Abstract
Chlorophenols (CPs) and their derivatives are persistent environmental pollutants which are used in the manufacture of dyes, drugs, pesticides and other industrial products. CPs, which include monochlorophenols, polychlorophenols, chloronitrophenols, chloroaminophenols and chloromethylphenols, are highly toxic to living beings due to their carcinogenic, mutagenic and cytotoxic properties. Several physico-chemical and biological methods have been used for removal of CPs from the environment. Bacterial degradation has been considered a cost-effective and eco-friendly method of removing CPs from the environment. Several bacteria that use CPs as their sole carbon and energy sources have been isolated and characterized. Additionally, the metabolic pathways for degradation of CPs have been studied in bacteria and the genes and enzymes involved in the degradation of various CPs have been identified and characterized. This review describes the biochemical and genetic basis of the degradation of CPs and their derivatives.
Keywords: Chlorophenol, Environmental pollutants, Bacterial degradation, Biodegradation
Introduction
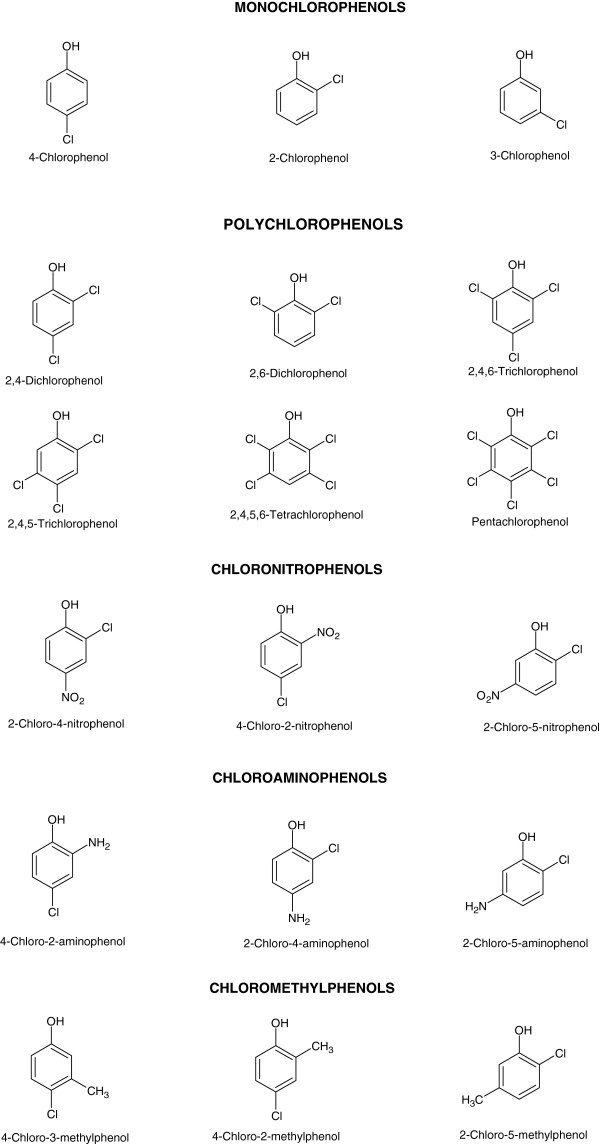
Chlorophenols (CPs) are aromatic ring structures containing at least one chlorine atom (-Cl) and one hydroxyl (-OH) group at the benzene rings. Five groups of CPs have been recognized on the basis of their chemical structures, monochlorophenols (MCPs), polychlorophenols (poly-CPs), chloronitrophenols (CNPs), chloroaminophenols (CAPs) and chloromethylphenols (CMPs) (Figure 1). These compounds are widely used (i) as mothproofing agents, miticides, germicides, algicides, fungicides and wood preservatives [1], as well as (ii) for the synthesis of dyes and drugs [2].
Figure 1.
Chemical structures of chlorophenols and their derivatives.
CPs have been introduced into the environment via anthropogenic activities [3]. The major sources of contamination are industrial wastes, pesticides, herbicides, and complex chlorinated hydrocarbons [3]. People may be exposed to CPs by eating or drinking substances that contain them or through skin contact [4]. CPs and their derivatives are highly toxic to living beings due to their carcinogenic, mutagenic and cytotoxic properties [5]. The World Health Organization and the International Agency for Research on Cancers have characterized several poly-CPs as potential human carcinogens [5]. Similarly, the United States Environmental Protection Agency has included several CPs in its list of priority pollutants.
Several conventional methods such as adsorption, ion exchange, liquid–liquid extraction, and chemical oxidation and advanced oxidation processes have been used for the removal of CPs from wastewater [3,6]. These methods are expensive and not eco-friendly due to the formation of hazardous compounds as by-products [3]. Conversely, bioremediation is an effective and eco-friendly method of removing CPs from the environment. Biodegradation of CPs has gained attention due to the complete mineralization of CPs by microorganisms in the environment.
Several reviews dealing with the degradation and toxicity of CPs and their derivatives have been published [3,5,7,8]; however, these reviews were focused on the biodegradation/toxicity of MCPs or poly-CPs or both. The present review describes the biochemical and genetic basis of bacterial degradation of CPs and their derivatives including MCPs, poly-CPs, CAPs, CNPs and CMPs. Both aerobic and anaerobic bacterial degradation of CPs are discussed.
Bacterial degradation of CPs
Aerobic degradation of CPs and their derivatives have been extensively investigated in bacteria, and many bacteria with the ability to utilize CPs as their sole carbon and energy sources have been isolated [8]. One of the following mechanisms may be involved in the bacterial degradation of CPs and their derivatives: (i) monooxygenases may catalyze hydroxylation at the ortho-positions of the chlorophenolic rings, which results in the formation of chlorocatechols that may be degraded further via ortho-[9] or meta-cleavage [10,11] or hydroxylated prior to ring cleavage [12]; (ii) monooxygenases may catalyze the hydroxylation at para-positions of the chlorophenolic rings, resulting in the formation of chlorohydroquinones that may be degraded further via hydroxylation [12] or dehalogenation [13] prior to ring cleavage; (iii) the degradation of CNPs may be initiated via hydroxylation [14], reductive dehalogenation [15] or reduction of the nitro group [16], (iv) The degradation of ACPs may be initiated with the removal of ammonium ions by the enzyme deaminase followed by the ring cleavage [17] or the dehalogenation [18]. In this section, we describe the bacterial degradation pathways for MCPs, poly-CPs, CNPs, CAPs and CMPs.
Bacterial degradation of MCPs
MCPs, which are the simplest form of CPs, contain one chlorine atom at the phenolic rings. MCPs include 2-chlorophenol (2CP), 3-chlorophenol (3CP), 4-chlorophenol (4CP), 3-chlorocatechol (3CC), 2-chlorocatechol (2CC) and 4-chlorocatechol (4CC). Chlorocatechols (CCs) were detected as intermediate products of bacterial degradation of MCPs, chlorobenzoates, mono-chlorobiphenyls, 4-chlorosalicylate and 5-chlorosalicylate [19-23]. In this section, we have described the bacterial degradation of 4CP, 2CP and 3CP.
