Abstract
Background
The current effective treatment options for posttraumatic stress disorder (PTSD) are limited and therefore the need to explore new treatment strategies is critical. Pharmacological inhibition of the renin-angiotensin system is a common approach to treat hypertension and emerging evidence highlights the importance of this pathway in stress and anxiety. A recent clinical study from our laboratory provides evidence supporting a role for the renin-angiotensin system in the regulation of the stress response in patients diagnosed with PTSD.
Methods
Using an animal model of PTSD and the selective angiotensin receptor type 1 (AT1) antagonist losartan, we investigated the acute and long-term effects of AT1 receptor inhibition on fear memory and baseline anxiety. Following losartan treatment, we performed classical Pavlovian fear conditioning pairing auditory cues with footshocks and examined extinction behavior, gene expression changes in the brain as well as neuroendocrine and cardiovascular responses.
Results
Following cued fear conditioning, both acute and 2-week administration of losartan enhanced the consolidation of extinction memory but had no effect on fear acquisition, baseline anxiety, blood pressure and neuroendocrine stress measures. Gene expression changes in the brain were also altered in mice treated with losartan for 2 weeks, in particular reduced amygdala AT1 receptor and bed nucleus stria terminalis c-Fos mRNA levels.
Conclusions
These data suggest that AT1 receptor antagonism enhances the extinction of fear memory and therefore maybe a beneficial therapy for PTSD patients who have impairments in extinction of aversive memories.
Keywords: Fear memory, PTSD, renin-angiotensin, angiotensin receptor type 1 (AT1), stress, cardiovascular disease
INTRODUCTION
There is increasing evidence that post-traumatic stress disorder (PTSD), a debilitating fear and stress-related psychiatric illness, is associated with cardiovascular disease and its major comorbidities (1–4). As highlighted in recent reviews of clinical studies, both civilian and non-civilian persons diagnosed with PTSD are more likely to have hyperlipidemia, obesity, hypertension and increased risk for stroke and heart attack (4–6). Despite these emerging clinical and epidemiologic studies, the mechanisms responsible for the association between cardiovascular disease risk and PTSD remain unclear. Moreover, at present the current effective treatment options for PTSD are limited, and therefore the need to explore new treatment strategies is critical for improving future prevention efforts.
The renin-angiotensin system is essential for cardiovascular regulation, and drugs targeting this pathway, for example angiotensin receptor blockers (ARBs) and angiotensin converting enzyme inhibitors (ACE-I), are common strategies for treating hypertension and cardiovascular-related diseases. However, there is increasing interest and evidence supporting the role of the renin-angiotensin pathway in stress-related and neurodegenerative pathologies independent of its cardiovascular effects (7–9). It is known that stressful situations can elicit an increase in plasma levels of renin, which catalyzes the formation of angiotensin, thus giving rise to elevated levels of circulating angiotensin II (10–12). Animal studies have demonstrated that in response to immobilization and isolation stress, AT1 receptor binding is increased in the paraventricular nucleus of the hypothalamus, and is reduced with ARBs (13–15). More, recently lentiviral knockdown of the AT1 receptor in the subfornical organ of the brain prevents the neuroendocrine response to restraint stress (16). Evidence also suggests the use of ARBs to prevent stress-related brain pathologies (7–9) and several pre-clinical and clinical reports have described protective effects of ARBs on cognition and memory (17; 18). In line with these studies, we recently completed a clinical retrospective observational study of over 500 traumatized patients and found a significant association between individuals taking ACE-I/ARB medication and decreased PTSD symptoms (19). To further understand the mechanism responsible for these clinical observations, the aim of the present study was to examine the role of the renin-angiotensin system, through inhibition of the angiotensin type 1 receptor (AT1) in an animal model of PTSD-like symptoms. AT1 receptor blockade has also been shown to influence the hypothalamic pituitary-adrenal (HPA) axis (13; 20), which could impact fear memory, therefore we also sought to examine the acute HPA stress response.
Although PTSD is a complex disorder that includes chronic development over time of hyperarousal, intrusive, and avoidance/numbing symptoms (21; 22), its cardinal pathology is thought to relate, in part, to the unmitigated fear response at the time of the trauma, and the inability to inhibit fear in the aftermath of trauma (23). Therefore, we utilize classical Pavlovian conditioning in mouse models to directly address the mechanisms of fear acquisition, expression, inhibition, and extinction. Numerous examples have now demonstrated that methods which facilitate extinction in rodent models may have great translational validity to enhancing extinction in human fear-related disorders marked by deficits in extinction processing (24; 25). In these studies, based on our prior human observational findings (19), we hypothesized that losartan, an AT1 receptor antagonist, would be associated with decreased fear consolidation, and/or enhanced extinction of fear.
METHODS
Animals
All experiments were performed on adult (3–4 months old) wild-type C57BL/6J male mice from Jackson Laboratory (Bar Harbor, ME). All procedures were approved by the Institutional Animal Care and Use Committee of Emory University and were in compliance with National Institutes of Health guidelines.
Drugs
We administered losartan (Sigma-Aldrich, catalog no. 61188), a selective AT1 receptor antagonist, intraperitoneally (i.p.) at a dose of 1 mg/kg and 10 mg/kg in a vehicle of 0.9 % isotonic sterile saline; the same vehicle was also used in control groups. In other experiments, losartan was subcutaneously infused via osmotic mini-pump (10 mg/kg/day) (Alzet, Model 2002) over a 2-week period. In experiments where losartan was given i.p, mice received a single dose 40 minutes before the appropriate behavioral procedure. See Supplement: Figure S1 for experimental design.
Cardiovascular Measures
Blood pressure was measured invasively using radiotelemetry to resolve minute-to-minute acute blood pressure changes in freely moving animals or non-invasively using the tail cuff method as previously described (27–29).
Anxiety Measures
The elevated-plus maze consisted of a platform with two walled, closed arms and two non-walled, open arms connected by an open center. The mice were placed onto the center between the plus maze arms and were recorded exploring the plus maze for 5 min. For analysis, the percentage of time spent exploring the open arms, was calculated by dividing the time spent in the open arms by the combined time spent in open and closed arm (31). The open field consisted of a circular arena (60 cm diameter) made of black Plexiglass with a wall 20 cm high. Mice were allowed to explore for 10 min. Activity data were obtained and analyzed using the Activity Software (Med Associates Inc.).
Cue Fear Conditioning and Extinction
As previously performed in our laboratory, fear conditioning was conducted in nonrestrictive acrylic cylinders (SR-LAB startle response system, San Diego Instruments) and extinction testing was performed 24 and 48 hours after fear conditioning (32; 33). Please refer to the online supplement for detailed description.
