Abstract
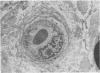
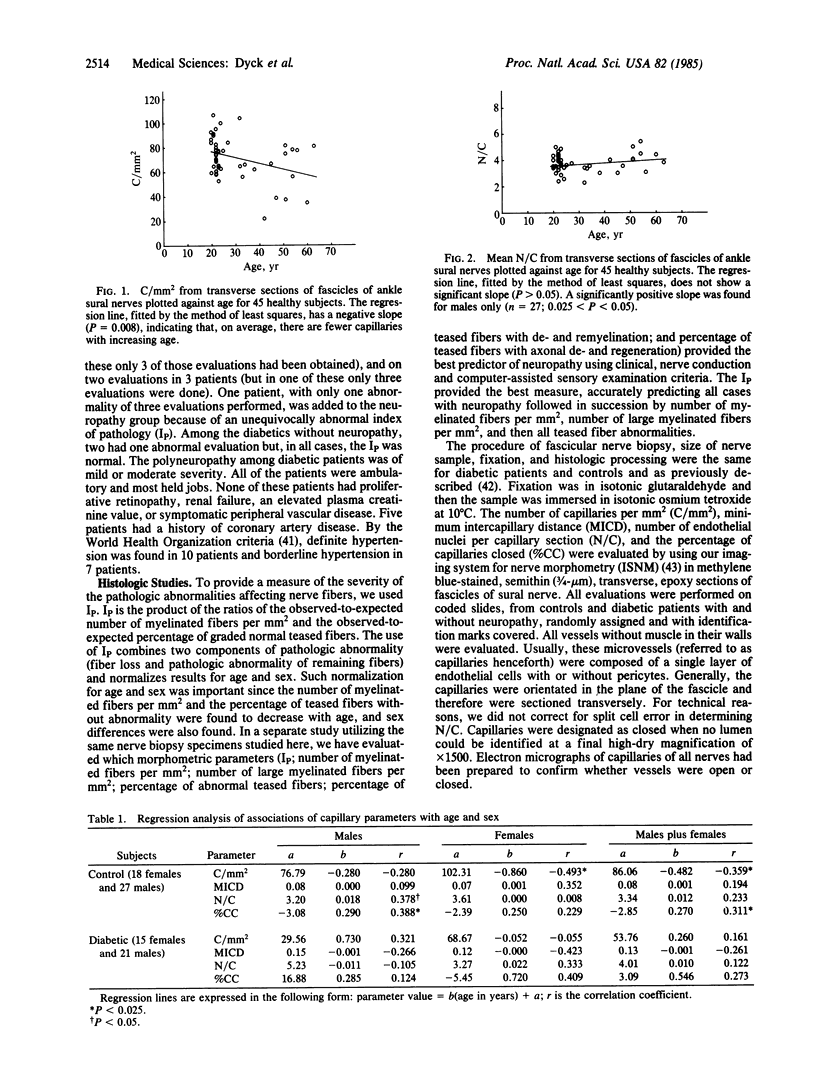
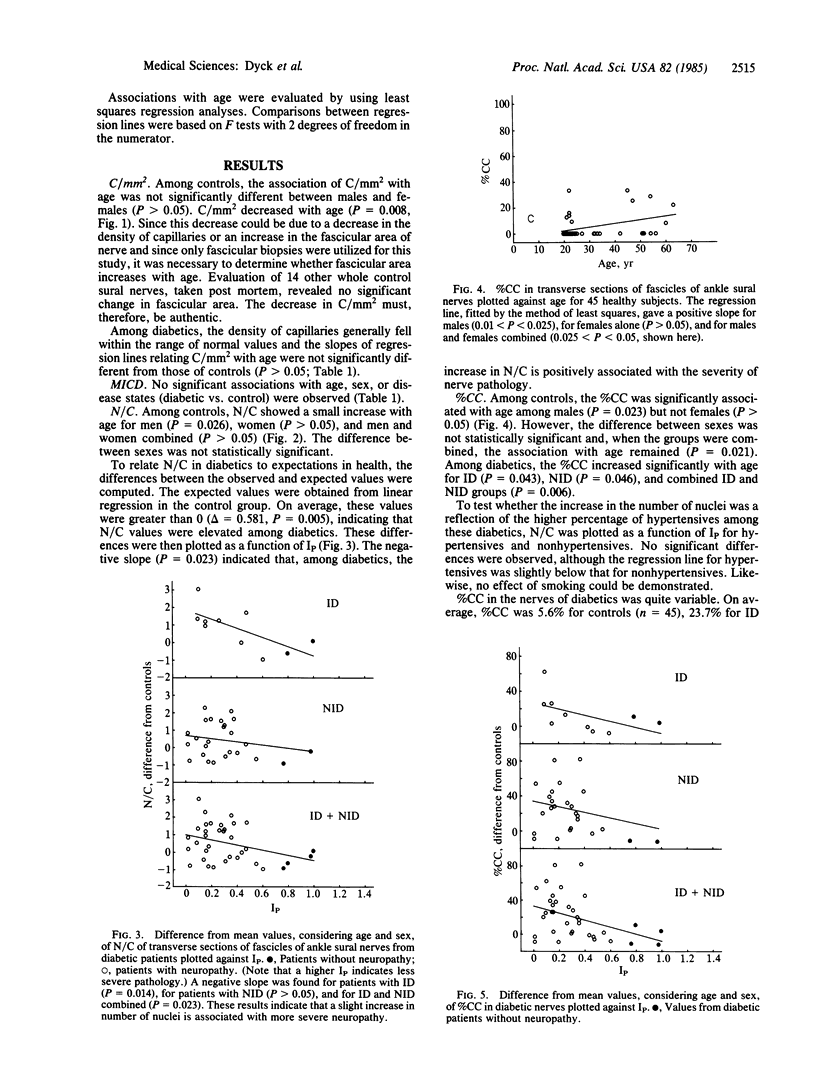
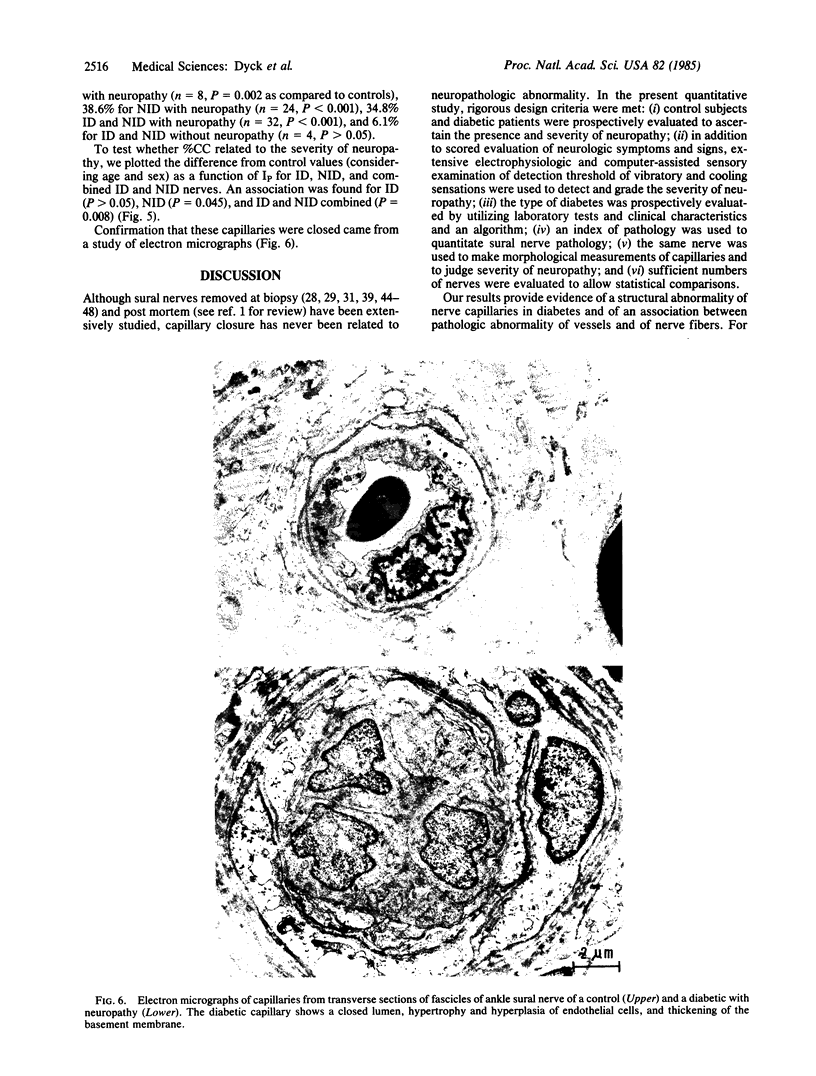
The number of capillaries per mm2, minimum intercapillary distance, number of endothelial nuclei per capillary section, and percentage of capillaries closed were evaluated in transverse sections of fascicles of 45 control and 36 diabetic sural nerves. All controls and patients were prospectively studied to ascertain their diabetic and neuropathic status. An index of pathology was introduced and it was found to provide a sensitive and reliable measurement of the presence and severity of neuropathy. The number of capillaries and minimum intercapillary distance of diabetic nerves were not significantly different from those of controls (P greater than 0.05). Diabetic nerves exhibited a small but statistically significant increase in the number of endothelial nuclei per capillary that was positively correlated with the severity of neuropathy. The most striking abnormality was the statistically significant increase in the percentage of capillaries closed in patients with neuropathy as compared to those without neuropathy and controls. Among diabetics, this percentage increased with the severity of neuropathy (P = 0.008). The two capillary abnormalities that have been demonstrated may play a role in the development of diabetic polyneuropathy.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHTON N. STUDIES OF THE RETINAL CAPILLARIES IN RELATION TO DIABETIC AND OTHER RETINOPATHIES. Br J Ophthalmol. 1963 Sep;47:521–538. doi: 10.1136/bjo.47.9.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpert J. S., Coffman J. D., Balodimos M. C., Koncz L., Soeldner J. S. Capillary permeability and blood flow in skeletal muscle of patients with diabetes mellitus and genetic prediabetes. N Engl J Med. 1972 Mar 2;286(9):454–460. doi: 10.1056/NEJM197203022860903. [DOI] [PubMed] [Google Scholar]