Bacterial degradation of 4CP
Many bacteria that utilize 4CP as their carbon and energy sources have been isolated, including Pseudomonas knackmussii B-13 (previously known as Pseudomonas sp. B-13) [24,25], Ralstonia pickettii LD1 (previously known as Pseudomonas pickettii LD1) [26], Rhodococcus opacus 1G [27], Alcaligenes sp. A7–2 [28], Alcaligenes xylosoxidans JH1 [29], Arthrobacter ureafaciens CPR706 [30], Arthrobacter chlorophenolicus A6 [31], and Herbaspirillum chlorophenolicum CPW301 (previously known as Comamonas testosteroni CPW301) [32,33]. The bacterial degradation of 4CP occurs via either the CC pathway [34] or the hydroquinone (HQ) pathway [35]. In the CC pathway, 4CP is first converted to 4CC by a 4CP-2-monooxygenase (EC = 1.14.13.-). Further degradation of 4CC then proceeds via the modified ortho-ring cleavage or meta-ring cleavage pathway [34]. In the modified ortho-cleavage pathway, 4CC is cleaved into 3-chloromuconate by a catechol-1,2-dioxygenase (EC 1.13.11.1) [7]. In the second step, 3-chloromuconate is transformed to cis-dienelactone through the release of chloride ion by a chloromuconate cycloisomerase (EC 5.5.1.7) [Figure 2a]. In the next step, cis-dienelactone is converted to maleylacetate by a dienelactone hydrolase (EC 3.1.1.45) [7]. Maleylacetate is then reduced to 3-oxoadipate by a maleylacetate reductase (EC = 1.3.1.32). In the meta-cleavage pathway, 4CC may be cleaved into a toxic compound, 5-chloro-2-hydoxymuconic semialdehyde (5C2HMS) by a catechol-2, 3-dioxygenase (EC = 1.13.11.2) [7,36]. In several cases, 5C2HMS has been identified as a dead end product in the degradation pathway of 4CP [7,36]. However, the complete degradation of 5C2HMS was observed in the 4CP degradation pathway in Comamonas testosteroni JH5 [10]. In strain JH4, 5C2HMS was converted to 5-chloro-2-hydroxypenta-2,4-dienoic acid, which was further degraded via intermediates of the TCA cycle [Figure 2b].
Figure 2.
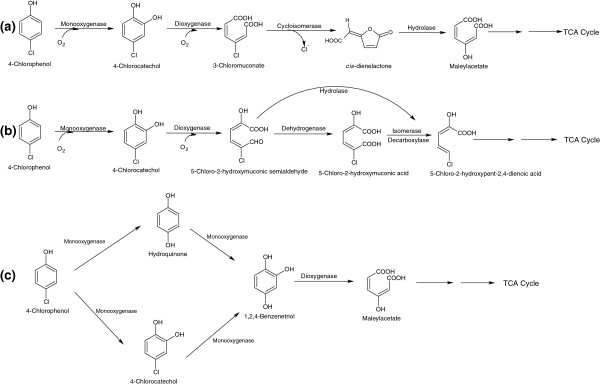
Bacterial degradation pathways for 4-chlorophenol. (a) 4-Chlorophenol degradation via modified ortho-cleavage, (b) 4-chlorophenol degradation via the meta-cleavage, (c) two pathways of degradation of 4-chlorophenol [4-Chlorocatechol-Benzenetriol pathway (lower) and Hydroquinone pathway (upper)].
In addition to the modified-ortho or meta-ring cleavage pathway of 4CC, there is another pathway for degradation of 4CC, which is here designated as the 4CC-Benzenetriol (4CC-BT) pathway. In this pathway, 4CC is first hydroxylated to 1,2,4-benzenetriol (BT) through the release of chloride ion [12]. BT is then further degraded via ring cleavage and the formation of maleylacetate [12]. The 4CC-BT pathway was observed in the degradation of 4CP in A. chlorophenolicus A6 [12].
The 4CP degradation can also occur through the HQ pathway [12,37]. The first step of the HQ pathway is the formation of HQ through the release of chloride ion from 4CP by a 4CP-4-monooxygenase [Figure 2c]. In the next step, HQ is converted to BT, which is then cleaved into maleylacetate by a BT-dioxygenase [12]. A few bacterial strains degrade 4CP via two pathways. For example, A. chlorophenolicus A6 degrades 4CP via the HQ pathway as well as the 4CC-BT pathway [12].
Bacterial degradation of 2CP and 3CP
Several 4CP-mineralizing bacteria including Ralstonia pickettii LD1 [26], Rhodococcus opacus 1G [27] and Alcaligenes xylosoxidans JH1 [29] also utilize 2CP and 3CP as their sole carbon and energy sources. Other 2CP-mineralizing bacteria include Alcaligenes sp. A7–2 [28] and Streptomyces rochei 303 [38]. The bacterial degradation of 2CP occurs via the formation of 3CC, which is further degraded via the modified ortho-cleavage pathway or the meta-cleavage pathway [7,39]. In the modified ortho-cleavage pathway, 3CC is cleaved into 2-chloro-cis,cis-muconate by a catchol-1,2-dioxygenase [7]. In the next step, a chloromuconate cycloisomerase catalyzes the conversion of 2-chloro-cis,cis-muconate to trans-dienelactone [7], which degrades further via formation of maleylacetate by dienelactone hydrolase [Figure 3a].
Figure 3.
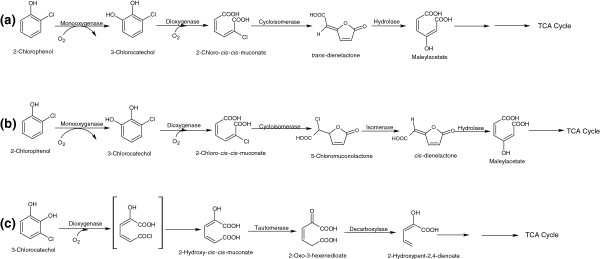
Bacterial degradation pathways for 2-chlorophenol via 3-chlorocatechol. (a) Modified ortho cleavage pathway, (b) new modified ortho cleavage pathway, and (c)meta-cleavage pathway of 3-chlorocatechol.
A new modified ortho-cleavage pathway of the 3CC was reported in Rhodococcus opacus 1CP that degraded 2CP via 3CC [40,41]. The key enzymes of this pathway are chlorocatechol-1,2-dioxygenase, chloromuconate cycloisomerase (CMCI), chloromuconolactone isomerase (CMLI), and dienelactone hydrolase (DELH) [40]. Specifically, chlorocatechol-1,2-dioxygenase catalyzes the conversion of 3CC to 2-chloromuconate, while CMCI converts 2-chloromuconate into 5-chloromuconolactone, CMLI converts 5-chloromuconolactone into cis-dienelactone and DELH converts cis-dienelactone into maleylacetate, which is further degraded via the TCA cycle [Figure 3b].
In the meta-cleavage pathway, there are two possibilities for 3CC degradation: (i) formation of a dead end product [39,42] and (ii) complete mineralization of 3CC [43]. In the first case, a suicide compound, 5-chloroformyl-2-hydroxypenta-2,4-dienoic acid, is formed due to the meta-cleavage of 3CC, which inactivates catechol-2,3-dioxygenase (EC = 1.13.11.2), resulting in 3CC accumulation in the media. In the second case, bacteria may utilize 3CC completely. This type of pathway has been observed in the degradation of chloro-aromatics by Pseudomonas putida GJ31 [43]. In strain GJ31, 3CC was cleaved into 2-hydroxy-cis-cis-muconate by a catechol-2,3-dioxygenase that was further degraded completely [43] [Figure 3c]. This pathway has been demonstrated in several other strains that are able to metabolize 3CC, including Pseudomonas sp. MG61, Pseudomonas fluorescens SK1 and Pseudomonas veronii 16-6A [44].
The degradation of 3CP occurred either via the formation of 3CC or via the formation of 4CC that may be further degraded via the modified ortho-cleavage pathway or the meta-cleavage pathway [7,39].