Restraint Stress
Mice were individually restrained in a well-ventilated 50 ml conical tube for 30 minutes. Immediately following the restraint period they were sacrificed, brains removed and frozen with powdered dry ice and stored at −80 °C. Brains were then cut on a freezing microtome in sections of 40 μm at −18 °C and tissue punches were obtained for mRNA and qPCR.
Reverse Transcription and qPCR Quantification
Gene expression changes in the amygdala, prefrontal cortex, and bed nucleus stria terminalis were detected by relative quantitative-RT-PCR (Applied Biosystems FAST 7500). Bilateral tissue punches were performed according to the Mouse Brain Atlas by Watson and Paxinos (26) (Figure 4D). Total RNA was isolated using Qiagen RNeasy kit. Please refer to the online supplement for detailed description.
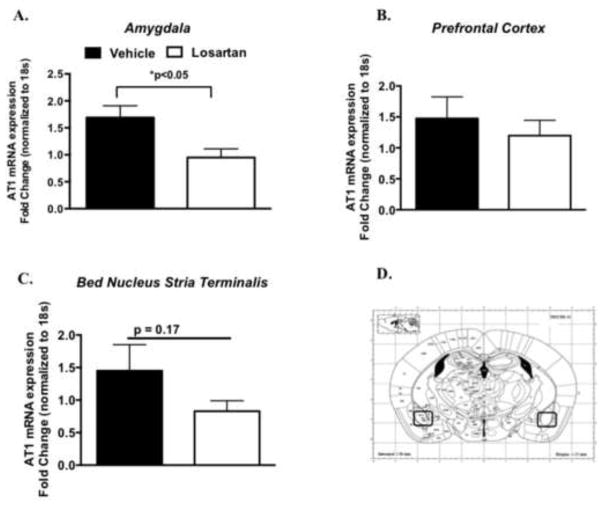
Figure 4. AT1 receptor mRNA gene expression following extinction training in losartan treated mice.
AT1 receptor messenger RNA (mRNA) levels in the amygdala (A.) Prefrontal Cortex (B.) and Bed nucleus stria terminalis of vehicle (n = 7) or losartan infused mice (n = 7) (A). Example of coronal brain section with black circles designating the surrounding regions of the amygdala isolated for reverse transcriptase quantitative polymerase chain reaction (B) (reprinted from Paxinos and Franklin (26) with permission from Elsevier, copyright 2006). *P < 0.05
Immunohistochemistry
Mice were perfused intracardially with ice-cold saline followed by 4 % paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The brains were removed and stored in the same fixative for 24 h at 4°C, and subsequently immersed in 30 % sucrose at 4 °C. To visualize the c-Fos protein, an immunohistochemical avidin-biotin-peroxidase staining procedure was used (Vector Labs Elite ABC Kit). The immunostaining reaction was developed using the oxidase-diaminobenzidine-nickel method (DAB; Sigma). Induction of c-Fos protein was evaluated by automated image analysis using Image J software (National Institutes of Health, Bethesda, MD). Please refer to the online supplement for detailed description.
Neuroendocrine Measures
Plasma serum corticosterone levels were measured by radioimmunoassay by the Emory University Biomarkers Core. Blood was collected on ice with 0.1 mol/L ethylenediaminetetraacetic acid (EDTA), and plasma was separated in a refrigerated centrifuge and stored at −70°C until analysis. Plasma renin activity was measured in heparinized plasma using a fluoenzymatic assay adapted from (30). Please refer to the online supplement for detailed description.
Data Presentation and Statistical Analysis
Data in the manuscript are expressed as the mean ± SEM and values of *P < 0.05 were considered statistically significant. Comparisons between more than 2 groups were made using ANOVA using Prism 6.0. When differences were observed, a Bonferroni post-hoc test was employed to compare specific groups. When identical measurements were made over time, we employed 1-way repeated measures ANOVA with a Bonferroni post hoc test. When 2 groups were compared we used an unpaired two-tailed Students T test.
RESULTS
Effect of Single Administration of Losartan on Learned Fear
To further understand the mechanism by which angiotensin II blockers reduce PTSD symptoms (19), we examined the effects of the selective AT1 receptor antagonist losartan in an animal model of PTSD-like symptoms. As shown in figure 1A, in the absence of drug, both groups exhibited normal acquisition of fear to the five tone-shock or CS-US pairings. Twenty-four hours later, we examined the effects of losartan on fear expression (also considered the extinction training session) to the presentation of 15 trials of CS cues in a different context. Groups were given either losartan (1 mg/kg or 10 mg/kg i.p.) or saline prior to fear expression/extinction training. During both extinction training, at 1 mg/kg losartan, and 24 hours later, during extinction retention testing, in the absence of drug there were no differences in freezing between groups (Figure 1B–D). Therefore in a separate group, a higher dose of losartan (10 mg/kg i.p.) was examined. As shown in Figure 1E, during extinction training/fear expression, total average freezing was similar between groups. However, 24 hours later in the absence of drug, total overall freezing was significantly reduced during extinction retention, an index of long-term fear memory (Figure 1F) (t(48) = 2.9; *P < 0.01). As determined by repeated-measures 2 way ANOVA, there was a significant main effect for treatment in the losartan group (10 mg/kg i.p), which exhibited significantly less freezing to CS presentation during extinction retention (F1,144 = 14.6; *P < 0.01) (Figure 1G). Bonferroni post hoc analysis revealed significant reductions in freezing during the first and third blocks of five CS tone presentations (*P < 0.05) (Figure 1G). Taken together, these data indicate that losartan does not affect fear expression, but enhances retention of fear extinction, in a dose-dependent manner, suggesting that AT1 receptor antagonism may reduce fear memory through enhancing the consolidation of extinction learning.
Figure 1. Angiotensin type 1 (AT1R) receptor inhibition enhances the extinction of learned fear.