- Asbury A. K. Proximal diabetic neuropathy. Ann Neurol. 1977 Sep;2(3):179–180. doi: 10.1002/ana.410020302. [DOI] [PubMed] [Google Scholar]
- BERGSTRAND A., BUCHT H. Electron microscopic investigations on the glomerular lesions in diabetes mellitus (diabetic glomerulosclerosis). Lab Invest. 1957 Jul-Aug;6(4):293–300. [PubMed] [Google Scholar]
- BISCHOFF A. DIE DIABETISCHE NEUROPATHIE. Praxis. 1965 Jun 17;54:723–729. [PubMed] [Google Scholar]
- Ballin R. H., Thomas P. K. Hypertrophic changes in diabetic neuropathy. Acta Neuropathol. 1968 Sep 2;11(2):93–102. doi: 10.1007/BF00690213. [DOI] [PubMed] [Google Scholar]
- Behse F., Buchthal F., Carlsen F. Nerve biopsy and conduction studies in diabetic neuropathy. J Neurol Neurosurg Psychiatry. 1977 Nov;40(11):1072–1082. doi: 10.1136/jnnp.40.11.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brismar T., Sima A. A. Changes in nodal function in nerve fibres of the spontaneously diabetic BB-Wistar rat: potential clamp analysis. Acta Physiol Scand. 1981 Dec;113(4):499–506. doi: 10.1111/j.1748-1716.1981.tb06928.x. [DOI] [PubMed] [Google Scholar]
- Brown M. J., Iwamori M., Kishimoto Y., Ostroff S. M., Moser H. W., Asbury A. K. Endoneurial lipid composition of normal human sural nerve. Ann Neurol. 1979 Mar;5(3):239–244. doi: 10.1002/ana.410050305. [DOI] [PubMed] [Google Scholar]
- Brown M. J., Martin J. R., Asbury A. K. Painful diabetic neuropathy. A morphometric study. Arch Neurol. 1976 Mar;33(3):164–171. doi: 10.1001/archneur.1976.00500030020004. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Gabbay K. H., Gallop P. M. The glycosylation of hemoglobin: relevance to diabetes mellitus. Science. 1978 Apr 7;200(4337):21–27. doi: 10.1126/science.635569. [DOI] [PubMed] [Google Scholar]
- Connolly D. C., Elveback L. R., Oxman H. A. Coronary heart disease in residents of Rochester, Minnesota, 1950-1975. III. Effect of hypertension and its treatment on survival of patients with coronary artery disease. Mayo Clin Proc. 1983 Apr;58(4):249–254. [PubMed] [Google Scholar]