Bacterial degradation of poly-CPs
poly-CPs such as dichlorophenols (DCPs), trichlorophenols (TCPs), tetrachlorophenols (TeCPs) and pentachlorophenol (PCP) are more recalcitrant to bacterial degradation than MCPs due to the presence of the two or more chlorine atoms at the phenolic rings. Here, we have described the degradation of 2,4-dichlorophenol (2,4-DCP), 2,4,6-trichlorophenol (2,4,6-TCP), 2,4,5-trichlorophenol (2,4,5-TCP) and PCP.
Bacterial degradation of 2,4-DCP
2,4-DCP is the first intermediate in the degradation pathway of a herbicide, 2,4-dichlorophenoxyacetic acid (2,4-D). Several bacteria that utilize 2,4-DCP as their sole source of carbon and energy have been isolated, including Pseudomonas sp. DP-4 [45], Rhodococcus opacus 1G [27], Rhodococcus erythropolis[46], and Pseudomonas sp. NCIB9340 [47]. Wang et al. [48] reported the removal of 2,4-DCP by the suspended and immobilized cells of Bacillus insolitus. They demonstrated that the immobilized cells showed faster degradation of lower concentrations of 2,4-DCP (10–50 mg/l), whereas the high concentrations (50–200 mg/ml) were removed by immobilized and suspended cells at the same rate [48]. The bacterial degradation of 2,4-D is initiated by the formation of 2,4-DCP by the enzyme, 2,4-dichlorophenoxyacetate-α-ketoglutarate dioxygenase (EC = 1.14.11.-) [49]. 2,4-DCP is further degraded via the formation of 3,5-dichlorocatechol by a 2,4-DCP-hydroxylase [EC = 1.14.13.20] [50]. In the third step, 3,5-dichlorocatechol is ortho-cleaved to 2,4-dichloromuconic acid by 3,5-dichlorocatechol dioxygenase (EC = 1.13.11.-) (Figure 4a). In the next step, 2,4-dichloromuconic acid isomerase (EC = 5.2.1.10) catalyzes the conversion of 2,4-dichloromuconic acid to trans-2-chlorodienelactone via the removal of one chloro group, which is further converted to cis-2-chlorodienelactone by an isomerase that is subsequently degraded via formation of chloromaleylacetate by a hydroxylase [50]. The chloromaleylacetate is further degraded to maleylacetate by removal of the chloro group and then to 3-oxodipic acid by a maleylacetate reductase (EC = 1.3.1.32). Koh et al. [51] reported that o-cresol grown cells of Cupriavidus necator JMP222 (a derivative of C. necator JMP134 that had lost plasmid pJP4) degraded 2,4-DCP via a distal meta-cleavage pathway. In that process, 2,4-DCP is first oxidized to 3,5-dichlorocatechol, which is subsequently degraded via a distal meta-cleavage pathway through the formation of 2-hydroxy-3,5-dichloro-6-oxo-hexa-2,4-dienoic acid (Figure 4b).
Figure 4.
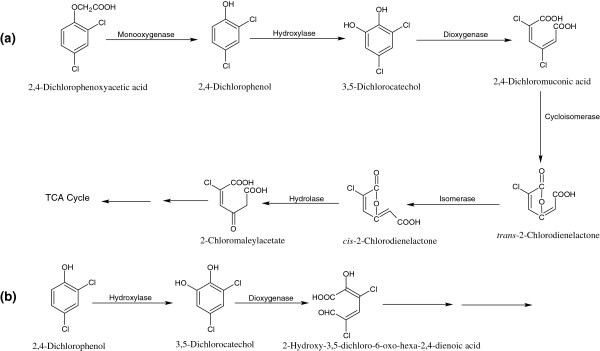
Bacterial degradation pathways for 2,4-dichlorophenol via ortho -cleavage (a) and the distal meta -cleavage (b).
Bacterial degradation of 2,4,6-TCP
Many bacteria that utilize 2,4,6-TCP as their sole carbon and energy source have been isolated and characterized including Azotobacter sp. Gp1 [52], Ralstonia pickettii[53], Cupriavidus necator[54,55], Nocardioides sp. K44 [56] and Novosphingobium lentum MT1 [57]. Bacterial degradation of 2,4,6-TCP was well-characterized in Cupriavidus necator JMP134 [54,55]. In the initial step of the TCP degradation, a reduced flavin adenine dinucleotide (FADH2)-utilizing monooxygenase catalyzes the conversion of 2,4,6-TCP to 6-chlorohydroxyquinol via the formation of 2,6-dichlorohydroquinone [58]. 6-Chlorohydroxyquinol is then further cleaved to 2-chloromaleylacetate by 6-chlorohydroxyquinol-1,2-dioxygenase, which is subsequently converted to maleylacetate by removal of the chloro group (Figure 5a).
Figure 5.
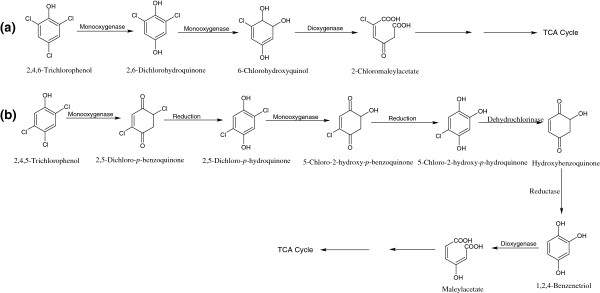
Bacterial degradation pathway for 2,4,6-trichlorophenol (a), and 2,4,5-trichlorophenol (b).
Bacterial degradation of 2,4,5-TCP
Burkholderia phenoliruptrix AC1100 (previously known as B. cepacia) uses 2,4,5-TCP as the sole source of carbon and energy [59,60]. The first step in degradation of 2,4,5-TCP involves conversion of 2,4,5-TCP to 2,5-dichloro-p-benzoquinone (DiCBQ) by FADH2-dependent-2,4,5-TCP-4-monooxygenase (TftD) [EC = 1.14.14.-] [60,61]. DiCBQ is then reduced to 2,5-dichloro-p-hydroquinone (2,5-DiCHQ) by NADH. In the next step, 2,5-DiCHQ is oxidized to 5-chloro-2-hydroxy-p-benzoquinone by TftD, which is then further reduced to 5-chloro-2-hydroxy-p-hydroquinone (CHHQ) [60,61]. Another enzyme, flavin reductase TftC, supplies FADH2 as a co-substrate to TftD [60,61]. In the next step, dehydrochlorinase TftG catalyzes the conversion of CHHQ to hydroxybenzoquinone, which is reduced to BT by hydroxylbenzoquinone reductase (EC = 1.6.5.7) [62]. BT is subsequently converted to maleylacetate by BT-1,2-dioxygenase (EC = 1.13.11.37) (Figure 5b).
Bacterial degradation of PCP
PCP degradation is initiated by the formation of tetrachlorohydroquinone (TeCHQ) due to hydroxylation at the para-position by either PCP-4-monooxygenase (EC = 1.14.13.50) [63-65] or cytochrome P-450 type enzyme [66,67] [Figure 6]. In Sphingomonas chlorophenolicum L-1 (previously known as Sphingomonas chlorophenolicum ATCC 39723), PCP-4-monooxygenase (PcpA) catalyzes the conversion of PCP to TeCHQ via the removal of chloride ions [63-65]. In the next step, TeCHQ is sequentially dehalogenated to 2,6-dichloro-1,4-hydroquinone (2,6-DCHQ) by a TeCHQ-reductive dehalogenase (EC = 1.8.99.-). The further degradation of 2,6-DCHQ occurs via ring cleavage by the 2,6-DCHQ-1,2-dioxygenase, leading to formation of 2-chloromaleylacetate that is further degraded via the TCA cycle [63-65]. In Mycobacterium chlorophenolicum PCP-1 and Mycobacterium fortuitum CG-2 (formerly Rhodoccocus strains), PCP is hydroxylated to TeCHQ by a membrane bound cytochrome P-450 type enzyme [66,67]. Subsequently, TeCHQ undergoes hydrolytic dehalogenation followed by reductive dehalogenation to form dichloro-1,2,4-trihydroxybenzene, which produces BT after two successive reductive dehalogenation [68].