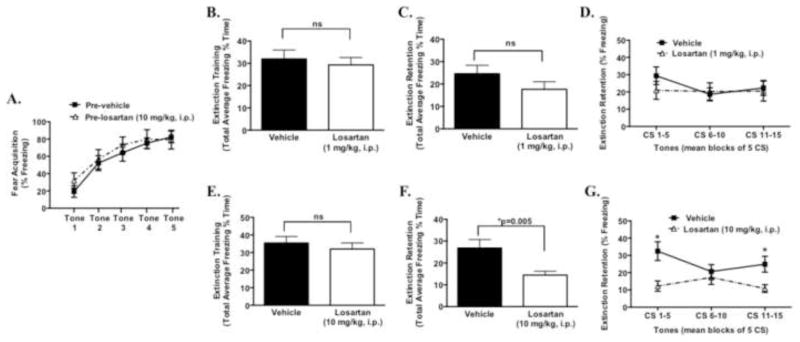
Prior to losartan treatment (pre-drug) acquired fear during cued fear conditioning with five tone-shock pairings in pre-vehicle (n = 10) and pre-losartan (n = 11) groups (A). 24hr following fear acquisition, losartan (1 mg/kg or 10 mg/kg i.p) was given prior to extinction training (B, E; n=20–25/group). 24 hr following extinction training, and in the absence of drug, mice were tested for extinction retention of learned fear, expressed as total average freezing and in blocks of 5 CS tones (C,D,F,G). *P < 0.05
No Blood Pressure or Anxiety-like Effects Following Acute Administration of Losartan
One possible hypothesis is that the above effects on extinction consolidation were due simply to compensatory changes following acutely lowered blood pressure or anxiety level. A dose of 10 mg/kg is commonly used in rodents, and when given acutely or chronically, some studies show reductions in baseline blood pressure (34–36) while others show no effect (37–39). In the current study, administration of losartan at a dose that enhanced extinction retention (i.p. 10 mg/kg) had no effect on baseline blood pressure (Figure 2A).
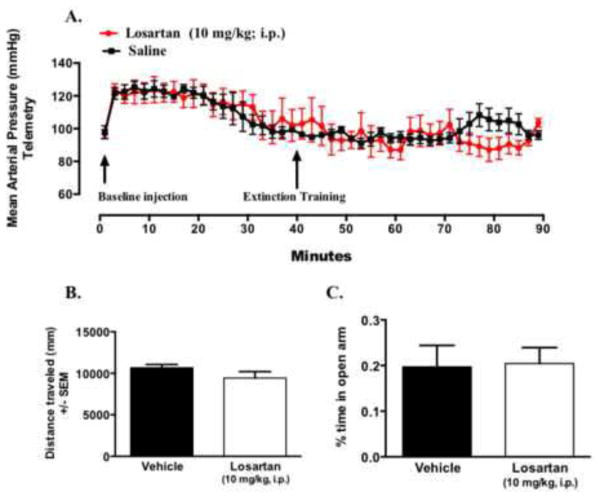
Figure 2. Acute administration of losartan (10 mg/kg, i.p.) does not affect baseline blood pressure or anxiety measures.
Minute analysis (every other minute) blood pressure measured by radiotelemetry following acute administration of saline (n = 4) or losartan (10 mg/kg, i.p n = 4) (A). Arrows represent time point of injection and start extinction training testing at minute 40 following injection of drug or vehicle. Distance travelled during open-field testing (B) and percent time in open arms of elevated plus maze test of vehicle (C) (n = 6–8/group).
In some animal studies, losartan has been previously shown to have anxiolytic effects (20), therefore, to determine whether the dose of losartan used in the present study affects baseline levels of generalized anxiety-like behavior, animals were tested in the elevated plus maze. We found no differences in anxiety-like behavior between losartan and saline treated groups as measured by distance traveled and time spent in open arms in the elevated plus maze (Figure 2B–C). Overall, these data suggest that acute administration of losartan at 10 mg/kg i.p, while enhancing extinction retention, does not affect baseline levels of blood pressure or measures of anxiety-like behavior in these animals.
Effect of Two Week Losartan Treatment on Learned Fear and Neuroendocrine measures
In our previous clinical study, most patients were on long-term treatment regimens of blood pressure lowering drugs, including angiotensin converting enzyme inhibitors or angiotensin receptor blockers (19). Therefore, we next sought to examine the repeated effects of AT1 receptor antagonism on the extinction of fear. Two-week osmotic mini-pumps were implanted and losartan (10 mg/kg/day) was subcutaneously infused and on day 10, animals underwent Pavlovian fear conditioning. The dose of 10 mg/kg/day was chosen based on our single i.p. administration study showing enhanced extinction retention at this bolus dose. As shown in Figure 3A, there was no difference in the acquisition of fear as determined by percent freezing in mice treated with losartan compared to vehicle. Furthermore, when we evaluated fear expression 24 hrs later, despite the decreased trend, there was no significant difference in total average freezing or when expressed in blocks of 5 CS trials (Figure 3B–C). However, consistent with the effects of acute administration of losartan on extinction, mice treated for 2 weeks with losartan displayed enhanced extinction retention of fear memory when expressed over the total average freezing period (Figure 3D, t(35) = 2.4; *P < 0.05). Repeated-measures ANOVA, during extinction retention testing, revealed a significant main effect of treatment in the losartan group as they exhibited significantly less freezing to CS presentation (F1,108 = 8.8; *P < 0.005) (Figure 3E). Bonferroni post hoc analysis revealed significant reductions in freezing during the second block of five CS tone presentations (Figure 3E, *P < 0.05). Similar to the acute administration, there were no differences between groups in generalized anxiety testing during the open field test or baseline blood pressure (Supplement: Figure S2). These data provide additional evidence that long-term AT1 receptor inhibition is involved in fear memory, independent of blood pressure or measures of baseline anxiety.
Figure 3. Chronic inhibition of angiotensin type 1 (AT1R) receptor enhances extinction of learned fear.
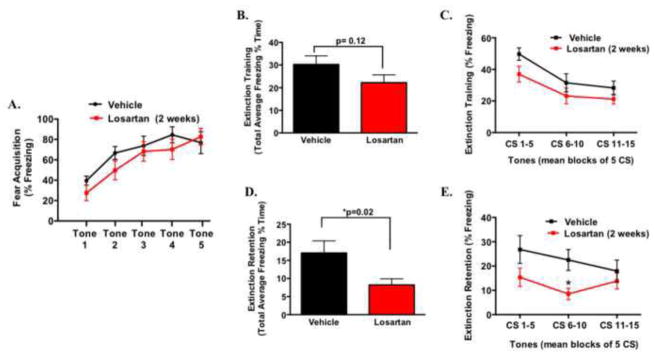
Following two weeks of losartan (10 mg/kg/day) (n = 17) or vehicle (n = 13) infusion, fear acquisition expressed as percent freezing during cued fear conditioning with five tone-shock pairings (A). Extinction training/fear expression was tested and total average freezing within the session (B) and represented in blocks of 5 CS tones (C) 24 hr following fear acquisition. Extinction retention of learned fear, expressed as total average freezing and in blocks of 5 CS tones are shown in panels (D) and (E) (n = 18–20). * P < 0.05
The question of how AT1 inhibition effects fear memory and whether it is due to a primary central effect (brain specific) or a secondary peripheral action is unknown. Therefore, we next examined AT1 receptor expression and other stress and fear memory-related genes in brain nuclei important in the consolidation of learned fear, such as the amygdala, prefrontal cortex (PFC) and bed nucleus stria terminalis (BNST) (40). As shown in Figure 4A, following extinction training (24 hr post fear conditioning), 14-day losartan treatment significantly reduced amygdala AT1R mRNA expression (t(11) = 2.8 *P < 0.05), however no change was observed in the PFC and a trend for reduced AT1R mRNA was observed in the BNST (Figure 4B–C). We also examined additional genes that maybe involved in the consolidation of fear memory in these brain regions. As shown in Figure 5A, losartan did not alter brain derived neurotrophic factor (bdnf) mRNA expression in the BNST, a gene in which we have previously shown be important in fear learning (32; 33; 41–43). The mRNA expression of corticotropin releasing hormone (crh), a gene involved in both fear and HPA stress activation in the BNST (44) was also not affected (Figure 5B). However, c-fos mRNA levels were significantly reduced in the BNST compared to the vehicle group (Figure 5C, t(13) = 2.2 *P < 0.05), whereas the expression levels of these genes were unchanged in the prefrontal cortex following extinction training (Figure 5D–F). Taken together, these gene expression studies suggest that losartan has a central effect on AT1 receptor and c-fos mRNA levels that appear to be region specific (Figure 4A).