- DOWNIE A. W., NEWELL D. J. Sensory nerve conduction in patients with diabetes mellitus and controls. Neurology. 1961 Oct;11:876–882. doi: 10.1212/wnl.11.10.876. [DOI] [PubMed] [Google Scholar]
- Dyck P. J., Lambert E. H., Windebank A. J., Lais A. A., Sparks M. F., Karnes J., Sherman W. R., Hallcher L. M., Low P. A., Service F. J. Acute hyperosmolar hyperglycemia causes axonal shrinkage and reduced nerve conduction velocity. Exp Neurol. 1981 Mar;71(3):507–514. doi: 10.1016/0014-4886(81)90028-5. [DOI] [PubMed] [Google Scholar]
- Dyck P. J., Sherman W. R., Hallcher L. M., Service F. J., O'Brien P. C., Grina L. A., Palumbo P. J., Swanson C. J. Human diabetic endoneurial sorbitol, fructose, and myo-inositol related to sural nerve morphometry. Ann Neurol. 1980 Dec;8(6):590–596. doi: 10.1002/ana.410080608. [DOI] [PubMed] [Google Scholar]
- ELIASSON S. G., HUGHES A. H. Cholesterol and fatty acid synthesis in diabetic nerve and spinal cord. Neurology. 1960 Feb;10:143–147. doi: 10.1212/wnl.10.2.143. [DOI] [PubMed] [Google Scholar]
- FAGERBERG S. E. Diabetic neuropathy: a clinical and histological study on the significance of vascular affections. Acta Med Scand Suppl. 1959;345:1–97. [PubMed] [Google Scholar]
- FARQUHAR M. G., HOPPER J., Jr, MOON H. D. Diabetic glomerulosclerosis: electron and light microscopic studies. Am J Pathol. 1959 Jul-Aug;35(4):721–753. [PMC free article] [PubMed] [Google Scholar]
- Gabbay K. H., Merola L. O., Field R. A. Sorbitol pathway: presence in nerve and cord with substrate accumulation in diabetes. Science. 1966 Jan 14;151(3707):209–210. doi: 10.1126/science.151.3707.209. [DOI] [PubMed] [Google Scholar]