Figure 6.
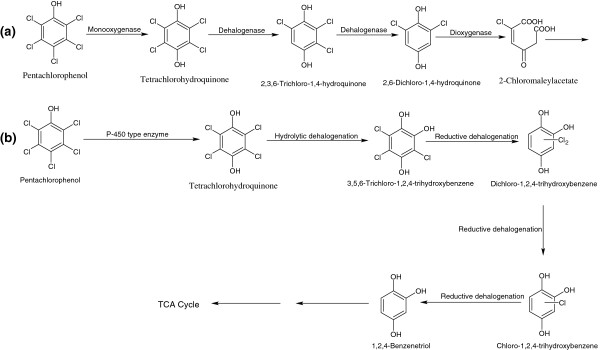
Bacterial degradation pathways for pentachlorophenol in Sphingomonas chlorophenolicum L-1 (a), and Mycobacterium strains (b).
Bacterial degradation of CNPs
CNPs are nitro derivatives of MCPs. Examples include 2-chloro-4-nitropheol (2C4NP), 4-chloro-2-nitrophnol (4C2NP), 4-chloro-3-nitrophenol (4C3NP), 2-chloro-5-nitrophenol (2C5NP) and 2-chloro-3-nitrophenol (2C3NP). In this section, we have summarized the bacterial degradation of various CNPs.
The degradation of 2C4NP has been studied in Burkholderia sp. SJ98 [15], Burkholderia sp. RKJ 800 [14], Arthrobacter nitrophenolicus SJCon [69] and Rhodococcus imtechensis RKJ300 [70]. Pandey et al. [15] proposed a degradation pathway of 2C4NP in Burkholderia sp. SJ98 that utilized 2C4NP as the sole carbon, nitrogen and energy sources. The first step of the 2C4NP degradation in strain SJ98 involves the reductive dehalogenation of 2C4NP by 2C4NP-dehalogenase that leads to the formation of 4-nitrophenol (4NP) [Figure 7a]. In the next step, 4NP is converted to 4-nitrocatechol and then to BT, which is further cleaved into maleylacetate by BT-1,2-dioxygenase. Maleylacetate is further degraded via the β-ketoadipic acid cycle [15]. Another pathway of the 2C4NP degradation was investigated in Rhodococcus imtechensis RKJ 300 [70] and Bukholderia sp. RKJ 800 [14]. In this pathway, 2C4NP is first transformed to chlorohydroquinone (CHQ) by 2C4NP-monooxygenase. CHQ is then dehalogenated to HQ by CHQ-dehalogenase [14]. In the next step, HQ is cleaved to γ-hydroxymuconic semialdehyde by HQ-1,2-dioxygenase (EC = 1.13.11.66) [Figure 7b]. Arora and Jain [69] reported a new degradation pathway of 2C4NP in Arthrobacter nitrophenolius sp. SJCon. In strain SJCon, 2C4NP is first converted to CHQ and then further cleaved to maleylacetate by CHQ-dioxygenase [Figure 7c].
Figure 7.
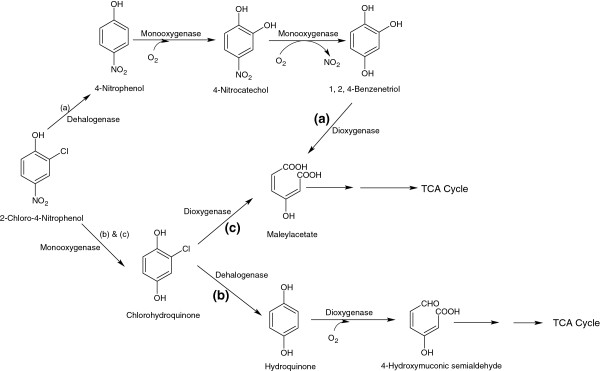
Bacterial degradation pathways for 2-chloro-4-nitrophenol in (a) Burkholderia sp. SJ98, (b) Burkholderia sp. RKJ 800 and Rhodococcus imtechensis RKJ300, and (c) Arthrobacter nitrophenolicus SJCon.
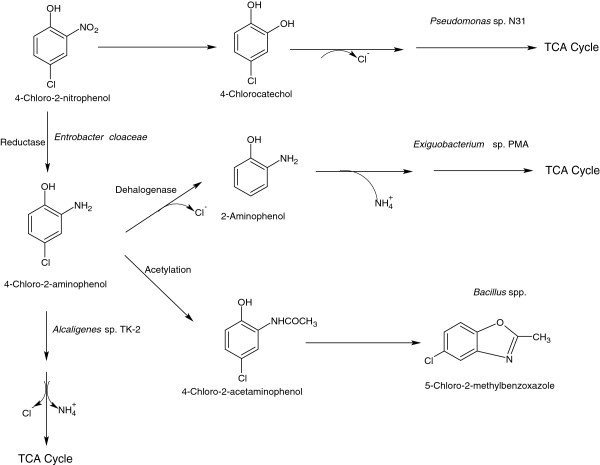
The first report of the 4C2NP degradation was documented in 1988 with construction of a genetically engineered bacterium, Pseudomonas sp. N31, which utilizes 4C2NP as a sole carbon, nitrogen and energy source [71]. The constructed strain degrades 4C2NP via the formation of 4CC and the release of chloride and nitrite ions [Figure 8]. Beunink and Rehm [72] reported 4C2NP degradation via the formation of 4-chloro-2-aminophenol (4C2AP) by a co-culture of Enterobacter cloaceae and Alcaligenes sp. TK-2 [Figure 8]. A detoxification mechanism for 4C2NP transformation has been proposed for two Bacillus species [73,74]. In this mechanism, detoxification is initiated by the formation of 4C2AP, which acetylates into 4-chloro-2-acetaminophenol (4C2AAP). 4C2AAP is then converted to a non-toxic compound, 5-chloro-2-methylbenzoxazole [Figure 8]. Another investigation of complete mineralization of 4C2NP was published following the isolation of a 4C2NP-mineralization bacterium, Exiguobacterium sp. PMA [16]. This strain initiates 4C2NP degradation by the formation of 4C2AP via a reduction mechanism, which is further dehalogenated into 2-aminophenol (2AP) through the release of chloride ions [16]. The further degradation of 2AP proceeds via ring cleavage and the removal of ammonium ions [Figure 8].
Figure 8.
Bacterial degradation pathways for 4-chloro-2-nitrophenol.