Figure 5. Messenger RNA (mRNA) levels in the bed nucleus stria terminalis (BNST) and prefrontal cortex (PFC) of vehicle or losartan infused mice following extinction training in losartan treated mice.
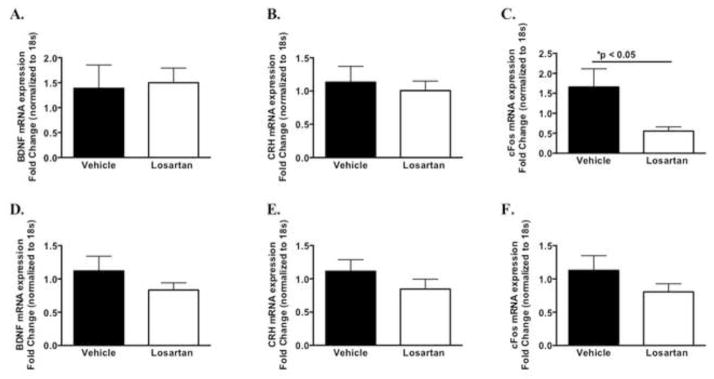
qPCR-determined quantitative levels (fold changes) of mRNAs encoding BNST (A–C) and PFC (D–F) (n = 7–8) *P < 0.05.
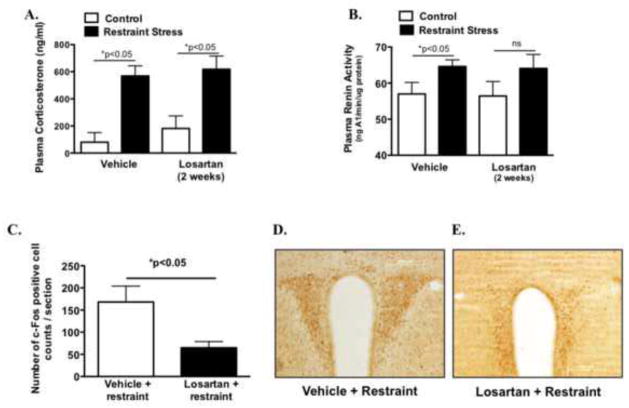
AT1 receptor inhibition has been previously found to inhibit the neuroendocrine stress response mediated by the hypothalamic pituitary-adrenal axis (HPA) (16), which could influence fear extinction. Using a standard, well-validated HPA stressor, we first examined the effects of losartan on plasma corticosterone as well as renin, a measure of endogenous angiotensin II activity. Following 30-minutes of restraint stress, significant elevations in corticosterone levels were observed but these levels were unaltered by losartan (F1,35 = 193.4; *P < 0.0001) (Figure 6A). Similarly plasma renin activity was elevated following restraint and unchanged by losartan (Figure 6B, (17)t = 2.1; *P < 0.05). As an index of central HPA neuronal activation, we then examined c-Fos activation in the paraventricular nucleus (PVN). As shown in Figure 6C, losartan treated animals exhibited reduced PVN c-Fos activation following restraint stress (t(10) = 2.3 *P < 0.05). These data suggest that chronic AT1 receptor inhibition does not prevent elevations in peripheral indices of the stress response but does influence central stress activation sites in the brain such as the PVN, which may influence other neuronal circuits (ie; amygdala, PFC, BNST) involved in the extinction of fear.
Figure 6. Effect of chronic losartan on central and peripheral indices of stress activation.
Plasma corticosterone levels (A) and plasma renin activity (B) in mice treated with vehicle or losartan for 2 weeks following acute restraint stress (n =9–10/group). Induction of c-Fos protein quantified as number of positive cell counts per section of paraventricular nucleus (C) (n = 5–7/group). Representative images showing reduced cFos protein induction in losartan versus vehicle animals exposed to acute restraint stress (D–E). *P < 0.05
DISCUSSION
Post-traumatic stress disorder (PTSD) is a debilitating psychiatric illness with increasing prevalence and limited treatment options. Moreover, PTSD and other chronic stress and anxiety disorders eventually lead to pathologies, including cardiovascular disease and hypertension (4; 6). In the present study we demonstrate that losartan, a selective angiotensin type I receptor antagonist and widely used anti-hypertensive drug, enhances extinction and reduces fear memory. These data illustrate a novel role of the AT1 receptor in an animal model of PTSD-like symptoms and suggest that this class of medications may be particularly useful for decreasing fear responses and enhancing the extinction of fear memories. These results also begin to provide a mechanistic understanding to support our previous clinical report suggesting that inhibition of the renin-angiotensin system may be beneficial for patients diagnosed with PTSD (19).
The renin-angiotensin system has long been known to play a key role in cardiovascular homeostasis. Angiotensin II, the main effector molecule, binds to its receptor subtypes, which include angiotensin type 1 receptor (AT1), angiotensin type 2 receptor (AT2) and angiotensin type 4 receptor (AT4)(46). The AT1 receptor is widely expressed across many organs including the heart, kidney and vasculature, and throughout many brain regions (48). The major systemic cardiovascular effects, including elevation in blood pressure, increased sympathetic activity, and fluid homeostasis as well as proliferative and hypertrophic effects, are mediated by AT1 signaling. Therefore, antagonists of this receptor are widely used to treat hypertension and cardiovascular related diseases. Several animal studies have also shown that independent of their beneficial effects on hypertension and cardiovascular related diseases, angiotensin receptor blockers can improve stress-related symptoms (15; 49; 50). A recent excellent review, highlights the beneficial effects of AT1 antagonists on brain disorders and are suggested as potential therapy for neurodegenerative diseases such as Alzheimers (7). In support of these data, we recently reported in a clinical population diagnosed with PTSD, that individuals taking medication that blocked the renin-angiotensin system, but not other blood pressure medications, had fewer PTSD symptoms (19). Our current study extends these findings as we demonstrate a role for AT1 receptor inhibition in the extinction of fear memories.
