Monocyte–endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc Med. 2008;18:228–232. doi: 10.1016/j.tcm.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller BS. Leukocytosis and ischemic vascular disease morbidity and mortality: is it time to intervene. Arterioscler Thromb Vasc Biol. 2005;25:658–670. doi: 10.1161/01.ATV.0000156877.94472.a5. [DOI] [PubMed] [Google Scholar]
- Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JL, Newby AC. Macrophage heterogeneity in atherosclerotic plaques. Curr Opin Lipidol. 2009;20:370–378. doi: 10.1097/MOL.0b013e3283309848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadl A, Meher AK, Sharma PR, Lee MY, Doran AC, Johnstone SR, et al. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res. 2010;107:737–746. doi: 10.1161/CIRCRESAHA.109.215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, et al. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- Combadiere C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Yoshida H, Major AS, Zhu T, Babaev VR, Linton MF, et al. Retrovirus-mediated expression of apolipoprotein A-I in the macrophage protects against atherosclerosis in vivo. J Biol Chem. 2001;276:36 742–36 748. doi: 10.1074/jbc.M106027200. [DOI] [PubMed] [Google Scholar]
- Belalcazar LM, Merched A, Carr B, Oka K, Chen KH, Pastore L, et al. Long-term stable expression of human apolipoprotein A-I mediated by helper-dependent adenovirus gene transfer inhibits atherosclerosis progression and remodels atherosclerotic plaques in a mouse model of familial hypercholesterolemia. Circulation. 2003;107:2726–2732. doi: 10.1161/01.CIR.0000066913.69844.B2. [DOI] [PubMed] [Google Scholar]
- Brenner S, Malech HL. Current developments in the design of onco-retrovirus and lentivirus vector systems for hematopoietic cell gene therapy. Biochim Biophys Acta. 2003;1640:1–24. doi: 10.1016/s0167-4889(03)00024-7. [DOI] [PubMed] [Google Scholar]
- Li AC, Guidez FR, Collier JG, Glass CK. The macrosialin promoter directs high levels of transcriptional activity in macrophages dependent on combinatorial interactions between PU. 1 and c-Jun. J Biol Chem. 1998;273:5389–5399. doi: 10.1074/jbc.273.9.5389. [DOI] [PubMed] [Google Scholar]
- Horvai A, Palinski W, Wu H, Moulton KS, Kalla K, Glass CK. Scavenger receptor A gene regulatory elements target gene expression to macrophages and to foam cells of atherosclerotic lesions. Proc Natl Acad Sci. 1995;92:5391–5395. doi: 10.1073/pnas.92.12.5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Qiang M, Ma W, Valente AJ, Quinones MP, Wang W, et al. Development of a synthetic promoter for macrophage gene therapy. Hum Gene Ther. 2006;17:949–959. doi: 10.1089/hum.2006.17.949. [DOI] [PubMed] [Google Scholar]
- Zhu W, Cheng KK, Vanhoutte PM, Lam KS, Xu A. Vascular effects of adiponectin: molecular mechanisms and potential therapeutic intervention. Clin Sci (Lond) 2008;114:361–374. doi: 10.1042/CS20070347. [DOI] [PubMed] [Google Scholar]
- Trujillo M, Scherer P. Adiponectin—journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med. 2005;257:167–175. doi: 10.1111/j.1365-2796.2004.01426.x. [DOI] [PubMed] [Google Scholar]
- Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29:2959–2971. doi: 10.1093/eurheartj/ehn387. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–2770. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- Hara S, Shike T, Takasu N, Mizui T. Lysophosphatidylcholine promotes cholesterol efflux from mouse macrophage foam cells. Arterioscler Thromb Vasc Biol. 1997;17:1258–1266. doi: 10.1161/01.atv.17.7.1258. [DOI] [PubMed] [Google Scholar]
- Turunen P, Jalkanen J, Heikura T, Puhakka H, Karppi J, Nyyssönen K, et al. Adenovirus-mediated gene transfer of Lp-PLA2 reduces LDL degradation and foam cell formation in vitro. J Lipid Res. 2004;45:1633–1639. doi: 10.1194/jlr.M400176-JLR200. [DOI] [PubMed] [Google Scholar]
- Yla-Herttuala S, Raty JK, Lesch HP, Wirth T. Improving safety of gene therapy. Curr Drug Saf. 2008;3:46–53. doi: 10.2174/157488608783333925. [DOI] [PubMed] [Google Scholar]
- Levin MC, Lidberg U, Jirholt P, Adiels M, Wramstedt A, Gustafsson K, et al. Evaluation of macrophage-specific promoters using lentiviral delivery in mice. Gene Therapy. 2012;19:1041–1047. doi: 10.1038/gt.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S-L, Valente AJ, Zhao S-J, Clark RA. PU. 1 is essential for p47 phox promoter activity in myeloid cells. J Biol Chem. 1997;272:17 802–17 809. doi: 10.1074/jbc.272.28.17802. [DOI] [PubMed] [Google Scholar]
- Li S-L, Schlegel W, Valente AJ, Clark RA. Critical flanking sequences of PU. 1 binding sites in myeloid-specific promoters. J Biol Chem. 1999;274:32 453–32 460. doi: 10.1074/jbc.274.45.32453. [DOI] [PubMed] [Google Scholar]
- Hattori Y, Suzuki M, Hattori S, Kasai K. Globular adiponectin upregulates nitric oxide production in vascular endothelial cells. Diabetologia. 2003;46:1543–1549. doi: 10.1007/s00125-003-1224-3. [DOI] [PubMed] [Google Scholar]
- Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45 021–45 026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y, et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, et al. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol. 2009;297:H1535–H1543. doi: 10.1152/ajpheart.00510.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]