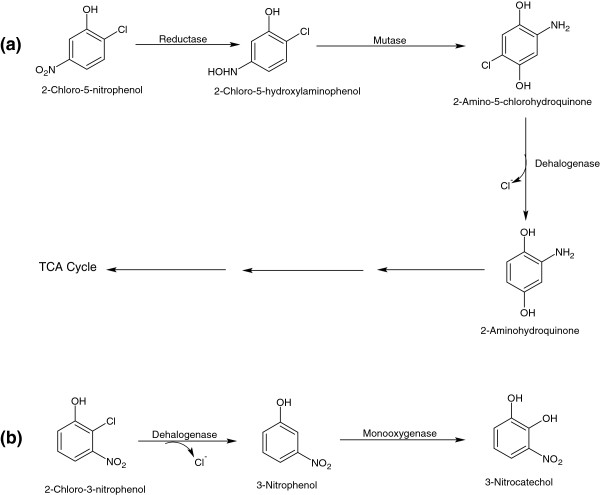
The metabolic pathway of 2C5NP has also been studied in C. necator JMP134, which utilizes 2C5NP as its sole carbon, nitrogen and energy source [75]. The first step of 2C5NP degradation involves the reduction of 2C5NP to 2-chloro-5-hydroxylaminophenol (2C5HAP) by 3NP-reductase [75]. In the second step, 2C5HAP undergoes Bamberger rearrangement to form 2-amino-5-chlorohydroquinone (2A5CHQ) by mutase [Figure 9a]. In the next step, 2A5CHQ is reductively dehalogenated to 2-aminohydroquinone, which is further degraded by ring cleavage and ammonia release [75].
Figure 9.
Bacterial degradation pathway for 2-chloro-5-nitrophenol (a), and 2-chloro-3-nitrophenol (b).
Pandey et al. [76] reported the biotransformation of 2C3NP to 3-nitrocatechol (3NC) in Burkholderia sp. SJ98. Initially, 2C3NP is reductively dehalogenated to 3NP, which is further hydroxylated to 3NC [Figure 9b].
Bacterial degradation of CAPs and CMPs
CAPs are amino derivatives of MCPs that are used in the manufacture of dyes. Examples include 4-chloro-2-aminophenol (4C2AP) and 2-chloro-4-aminophenol (2C4AP). Bacterial degradation of 4C2AP was studied in the Gram negative bacterium, Burkholderia sp. RKJ 800, which utilizes 4C2AP as a sole carbon and energy source [17]. The degradation of 4C2AP is initiated by the release of ammonium ion and the formation of 4CC by a deaminase. In the next step, 4CC is cleaved to cis, cis-chloromuconic acid by 4CC-1,2-dioxygenase (Figure 10a). Conversely, the bacterial degradation of 2C4AP was studied in a Gram positive bacterium, Arthrobacter sp. SPG, which utilized 2C4AP as its sole source of carbon and energy [18]. The first step of 2C4AP degradation involves removal of the ammonium ion by deaminase, which leads to formation of CHQ that is then dehalogenated to HQ by a CHQ-dehalogenase (Figure 10b). In the next step, HQ is cleaved to γ-hydroxymuconic semialdehyde by HQ-1,2-dioxygenase (EC = 1.13.11.66) [18].
Figure 10.
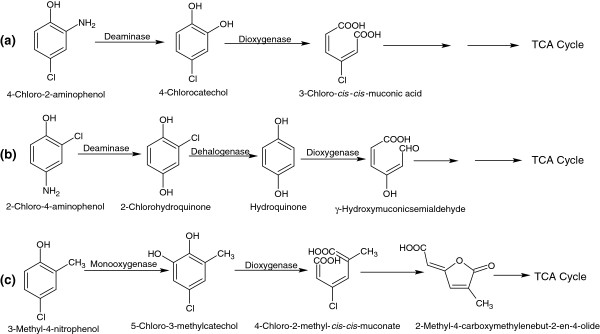
Bacterial degradation pathways for 4-chloro-2-aminophenol (a), 2-chloro-4-aminophenol (b), and 4-chloro-2-methylphenol (c).
CMPs are methyl derivatives of CPs used for the manufacture of herbicides such as 4-chloro-3-methylphenol and 4-chloro-2-methylphenol. Lechner et al. [77] investigated the degradation pathway of 4C2MP in a Gram negative strain, S-1. 4C2MP is first converted to 5-chloro-3-methylcatechol, which is ortho-cleaved into 4-chloro-2-methyl-cis-cis-muconate and then further degraded via the formation of 2-methyl-4-carboxymethylenebut-2-en-4-olide (Figure 10c).
Anaerobic degradation of CPs
Anaerobic degradation of CPs is well studied in bacteria or various enrichment cultures derived from sediments collected from a variety of the sources [8]. Anaerobic degradation of various CPs proceeds via reductive dehalogenation in which chlorine atoms are replaced by hydrogen atoms [8]. In fact, the reductive dehalogenation is a crucial step for the anaerobic biodegradation of CPs especially for poly-CPs. Several poly-CPs are recalcitrant towards aerobic bacterial attack and can be reductively dehalogenated into lesser chlorinated phenols that further mineralized easily. PCP may be reductively dehalogenated to 2,3,4,5-tetrachlorophenol (2,3,4,5-TeCP), then to 3,4,5-trichlorophenol (3,4,5-TCP), then to 3,5-dichlorophenol, then to 3CP and finally to phenol which further degraded to CH4 and CO2 by anaerobic bacteria [78,79] (Figure 11a). The combination of phenol-dehalogenating and phenol-degrading cultures was used for complete mineralization of PCP under anaerobic conditions [80]. In this process, a phenol-dehalogenating culture dehalogenates PCP to phenol under anaerobic conditions. The phenol is then further degraded by phenol-degrading culture under iron reducing or sulfate reducing conditions [80]. Becker et al. [81] studied two biotransformation pathways for 2CP in the anaerobic sediment slurry reactors. In the first pathway, 2CP is reductively dehalogenated to phenol, then carboxylated to 4-hydroxybenzoate and finally dehydroxylated to benzoate (Figure 11b). In the second pathway, 2CP is para-carboxylated to 3-chloro-4-hydroxybenzoate, which is further dehydroxylated to 3-chlorobenzoate.The mineralization of 14C-radiolabeled 4CP, 2CP, and 2,4-DCP to 14CH4 and 14CO2 was studied in acclimated sludge [82]. In this process, 4CP is mineralized via phenol, 4-hydroxybenzoate and benzoate, while 2,4-DCP is mineralized via 4CP, phenol, 4-hydroxybenzoate and benzoate [82] (Figure 11c).
Figure 11.
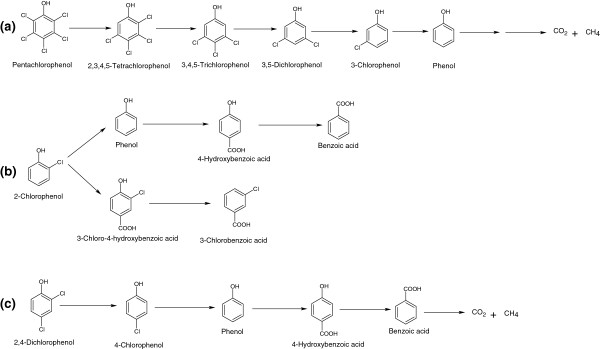
Anaerobic degradation of CPs and poly-CPs. (a) Anaerobic dehalogenation and degradation of pentachlorophenol, (b) Two biotransformation pathways for 2-chlorophenol, and (c) Anaerobic mineralization for 2,4-dichlorophenol.
Methanogenic, sulfate reducing, iron-reducing and denitrifying conditions favor anaerobic degradation and reductive dechlorination of CPs. Anaerobic degradation and dechlorination of CPs have been extensively studied under methanogenic conditions [78,83-87]. A PCP acclimated methanogenic consortium reductively dechlorinated PCP and TeCPs [83]. In this process, PCP is first dechlorinated to 2,3,4,5-TeCP, 2,3,4,6-tetrachlorophenol and 2,3,5,6-tetrachlorophenol. These TeCPs are then further dehalogenated to TCPs, DCPs and MCPs. Another methanogenic enrichment culture derived from sewage sludge transformed 2,4,6-TCP, 2,4,5-TCP and 3,4,5-TCP [84]. In this process, 2,4,6-TCP is reductively dechlorinated to 4CP via 2,4-DCP, whereas 2,4,5-TCP and 3,4,5-TCP are dehalogenated to 3CP via 3,4-DCP [84]. The reductive dechlorination of 12 isomers of CPs and poly-CPs including MCPs, DCPs, TCPs, TeCPs and PCP was also investigated using methanogenic cultures [85]. Takeuchi et al. [86] reported dehalogenation and transformation of 19 isomers of CPs under methanogenic conditions. A fresh water sediment mineralized 2,4-DCP into CO2 and methane via 4CP, phenol and benzoate [87].