Patients with PTSD and other anxiety disorders are thought to have deficits in extinction of aversive memories (52–55). Similarly, rodents with anxiety-like behavior or trauma exposure demonstrate a deficit in extinction of conditioned fear (56). In the current study, mice treated with losartan one time or over 2 weeks, show less retention of fear memory or enhanced extinction of fear. Interestingly, these effects were independent of baseline blood pressure, anxiety or locomotor activities. Losartan has been previously shown to have anxiolytic effects (38; 57), therefore it is possible that AT1 receptor antagonism could influence the level of fear acquired during fear training. However, at the doses we used, animals exposed to losartan for 2 weeks acquired fear similarly to control groups. These data are in line with other studies showing that losartan does not influence baseline anxiety levels in rodents when given acutely or chronically (48; 58; 59) or on acquisition of an aversive memory (60). Moreover, these data demonstrate that AT1 receptor inhibition during fear conditioning enhances the extinction of an aversive memory and improves emotional learning, thus suggesting a role for endogenous angiotensin II in fear-related neurobiological processes.
In many animal studies examining the role of angiotensin II and AT1 antagonism in learning and memory, inhibitory avoidance learning paradigms have been utilized and these data have produced mixed results. For example, some have shown that angiotensin II improves avoidance memory (60–63), while others using similar learning paradigms have shown that angiotensin II impairs or has no effect on learning and memory (60; 64–66). Given that one of the main aims of the current study was to further understand the role of the renin-angiotensin system in the fear response, we utilized Pavlovian fear conditioning, a robust animal model for assessing memory in fear learning in both animals and humans. We demonstrate for the first time that inhibition of the AT1 receptor plays a role in the extinction of fear memory. In comparing these data to studies using inhibitory avoidance, our results would support studies suggesting that angiotensin II improves memory because in the presence of the AT1 antagonist, memory retention as determined by freezing behavior was attenuated. On the other hand, our results are in contrast to some studies using inhibitory avoidance, that show improvements in aversive memory following AT1 antagonism (67–69). These opposing results could be due to study differences in aversive learning paradigms, the dose of the AT1 antagonist and/or whether the drug was delivered via brain injection or systemically and time of antagonist administration (ie, prior to acquisition or retention).
Studies have also shown that avoidance learning paradigms have produced inconsistent results with regard to the involvement of brain structures essential to the fear response such as the amygdala (70). The amygdala is an integral part of the fear circuitry (23) and key inputs to the amygdala from the medial prefrontal cortex are thought to be required for the extinction of fear (71; 72). Immunohistological studies have revealed that brain AT1 receptors are expressed throughout regions involved in emotional learning including the amygdala and hippocampus (47; 73). In the current study, following extinction, losartan treated animals showed decreased amygdala AT1 receptor mRNA expression as well as reduced c-fos mRNA levels in another key limbic structure involved in learned fear, the bed nucleus stria terminalis (BNST)(74; 75). The mechanism for this reduction in amygdala AT1 and BNST c-fos mRNA levels and whether it is a direct or indirect result of AT1 inhibition with systemic losartan is unclear. Although speculative, these data suggest that reduced AT1 receptor interaction may be contributing to the enhanced extinction, however further AT1 receptor binding studies would be required and likely involves other brain angiotensin receptor subtypes such as AT2 and AT4 (76).
AT1 receptor inhibition can also inhibit the central and peripheral neuroendocrine HPA stress response in rodents (13; 15; 49) and therefore we speculated that these effects could influence learned fear. We demonstrate that in response to restraint stress, mice treated with losartan for 14 days have decreased PVN c-fos activation, but surprisingly this treatment did not alter the downstream peripheral adrenal corticosterone response. This dissociation between central and peripheral indices of HPA activation may reflect the time frame chosen to analyze c-fos in our study or other neural input unrelated to the HPA response could be affecting c-fos activation thus reflecting a limitation of this technique.
Brain angiotensin II can also interfere with different neurotransmitters and hormones such as norepinephrine, serotonin, vasopressin, and dopamine which are all involved in memory consolidation (48; 62). Moreover, angiotensin II has been found to modulate neurotrophic factors such as BDNF a critical molecular mediator in learning and memory (77). In our study, 14-day losartan treatment did not alter bdnf mRNA levels in the BNST or PFC following extinction training, although it is possible that amygdala bdnf maybe involved in this pathway. In addition to bdnf, angiotensin II can activate multiple other signaling pathways that may influence AT1 or AT2 receptor expression and function in fear memory. For example, Nostramao and colleagues recently showed in vitro in rat adrenal medulla cells, that pituitary adenylate cyclase-activating polypeptide (PACAP), a key peptide in the cellular stress response, modulates the AT2 receptor (78). These authors suggested that in response to stress, PACAP-triggered elevations in cAMP in chromaffin cells of the adrenal medulla mediate a down regulation of AT2 receptor. Interestingly, in a clinical study, a single nucleotide polymorphism in the PACAP receptor gene as well as differential levels of circulating PACAP peptide have recently been linked to level of fear physiology and PTSD severity.
In summary, we show that inhibition of the AT1 receptor in mice enhances the extinction of fear memory, a process that is dysregulated in humans with PTSD. This was shown following both acute and repeated administration of losartan and was independent of effects on blood pressure, measures of anxiety and fear acquisition. Furthermore, amygdala AT1 and c-fos BNST mRNA expression is reduced in losartan treated animals following extinction training, which implies that downstream AT1 signaling events maybe important in consolidation of extinction of fear. Importantly, these data also support the recent clinical observation that this class of medication may improve symptoms of PTSD (19). Future questions for translating our current findings into potential novel therapies, include identifying the appropriate timing and use of AT1 receptor antagonists as therapy following trauma exposure, determine if circulating levels of angiotensin II are altered in patients diagnosed with PTSD and whether angiotensin receptor polymorphisms are present in these patients. Future studies are therefore aimed at further understanding these questions and the mechanism as inhibition of this pathway may serve as a safe, powerful and novel treatment for PTSD.