- Greene D. A., De Jesus P. V., Jr, Winegrad A. I. Effects of insulin and dietary myoinositol on impaired peripheral motor nerve conduction velocity in acute streptozotocin diabetes. J Clin Invest. 1975 Jun;55(6):1326–1336. doi: 10.1172/JCI108052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A. Impaired rat sciatic nerve sodium-potassium adenosine triphosphatase in acute streptozocin diabetes and its correction by dietary myo-inositol supplementation. J Clin Invest. 1983 Sep;72(3):1058–1063. doi: 10.1172/JCI111030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen J. Axonal dwindling in early experimental diabetes. II. A study of isolated nerve fibres. Diabetologia. 1976 Dec;12(6):547–553. doi: 10.1007/BF01220630. [DOI] [PubMed] [Google Scholar]
- Jakobsen J., Sidenius P. Decreased axonal transport of structural proteins in streptozotocin diabetic rats. J Clin Invest. 1980 Aug;66(2):292–297. doi: 10.1172/JCI109856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low P. A., Tuck R. R., Dyck P. J., Schmelzer J. D., Yao J. K. Prevention of some electrophysiologic and biochemical abnormalities with oxygen supplementation in experimental diabetic neuropathy. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6894–6898. doi: 10.1073/pnas.81.21.6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULDER D. W., LAMBERT E. H., BASTRON J. A., SPRAGUE R. G. The neuropathies associated with diabetes mellitus. A clinical and electromyographic study of 103 unselected diabetic patients. Neurology. 1961 Apr;11(4):275–284. doi: 10.1212/wnl.11.4.275. [DOI] [PubMed] [Google Scholar]
- P ARVING H. H., Rasmussen S. M. Transcapillary escape rate of albumin and plasma volume in short- and long-term juvenile diabetics. Scand J Clin Lab Invest. 1973 Aug;32(1):81–87. doi: 10.3109/00365517309082454. [DOI] [PubMed] [Google Scholar]
- PIRART J. DIABETIC NEUROPATHY: A METABOLIC OR A VASCULAR DISEASE? Diabetes. 1965 Jan;14:1–9. doi: 10.2337/diab.14.1.1. [DOI] [PubMed] [Google Scholar]