The mineralization of CPs has been found to be coupled with sulfate reduction. Haggblom and Young [88] developed a CPs-mineralizing sulfate reducing consortia from estuarine sediment that was maintained on 2CP, 3CP or 4CP as the only source of carbon and energy for several years [89]. Their experiments utilizing a 4CP-utilizing consortium revealed that mineralization of 4CP into CO2 was coupled to sulfate reduction, and that 4CP depletion did not occur in the absence of sulfate. In this reaction, sulfate, thiosulfate or sulfite were used as electron acceptors [89]. The coupling of sulfate reduction with mineralization of CPs was also observed in degradation of 2CP or 4CP by sulfate reducing enrichment cultures derived from Hudson River sediment [90].
Under denitrifying conditions, the 2CP degradation was studied in enrichment cultures derived from activated sludge samples [91]. The presence of nitrate was essential as electron acceptors for the mineralization of 2CP into CO2[91]. Sanford and Tiedje [92] studied dechlorination and subsequent degradation of MCPs and DCPs in anaerobic microcosms supplemented with 1 mM or 5 mM nitrate.
CPs degradation is associated with reduction of Fe3+ to Fe2+. An anaerobic enrichment culture derived from Hudson River sediments mineralized 2CP, 3CP and 4CP with concomitant reduction of Fe3+ to Fe2+[93]. Several factors may affect dechlorination of CPs and reduction of Fe. For example, a low amount of nitrate enhances reductive dechlorination of PCP and Fe(III) reduction, while high concentrations of nitrate inhibit reductive dechlorination and Fe(III) reduction [94].
The reductive dehalogenation of MCPs and DCPs was investigated in the anaerobic sediment samples of estuarine Lake Shinji and Lake Nakaum [95]. Estuarine sediment enrichment cultures of lake Shinji dehalogenated 2CP, 3CP and 2,6-DCP, whereas enrichment cultures of Lake Nakaum dehalogenated 3CP and 2,6-DCP [95]. The dehalogenated product of MCPs was phenol, which was further degraded by the formation of benzoic acid. Itoh et al. [96] identified the bacterial consortia involved in dehalogenation of MCP into phenol and transformation of phenol to benzoic acid using polymerase chain reaction-denaturing gradient gel electrophoresis (PCR-DGGE) of the 16S rRNA gene in the enrichment sample of Lake Shinji. The 4CP-dechlorinating culture had two dominant bacteria, in which one belonged to Dehalobacter sp. In the phenol transforming culture, Cryptanaerobacter phenolicass was present.
Li et al. [97] established a simple anaerobic upflow column system (15 cm long, 5 cm inner diameter) for complete PCP-mineralization using a microbial consortium requiring only lactate as an external nutrient. Anaerobic microbes dehalogenated PCP to 3CP and phenol using external lactate as an electron donor [97]. The further degradation of 3CP and phenol proceeded without an external electron donor and the nitrogen required for degradation was supplied by nitrogen-fixation [97]. The potential dechlorinators, Dehalobacter and Desulfitobacterium, and the phenol/3CP fermentative or syntrophic degraders, Cryptanaerobacter and Syntrophus, were found at the bottom of the column, whereas the nitrogen-fixing facultative anaerobe, Rhizobiales, was detected in the top of the upflow column, and other possible nitrogen-fixers were found at both the bottom and top of the upflow column [97].
A variety of pure bacterial cultures have been characterized for their ability to dechlorinate CPs under anaerobic conditions [98-101]. For example, several species of Desulfitobacterium with dehalogenating capabilities toward various CPs have been isolated and characterized, including Desulfitobacterium hafniense PCP-1 [98], D. hafniense DCB-2 [99], D. dehalogenans IW/IU-DC1 [102] and D. chlororespirans[103]. These strains utilize CPs as electron acceptors for growth during the oxidation of electron donating chemicals in a process known as halorespiration [8]. Villemur [98] reported that D. hafniense PCP-1 isolated from a methanogenic consortium was able to dehalogenate PCP to 3CP via the formation of 3,4,5-TCP and 3,5-DCP. Strain PCP-1 was also capable of dehalogenation of other TCPs and DCPs, but unable to dehalogenate MCPs [98]. D. hafniense strain DCB-2 removed the ortho-substituted chorine from 2,4,6-TCP, 2,4,5-TCP, PCP, 2,4-DCP, and meta-substituted chlorine from 3,5-DCP [98]. Apart from Desulfitobacterium spp., other dehalorespirating bacteria include Desulfomonile tiedje DCB-1 [100] and Anaeromyxobacter dehalogenans[101]. Mohn and Kennedy [100] reported the dehalogenation of PCP into 2,4,6-TCP by 3-chlorobenzoate-induced cells of a sulfate reducing bacterium, Desulfomonile tiedje DCB-1. He and Sandford [104] reported ortho-dehalogenation of 2,6-DCP and 2CP to phenol by a facultative anaerobic bacterium, Anaeromyxobacter dehalogenans 2CP-C. Recently, Wang et al.[105] reported the removal of the ortho-chlorines from 2,4,6-TCP by Dehalobacter sp. PCP-1 that converted 2,4,6-TCP to 4CP via 2,4-DCP.
Genetics of bacterial degradation of CPs
The genes responsible for degradation of CPs are located on either plasmids or chromosomal DNA. Plasmid encoded genes include (i) two tdf operons on the C. nector JMP134 plasmid pJP4 [106], (ii) tcpRXABCYD cluster on C. necator JMP134 (pJP4) [107], (ii) the clc operon on the Pseudomonas knackmussii plasmid pB13 (pWR1) [108], and (iv) the tcb operon on the Pseudomonas sp. P51 plasmid pP51 [109]. Two tdf gene clusters (tfdC I D I E I F I and tfdD II C II E II F II ) identified on plasmid pJP4 of strain JMP134 encode enzymes for 4CC metabolism. The genes tfdC, tfdD, tfdE and tfdF encode the enzymes chlorocatechol-1,2-dioxygenase (EC 1.13.11.-) (TfdC), chloromuconate cycloisomerase (TfdD) (EC = 5.5.1.7), dienelactone hydrolase (TfdE) (EC = 3.1.1.45), and maleylacetate reductase (TfdF) (EC = 1.3.1.32), respectively [106]. Van der Meer et al. [109] reported that genes tcbC, tcbD and tcbE located on the operon tcb (pP51) encoded a catechol 1,2-dioxygenase II (EC 1.13.11.1), a cycloisomerase II (EC = 5.5.1.7), and a hydrolase II (EC = 3.1.1.45), respectively which degraded 3,4-dichlorocatechol and 3,4,6-trichlorocatechol to chloromaleylacetate. The clc operon contains the genes for utilization of CCs on the Pseudomonas sp. P51 plasmid pP51 [108]. These genes include cicA, the gene encoding catechol oxygenase II (EC 1.13.11.1), clcB, the gene encoding muconate cycloisomerase II (EC = 5.5.1.7), and clcD, the gene encoding dienelactone hydrolase (EC = 3.1.1.45). The genes for degradation of 2,4,6-TCP are located on the tcpRXABCYD cluster from C. necator JMP134 (pJP4) [107]. The gene tcpA encodes a reduced flavin adenine dinucleotide (FADH2)-dependent monooxygenase (TcpA) (EC = 1.14.13-) that converts 2,4,6-TCP to 6-chlorohydroxyquinol. TcpA needs FADH2 that is supplied by the putative flavin reductase (TcpX) encoded by the tcpX gene [107]. The tcpB gene may also encode flavin reductase activity because it showed sequence similarity to genes coding for nitroreductases [107]. The gene tcpC encodes an enzyme 6-chlorohydroxyquinol-1,2-dioxygenase (TcpC) (EC = 1.13.11.-) that cleaves chlorohydoxyquinol to 2-chloromaleylacetate. The gene tcpD encodes an enzyme maleylacetate reductase (TcpD) (EC = 1.3.1.32) that converts chloromaleylacetate to β-ketoadipate. The gene tcpR is a regulator that controls the expression of all tcp genes whereas the function of tcpY is not clear [107]. In Ralstonia picketti DTP0602, two gene clusters (hadXABC and hadYD) are involved in the conversion of 2,4,6-TCP to 3-oxoadipate, where hadXABC and hadYD are regulated by hadR and hadS, respectively [110]. Torii et al. [111] investigated how HadR regulates 2,4,6-TCP catabolic pathway gene expression in Ralstonia pickettii DTP0602. They found that purified HadR binds to the hadX promoter and HadR–DNA complex formation is induced in the presence of 16 types of substituted phenols, including CPs, nitrophenols and tribromophenols.