Supplementary Material
Acknowledgments
This work was financially supported by The National Institutes of Health (K99 HL107675-01), (R01 MH096764) and the Burroughs Wellcome Foundation. We thank the Emory University Biomarkers Yerkes Core supported by a National Primate Research Center Base Grant 2P51RR000165-51and Rodent Behavioral Cores for their contribution to this manuscript.
Footnotes
CONFLICT OF INTEREST:
All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahmadi N, Hajsadeghi F, Mirshkarlo HB, Budoff M, Yehuda R, Ebrahimi R. Post-traumatic stress disorder, coronary atherosclerosis, and mortality. Am J Cardiol. (2011) 2011;108:29–33. doi: 10.1016/j.amjcard.2011.02.340. [DOI] [PubMed] [Google Scholar]
- 2.Sawchuk CN, Roy-Byrne P, Goldberg J, Manson S, Noonan C, Beals J, Buchwald D. The relationship between post-traumatic stress disorder, depression and cardiovascular disease in an American Indian tribe. Psychol Med. (2005) 2005;35:1785–1794. doi: 10.1017/S0033291705005751. [DOI] [PubMed] [Google Scholar]
- 3.Buckley TC, Kaloupek DG. A meta-analytic examination of basal cardiovascular activity in posttraumatic stress disorder. Psychosom Med. (2001) 2001;63:585–594. doi: 10.1097/00006842-200107000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Edmondson D, Cohen BE. Posttraumatic stress disorder and cardiovascular disease. Prog Cardiovasc Dis. 2013;55:548–556. doi: 10.1016/j.pcad.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wentworth BA, Stein MB, Redwine LS, Xue Y, Taub PR, Clopton P, et al. Post-traumatic Stress Disorder: A Fast Track to Premature Cardiovascular Disease? Cardiol Rev. (2012) 2012 doi: 10.1097/CRD.0b013e318265343b. [DOI] [PubMed] [Google Scholar]
- 6.Coughlin SS. Post-traumatic Stress Disorder and Cardiovascular Disease. Open Cardiovasc Med J. 2011;5:164–170. doi: 10.2174/1874192401105010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saavedra JM. Angiotensin II AT(1) receptor blockers as treatments for inflammatory brain disorders. Clin Sci. 2012;123:567–590. doi: 10.1042/CS20120078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright JW, Harding JW. The angiotensin AT4 receptor subtype as a target for the treatment of memory dysfunction associated with Alzheimer’s disease. J Renin Angiotensin Aldosterone Syst. 2008;9:226–237. doi: 10.1177/1470320308099084. [DOI] [PubMed] [Google Scholar]
- 9.Gard PR. Angiotensin as a target for the treatment of Alzheimer’s disease, anxiety and depression. Expert Opin Ther Targets. 2004;8:7–14. doi: 10.1517/14728222.8.1.7. [DOI] [PubMed] [Google Scholar]
- 10.Aguilera G. The Renin Angiotensin System and the Stress Response. Annals of the New York Academy of Sciences. 1995;771:173–186. doi: 10.1111/j.1749-6632.1995.tb44679.x. [DOI] [PubMed] [Google Scholar]
- 11.Yang G, Xi ZX, Wan Y, Wang H, Bi G. Changes in circulating and tissue angiotensin II during acute and chronic stress. Biol Signals. 1993;2:166–172. doi: 10.1159/000109488. [DOI] [PubMed] [Google Scholar]
- 12.Saavedra JM, Benicky J. Brain and peripheral angiotensin II play a major role in stress. Stress. 2007;10:185–193. doi: 10.1080/10253890701350735. [DOI] [PubMed] [Google Scholar]
- 13.Armando I, Volpi S, Aguilera G, Saavedra JM. Angiotensin II AT1 receptor blockade prevents the hypothalamic corticotropin-releasing factor response to isolation stress. Brain Research. (2007) 2007;1142:92–99. doi: 10.1016/j.brainres.2007.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dumont EC, Rafrafi S, Laforest S, Drolet G. Involvement of central angiotensin receptors in stress adaptation. NSC. (1999) 1999;93:877–884. doi: 10.1016/s0306-4522(99)00206-7. [DOI] [PubMed] [Google Scholar]
- 15.Bregonzio C, Seltzer A, Armando I, Pavel J, Saavedra JM. Angiotensin II AT(1) receptor blockade selectively enhances brain AT(2) receptor expression, and abolishes the cold-restraint stress-induced increase in tyrosine hydroxylase mRNA in the locus coeruleus of spontaneously hypertensive rats. Stress. 2008;11:457–466. doi: 10.1080/10253890801892040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krause EG, de Kloet AD, Scott KA, Flak JN, Jones K, Smeltzer MD, et al. Blood-Borne Angiotensin II Acts in the Brain to Influence Behavioral and Endocrine Responses to Psychogenic Stress. Journal of Neuroscience. 2011;31:15009–15015. doi: 10.1523/JNEUROSCI.0892-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amenta F, Mignini F, Rabbia F, Tomassoni D, Veglio F. Protective effect of anti-hypertensive treatment on cognitive function in essential hypertension: analysis of published clinical data. J Neurol Sci. 2002;203–204:147–151. doi: 10.1016/s0022-510x(02)00281-2. [DOI] [PubMed] [Google Scholar]
- 18.Anderson C, Teo K, Gao P, Arima H, Dans A, Unger T, et al. Renin-angiotensin system blockade and cognitive function in patients at high risk of cardiovascular disease: analysis of data from the ONTARGET and TRANSCEND studies. Lancet Neurol. 2011;10:43–53. doi: 10.1016/S1474-4422(10)70250-7. [DOI] [PubMed] [Google Scholar]
- 19.Khoury NM, Marvar PJ, Gillespie CF, Wingo A, Schwartz A, Bradley B, et al. The renin-angiotensin pathway in posttraumatic stress disorder: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are associated with fewer traumatic stress symptoms. J Clin Psychiatry. 2012;73:849–855. doi: 10.4088/JCP.11m07316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gard P. Strain differences in the anxiolytic effects of losartan in the mouse. Pharmacol Biochem Behav. 2001;69:35–40. doi: 10.1016/s0091-3057(01)00491-9. [DOI] [PubMed] [Google Scholar]
- 21.Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry. 1998;55:626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- 22.Brunello N, Davidson JR, Deahl M, Kessler RC, Mendlewicz J, Racagni G, et al. Posttraumatic stress disorder: diagnosis and epidemiology, comorbidity and social consequences, biology and treatment. Neuropsychobiology. 2001;43:150–162. doi: 10.1159/000054884. [DOI] [PubMed] [Google Scholar]