- Palumbo P. J., Elveback L. R., Chu C. P., Connolly D. C., Kurland L. T. Diabetes mellitus: incidence, prevalence, survivorship, and causes of death in Rochester, Minnesota, 1945-1970. Diabetes. 1976 Jul;25(7):566–573. doi: 10.2337/diab.25.7.566. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Asbury A. K. Ischemic mononeuropathy and mononeuropathy multiplex in diabetes mellitus. N Engl J Med. 1968 Jul 4;279(1):17–21. doi: 10.1056/NEJM196807042790104. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Sangalang V., Asbury A. K. Ischemic mononeuropathy multiplex associated with diabetes mellitus. Arch Neurol. 1968 May;18(5):487–499. doi: 10.1001/archneur.1968.00470350045004. [DOI] [PubMed] [Google Scholar]
- Siperstein M. D., Unger R. H., Madison L. L. Studies of muscle capillary basement membranes in normal subjects, diabetic, and prediabetic patients. J Clin Invest. 1968 Sep;47(9):1973–1999. doi: 10.1172/JCI105886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trap-Jensen J., Lassen N. A. Increased capillary diffusion capacity for small ions in skeletal muscle in long-term diabetics. Scand J Clin Lab Invest. 1968;21(2):116–122. doi: 10.3109/00365516809084273. [DOI] [PubMed] [Google Scholar]
- Tuck R. R., Schmelzer J. D., Low P. A. Endoneurial blood flow and oxygen tension in the sciatic nerves of rats with experimental diabetic neuropathy. Brain. 1984 Sep;107(Pt 3):935–950. doi: 10.1093/brain/107.3.935. [DOI] [PubMed] [Google Scholar]
- Ward J. D., Baker R. W., Davis B. H. Effect of blood sugar control on the accumulation of sorbitol and fructose in nervous tissues. Diabetes. 1972 Dec;21(12):1173–1178. doi: 10.2337/diab.21.12.1173. [DOI] [PubMed] [Google Scholar]
- Wieland O. H. Protein modification by non-enzymatic glucosylation: possible role in the development of diabetic complications. Mol Cell Endocrinol. 1983 Feb;29(2):125–131. doi: 10.1016/0303-7207(83)90207-1. [DOI] [PubMed] [Google Scholar]
- Zimmerman I. R., Karnes J. L., O'Brien P. C., Dyck P. J. Imaging system for nerve and fiber tract morphometry: components, approaches, performance, and results. J Neuropathol Exp Neurol. 1980 Jul;39(4):409–419. doi: 10.1097/00005072-198007000-00002. [DOI] [PubMed] [Google Scholar]