A gene cluster containing four genes (clcA2, clcB2, clcD2 and clcF) involved in a new modified ortho-cleavage pathway of 3CC was identified in Rhodococcus opacus 1CP [47]. The genes clcA2, clcB2, clcD2 and clcF encode the enzymes 3-chlorocatechol-1,2-dioxygenase (ClcA2), chloromuconate cycloisomerase (ClcB2), dienelactone hydrolase (ClcD2) and muconolactone isomerase-related enzyme (ClcF), respectively. This organism also contains a second cluster of chlorocatechol degradation genes that are similar to the proteobacterial genes [41].
A 4CP-degradation gene cluster (cph genes) was identified in A. chlorophenolicus[12]. This gene cluster contains 10 open reading frames that show similarity to the genes encoding the enzymes involved in CP degradation. Several open reading frames encode enzymes with similar functions. For example, two genes, cphA-1 and cph-11, encode functional hydroxyquinol-1,2-dioxygenase. A mutant strain constructed by disturbing the gene cphA-1 by site-directed mutagenesis was unable to utilize 4CP as the sole source of carbon energy. Other genes present on this cluster include cphC-I, cphC-II, cphF-I, cphF-II, Cph B, CphX, CphR and CphS. The genes cphC-I and cphC-II encode putative monooxygenase, whereas cphF-1 and cphF-11 encode putative maleylacetate reductase and cphB encodes a NADH:flavin adenine dinucleotide oxidoreductase. The roles of the remaining genes in the cph gene cluster have yet to be determined [112].
The ccaBARCD gene cluster is involved in CC degradation in Pseudomonas reinekei MT1 [112]. The genes ccaA, ccaB, ccaC and ccaD encode the enzymes catechol-1,2-dioxygenase, (chloro) muconate cycloisomerase, trans-dienelactone hydrolase and maleylacetate reductase, respectively. The gene, ccaR is a putative regulator homologous to regulators of the IclR-type family [112].
Genes for degradation of 2,4,5-TCP have been identified and characterized from Burkholderia phenoliruptrix AC1100 [61,62,113-115]. Two gene clusters, tftCD, and tftEFGH, are involved in conversion of 2,4,5-TCP to 3-oxoadipate in B. phenoliruptrix AC1100 [61,62,113-115].
Several genes (pcpB, pcpC, pcpA and pcp E) involved in PCP degradation have been identified and characterized from Sphingomonas chlorophenolicum L-1 [116]. The genes pcpB, pcpC, pcpA and pcpE encode the enzymes PCP-4-monooxygenase (PcpB) (EC = 1.14.13.50), TeCH-reductive dehalogenase (PcpC) (EC = 1.8.99-), DiCHQ-1,2-dioxygenase (PcpA) (1.13.11.-), maleylacetate reductase (EC = 1.3.1.32), respectively. PcpB is a flavin monooxygenase that converts PCP to TeCHQ via hydroxylation at the para-position with removal of the chloride ion in the first step of the bacterial degradation of PCP. PcpB has broad substrate specificity and catalyzes reaction of various substituted aromatic compounds [117]. The pcpB gene has also been detected in three other strains of Sphingonium chlorophenolicum (RP-2, SR-3 and ATCC 33790). An identical pcpB gene sequence was found in three strains (L-1, RP-2, SR-3) [118,119], whereas the pcpB gene sequence of Sphinogomonads strain UG-30 showed 90% sequence similarity with that of Sphingonium chlorophenolicum ATCC 39723 [120-122]. Homologues of the pcpB gene have also been detected in the polychlorinated degrading bacterium, Novosphingonium sp. strain MT1 [123], and in two non-PCP degrading β- and γ- proteobacterial strains [124]. In the second step of the PCP degradation, PcpC catalyzes the reductive dehalogenation of TeCHQ to 2,6-DCHQ, which is further cleaved to 2-chloromaleylacetate by PcpA. PcpE converts 2-chloromaleylacetate to 3-oxoadipate via maleylacetate. Another gene, pcpR is a LysR-type regulator that is essential to the induction of pcpB, pcpA, and pcpE.
Genetics of reductive dehalogenation
Reductive dehalogenation of CPs and poly-CPs is generally carried out by chlorophenol reductive dehalogenases (CprA) encoded by the cprA gene, which have been well-studied in Desulfitobacterium hafniense PCP-1, D. dehalognase IW/IU-DC1, and D. chlororespirans[98]. The cprA genes are associated with cpr gene clusters that also encode several accessory proteins (e.g., CprA-anchor protein [98,125], chaperones, regulators [126]). The cpr gene clusters composed of eight genes (cprT, cprK, cprZ, cprE, cprB, cprA, cprC, and cprD) have been identified in the genome of Desulfitobacterium dehalogenans IW/IU-DC1 and Desulfitobacterium hafniense DCB-2 [126,127]. CprK, a member of the CRP-FNR (cAMP-binding protein/fumarate nitrate reduction regulatory protein) family regulators, control transcription of the cpr genes [128]. The mechanism responsible for regulation of transcription of cpr genes has been investigated [128]. An effector domain of CprK interacts with a chlorinated aromatic compound with high affinity which induces its binding to an upstream target DNA sequence known as the “dehalobox to activate the transcriptions of the cpr genes [128].