- 23.Jovanovic T, Ressler KJ. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am J Psychiatry. 2010;167:648–662. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Annals of the New York Academy of Sciences. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- 25.Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. BPS. 2006;60:369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 26.Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates. 2. San Diego, CA: Academic Press; 2006. [Google Scholar]
- 27.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. Journal of Experimental Medicine. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, et al. Central and Peripheral Mechanisms of T-Lymphocyte Activation and Vascular Inflammation Produced by Angiotensin II-Induced Hypertension. Circ Res. 2010;107:263–270. doi: 10.1161/CIRCRESAHA.110.217299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marvar PJ, Vinh A, Thabet S, Lob HE, Geem D, Ressler KJ, Harrison DG. T lymphocytes and vascular inflammation contribute to stress-dependent hypertension. Biol Psychiatry. 2012;71:774–782. doi: 10.1016/j.biopsych.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang GT, Chung CC, Holzman TF, Krafft GA. A continuous fluorescence assay of renin activity. Anal Biochem. 1993;210:351–359. doi: 10.1006/abio.1993.1207. [DOI] [PubMed] [Google Scholar]
- 31.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 32.Choi DC, Maguschak KA, Ye K, Jang SW, Myers KM, Ressler KJ. Prelimbic cortical BDNF is required for memory of learned fear but not extinction or innate fear. Proceedings of the National Academy of Sciences. 2010;107:2675–2680. doi: 10.1073/pnas.0909359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12:656–670. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xia H, Feng Y, Obr TD, Hickman PJ, Lazartigues E. Angiotensin II Type 1 Receptor-Mediated Reduction of Angiotensin-Converting Enzyme 2 Activity in the Brain Impairs Baroreflex Function in Hypertensive Mice. Hypertension. 2008;53:210–216. doi: 10.1161/HYPERTENSIONAHA.108.123844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soltis EE, Jewell AL, Dwoskin LP, Cassis LA. Acute and chronic effects of losartan (DuP 753) on blood pressure and vascular reactivity in normotensive rats. Clin Exp Hypertens. 1993;15:171–184. doi: 10.3109/10641969309041618. [DOI] [PubMed] [Google Scholar]
- 36.Collister JP, Soucheray SL, Osborn JW. Chronic hypotensive effects of losartan are not dependent on the actions of angiotensin II at AT 2 receptors. J Cardiovasc Pharmacol. (2001) 2002;39:107–116. doi: 10.1097/00005344-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 37.Senador D, Kanakamedala K, Irigoyen MC, Morris M, Elased KM. Cardiovascular and autonomic phenotype of db/db diabetic mice. Exp Physiol. 2009;94:648–658. doi: 10.1113/expphysiol.2008.046474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srinivasan J, Jayadev S, Kumaran D, Ahamed KF, Suresh B, Ramanathan M. Effect of losartan and enalapril on cognitive deficit caused by Goldblatt induced hypertension. Indian J Exp Biol. (2005) 2005;43:241–246. [PubMed] [Google Scholar]
- 39.Wong PC, Price WA, Chiu AT, Duncia JV, Carini DJ, Wexler RR, et al. Nonpeptide angiotensin II receptor antagonists. VIII. Characterization of functional antagonism displayed by DuP 753, an orally active antihypertensive agent. J Pharmacol Exp Ther. 1990;252:719–725. [PubMed] [Google Scholar]
- 40.Mahan AL, Ressler KJ. Trends in Neurosciences. Vol. 35. Elsevier Ltd; 2012. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder; pp. 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rattiner LM. Brain-Derived Neurotrophic Factor and Tyrosine Kinase Receptor B Involvement in Amygdala-Dependent Fear Conditioning. Journal of Neuroscience. 2004;24:4796–4806. doi: 10.1523/JNEUROSCI.5654-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chhatwal JP, Stanek-Rattiner L, Davis M, Ressler KJ. Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nat Neurosci. 2006;9:870–872. doi: 10.1038/nn1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi DC, Gourley SL, Ressler KJ. Prelimbic BDNF and TrkB signaling regulates consolidation of both appetitive and aversive emotional learning. Transl Psychiatry. 2012;2:e205. doi: 10.1038/tp.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sink KS, Walker DL, Freeman SM, Flandreau EI, Ressler KJ, Davis M. Effects of continuously enhanced corticotropin releasing factor expression within the bed nucleus of the stria terminalis on conditioned and unconditioned anxiety. Mol Psychiatry. 2013;18:308–319. doi: 10.1038/mp.2011.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coughlin SS. Post-traumatic Stress Disorder and Cardiovascular Disease. Open Cardiovasc Med J. 2011;5:164–170. doi: 10.2174/1874192401105010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wright JW, Harding JW. Brain angiotensin receptor subtypes AT1, AT2, and AT4 and their functions. Regul Pept. 1995;59:269–295. doi: 10.1016/0167-0115(95)00084-o. [DOI] [PubMed] [Google Scholar]
- 47.Marc Y, Llorens-Cortes C. Progress in Neurobiology. Vol. 95. Elsevier Ltd; 2011. The role of the brain renin-angiotensin system in hypertension: Implications for new treatment; pp. 89–103. [DOI] [PubMed] [Google Scholar]
- 48.Gard PR. The role of angiotensin II in cognition and behaviour. European Journal of Pharmacology. 2002;438:1–14. doi: 10.1016/s0014-2999(02)01283-9. [DOI] [PubMed] [Google Scholar]
- 49.Armando I, Carranza A, Nishimura Y, Hoe KL, Barontini M, Terrón JA, et al. Peripheral administration of an angiotensin II AT(1) receptor antagonist decreases the hypothalamic-pituitary-adrenal response to isolation Stress. Endocrinology. 2001;142:3880–3889. doi: 10.1210/endo.142.9.8366. [DOI] [PubMed] [Google Scholar]
- 50.Shekhar A. Angiotensin-II Is a Putative Neurotransmitter in Lactate-Induced Panic-Like Responses in Rats with Disruption of GABAergic Inhibition in the Dorsomedial Hypothalamus. Journal of Neuroscience. 2006;26:9205–9215. doi: 10.1523/JNEUROSCI.2491-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davies NM, Kehoe PG, Ben-Shlomo Y, Martin RM. Associations of anti-hypertensive treatments with Alzheimer’s disease, vascular dementia, and other dementias. J Alzheimers Dis. 2011;26:699–708. doi: 10.3233/JAD-2011-110347. [DOI] [PubMed] [Google Scholar]
- 52.Wessa M, Flor H. Failure of extinction of fear responses in posttraumatic stress disorder: evidence from second-order conditioning. Am J Psychiatry. 2007;164:1684–1692. doi: 10.1176/appi.ajp.2007.07030525. [DOI] [PubMed] [Google Scholar]
- 53.Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behav Res Ther. 2007;45:2019–2033. doi: 10.1016/j.brat.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 54.Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, Ressler KJ. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol Psychiatry. 2011;69:556–563. doi: 10.1016/j.biopsych.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Glover EM, Jovanovic T, Mercer KB, Kerley K, Bradley B, Ressler KJ, Norrholm SD. Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biol Psychiatry. 2012;72:19–24. doi: 10.1016/j.biopsych.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andero R, Ressler KJ. Fear extinction and BDNF: translating animal models of PTSD to the clinic. Genes Brain Behav. 2012;11:503–512. doi: 10.1111/j.1601-183X.2012.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lopez LH, Caif F, Garcia S, Fraile M, Landa AI, Baiardi G, et al. Anxiolytic-like effect of losartan injected into amygdala of the acutely stressed rats. Pharmacol Rep. (2012) 2012;64:54–63. doi: 10.1016/s1734-1140(12)70730-2. [DOI] [PubMed] [Google Scholar]
- 58.Braszko JJ, Wincewicz D, Jakubów P. Candesartan prevents impairment of recall caused by repeated stress in rats. Psychopharmacology (Berl) 2012 doi: 10.1007/s00213-012-2829-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Braszko JJ. Valsartan Abolishes Most of the Memory-Improving Effects of Intracerebroventricular Angiotensin II in Rats. Clin Exp Hypertens. 2005;27:635–649. doi: 10.1080/10641960500298723. [DOI] [PubMed] [Google Scholar]
- 60.Kulakowska A, Karwowska W, Wisniewski K, Braszko JJ. Losartan influences behavioural effects of angiotensin II in rats. Pharmacol Res. (1996) 1997;34:109–115. doi: 10.1006/phrs.1996.0073. [DOI] [PubMed] [Google Scholar]
- 61.Georgiev V, Yonkov D. Participation of angiotensin II in learning and memory. I. Interaction of angiotensin II with saralasin. Methods Find Exp Clin Pharmacol. 1985;7:415–418. [PubMed] [Google Scholar]
- 62.Braszko JJ, Wisniewski K. Effect of angiotensin II and saralasin on motor activity and the passive avoidance behavior of rats. Peptides. 1988;9:475–479. doi: 10.1016/0196-9781(88)90150-7. [DOI] [PubMed] [Google Scholar]
- 63.DeSouza FAM, Sanchis-Segura C, Fukada SY, de Bortoli VC, Zangrossi H, de Oliveira AM. Intracerebroventricular effects of angiotensin II on a step-through passive avoidance task in rats. Neurobiol Learn Mem. 2004;81:100–103. doi: 10.1016/j.nlm.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 64.Morgan JM. Angiotensin Injected into the Neostriatum After Learning Disrupts Retention Performance. Science. 1977;196:87–89. doi: 10.1126/science.402696. [DOI] [PubMed] [Google Scholar]
- 65.Kerr DS, Bevilaqua LRM, Bonini JS, Rossato JI, Köhler CA, Medina JH, et al. Angiotensin II blocks memory consolidation through an AT2 receptor-dependent mechanism. Psychopharmacology (Berl) 2004;179:529–535. doi: 10.1007/s00213-004-2074-5. [DOI] [PubMed] [Google Scholar]
- 66.Wright JW, Miller-Wing AV, Shaffer MJ, Higginson C, Wright DE, Hanesworth JM, Harding JW. Angiotensin II(3-8) (ANG IV) hippocampal binding: potential role in the facilitation of memory. Brain Res Bull. 1993;32:497–502. doi: 10.1016/0361-9230(93)90297-o. [DOI] [PubMed] [Google Scholar]
- 67.Barnes NM, Champaneria S, Costall B, Kelly ME, Murphy DA, Naylor RJ. Cognitive enhancing actions of DuP 753 detected in a mouse habituation paradigm. NeuroReport. 1990;1:239–242. doi: 10.1097/00001756-199011000-00017. [DOI] [PubMed] [Google Scholar]
- 68.Raghavendra V, Chopra K, Kulkarni SK. Comparative studies on the memory-enhancing actions of captopril and losartan in mice using inhibitory shock avoidance paradigm. Neuropeptides. 2001;35:65–69. doi: 10.1054/npep.2000.0845. [DOI] [PubMed] [Google Scholar]
- 69.Bonini JS, Bevilaqua LR, Zinn CG, Kerr DS, Medina JH, Izquierdo I, Cammarota M. Angiotensin II disrupts inhibitory avoidance memory retrieval. Horm Behav. 2006;50:308–313. doi: 10.1016/j.yhbeh.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 70.Tinsley MR. The Role of Muscarinic and Nicotinic Cholinergic Neurotransmission in Aversive Conditioning: Comparing Pavlovian Fear Conditioning and Inhibitory Avoidance. Learning & Memory. 2004;11:35–42. doi: 10.1101/lm.70204. [DOI] [PubMed] [Google Scholar]
- 71.Likhtik E, Pelletier JG, Paz R, Paré D. Prefrontal control of the amygdala. Journal of Neuroscience. 2005;25:7429–7437. doi: 10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Paré D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gonzalez AD, Wang G, Waters EM, Gonzales KL, Speth RC, Van Kempen TA, et al. Distribution of angiotensin type 1a receptor-containing cells in the brains of bacterial artificial chromosome transgenic mice. Vol. 226. IBRO; 2012. pp. 489–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. European Journal of Pharmacology. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- 75.Davis M, Walker DL, Lee Y. Amygdala and bed nucleus of the stria terminalis: differential roles in fear and anxiety measured with the acoustic startle reflex. Philosophical Transactions of the Royal Society B: Biological Sciences. 1997;352:1675–1687. doi: 10.1098/rstb.1997.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wright JW, Harding JW. The angiotensin AT4 receptor subtype as a target for the treatment of memory dysfunction associated with Alzheimer’s disease. J Renin Angiotensin Aldosterone Syst. 2008;9:226–237. doi: 10.1177/1470320308099084. [DOI] [PubMed] [Google Scholar]
- 77.Szekeres M, Nadasy GL, Turu G, Supeki K, Szidonya L, Buday L, et al. Angiotensin II-Induced Expression of Brain-Derived Neurotrophic Factor in Human and Rat Adrenocortical Cells. Endocrinology. 2010;151:1695–1703. doi: 10.1210/en.2009-1060. [DOI] [PubMed] [Google Scholar]
- 78.Nostramo R, Tillinger A, Saavedra JM, Kumar A, Pandey V, Serova L, et al. Regulation of angiotensin II type 2 receptor gene expression in the adrenal medulla by acute and repeated immobilization stress. Journal of Endocrinology. 2012;215:291–301. doi: 10.1530/JOE-12-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.