Four genes (cprA2, cprA3, cprA4 and cprA5) encoding the putative chloroaromatic reductive dehalogenases (CprA2-A5) have been identified in D. hafniense PCP-1 [129-132]. Two gene products (CprA3 and CprA5) have been purified and characterized. CprA3 catalyzes ortho-dechlorination of highly chlorinated phenols including PCP, 2,3,4,5-TeCP, 2,3,4-TCP, 2,4,6-TCP and 2,3,6-TCP, whereas CprA5 catalyzes meta-dechlorination of 3,5-DCP and 2,3,5-TCP, para-dechlorination of PCP, 2,3,4,5-TeCP and 3,4,5-TCP, and ortho-dechlorination of 2,4,6-TCP, 2,4,5-TCP and 2,4-DCP [131,132]. The dehalogenation activities of the products of another two genes (cprA2 and cprA3) are not yet known. The transcription levels of the cprA2, cprA3, cprA4 and cprA5 genes were measured in strain PCP cultures exposed to CPs by reverse transcription-quantitative PCR [133]. The genes cprA2 and cprA3 were upregulated in cultures amended with 2,4,6-TCP, whereas only cprA5 was upregulated in 3,5-DCP-amended cultures. In PCP-amended cultures grown for 12 h, cprA2 and cprA3 were upregulated, but cprA5 was not. The gene, cprA4 was not upregulated significantly in cultures containing any tested CPs [133].
A non-CprA reductive dehalogenase known as CrdA from D. hafniense strain PCP-1 cultures amended with 2,4,6 TCP has been isolated and characterized. CrdA catalyzes ortho-dehalogenation of PCP and 2,4,6-TCP [134]. The gene (crdA) encoding CrdA has been cloned and sequenced from strain PCP-1 and also detected in several other strains of Desulfitobacterium[134]. Gauthier et al. [129] monitored the expression of the crd gene in Desulfitobacterium strains and transcripts of crdA were detected in D. hafniense strains PCP-1, DCB-2 and TCE-1.
Conclusions
The bacterial degradation of MCPs and poly-CPs has been extensively studied and several pathways have been proposed for degradation of MCPs and poly-CPs. The bacterial degradation of CPs and poly-CPs proceeded via formation of the corresponding CCs or the corresponding (chloro)HQs. The genes involved in the degradation of MCPs and poly-CPs have also been identified and characterized from CPs-degrading bacteria.
CAPs and CMPs are highly toxic compounds, and few studies have been conducted to investigate the biodegradation of these compounds. More CAPs and CMPs-degrading bacteria must be isolated to investigate the genetic and biochemical mechanism by which these compounds are degraded.
Anaerobic degradation of CPs has also been studied, and it has been established that MCPs and poly-CPs are initially dehalogenated to phenol, which is further transformed to benzoic acid and then mineralized to CO2 under anaerobic conditions. However, further study is needed to elucidate the genetic and enzymatic basis of this mechanism. Furthermore, anaerobic degradation of other CPs such as CNPs, CAPs and CMPs should also be studied.
Abbreviations
CPs: Chlorophenols; MCPs: Monochlorophenols; poly-CPs: Polychlorophenols; CNPs: Chloronitrophenols; CAPs: Chloroaminophenols; CMPs: Chloromethylphenols; 2CP: 2-Chlorophenol; 3CP: 3-Chlorophenol; 4CP: 4-Chlorophenol; CC: Chlorocatechol; 4CC: 4-Chlorocatechol; 3CC: 3-Chlorocatchol; 2CC: 2-Chlorocatechol; HQ: Hydroquinone; 5C2HMS: 5-Chloro-2-hydroxymuconic semialdehyde; BT: 1,2,4-Benzenetriol; 4CC-BT pathway: 4-Chlorocatechol-benzenetriol pathway; CMCI: Chloromuconate cycloisomerase; CMLI: Chloromuconolactone isomerase; DELH: Dienelactone hydrolase; DCPs: Dichlorophenols; TCPs: Trichlorophenols; TeCPs: Tetrachlorophenols; PCP: Pentachlorophenol; 2,4-DCP: 2,4-Dichlorophenol; 2,4,6-TCP: 2,4,6-Trichlorophenol; 2,4,5-TCP: 2,4,5-Trichlorophenol; 2,4-D: 2,4-Dichlorophenoxyacetic acid; DiCBQ: 2,5-Dichloro-p-benzoquinone; 2,5-DiCHQ: 2,5-Dichlorohydroquinone; CHHQ: 5-Chloro-2-hydroxy-p-hydroquinone; TeCHQ: Tetrachlorohydroquinone; 2,6-DCHQ: 2,6-Dichloro-1,4-hydroquinone; 2C4NP: 2-Chloro-4-nitropheol; 4C2NP: 4-Chloro-2-nitrophnol; 4C3NP: 4-Chloro-3-nitrophenol; 2C5NP: 2-Chloro-5-nitrophenol; 2C3NP: 2-Chloro-3-nitrophenol; 4NP: 4-Nitrophenol; CHQ: Chlorohydroquinone; 4C2AP: 4-Chloro-2-aminophenol; 4C2AAP: 4-Chloro-2-acetaminophenol; 2AP: 2-Aminophenol; 2C5HAP: 2-Chloro-5-hydroxylaminophenol; 2A5CHQ: 2-Amino-5-chlorohydroquinone; 3NC: 3-Nitrocatechol; 4C2AP: 4-Chloro-2-aminophenol; 2C4AP: 2-Chloro-4-aminophenol; 2,3,4,5-TeCP: 2,3,4,5-Tetrachlorophenol; 3,4,5-TCP: 3,4,5-Trichlorophenol; TCA Cycle: Tricarboxylic acid cycle.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
PKA collected all the relevant publications, arranged the general structure of the review, drafted the text and produced figures. HHB revised and formatted the review and also help to draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
Pankaj Kumar Arora, Email: arora484@gmail.com.
Hanhong Bae, Email: hanhongbae@ynu.ac.kr.
Acknowledgement
This research was supported by a Yeungnam University Research Grant (213A345032).
References
- National Pollutant Inventory. Department of the environment, water, heritage and the arts, Australia. [ http://www.npi.gov.au/resource/chlorophenols-di-tri-tetra]
- Muller F, Caillard L. Ullmann’s Encyclopedia of Industrial Chemistry. John Wiley & Sons, Inc; 2011. Chlorophenols. DOI: 10.1002/14356007.a07_001.pub2. [Google Scholar]
- Olaniran AO, Igbinosa EO. Chlorophenols and other related derivatives of environmental concern: properties, distribution and microbial degradation processes. Chemosphere. 2011;83:1297–1306. doi: 10.1016/j.chemosphere.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for Chlorophenols. Atlanta, GA: U.S Department of Health and Human Services, Public Health Service; 1999. [Google Scholar]
- Igbinosa EO, Odjadjare EE, Chigor VN, Igbinosa IH, Emoghene AO, Ekhaise FO, Igiehon NO, Idemudia OG. Toxicological profile of chlorophenols and their derivatives in the environment: the public health perspective. Sci World J. 2013;2013:11. doi: 10.1155/2013/460215. doi:10.1155/2013/460215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera-Titus M, García-Molina V, Baños MA, Giménez J, Esplugas S. Degradation of chlorophenols by means of advanced oxidation processes: a general review. Appl Catal B Environ. 2004;47(4):219–256. [Google Scholar]
- Solyanikova IP, Golovleva LA. Bacterial degradation of chlorophenols: pathways, biochemica, and genetic aspects. J Environ Sci Health B. 2004;39(3):333–351. doi: 10.1081/pfc-120035921. [DOI] [PubMed] [Google Scholar]
- Field JA, Sierra-Alvarez R. Microbial degradation of chlorinated phenols. Rev Environ Sci Biotechnol. 2008;7:211–241. [Google Scholar]
- Solyanikova I, Golovleva L. Biochemical features of the degradation of pollutants by Rhodococcus as a basis for contaminated wastewater and soil cleanup. Mikrobiologiia. 2011;80(5):579–594. [PubMed] [Google Scholar]
- Hollender J, Hopp J, Dott W. Degradation of 4-Chlorophenol via the meta Cleavage Pathway by Comamonas testosteroni JH5. Appl Environ Microbiol. 1997;63(11):4567–4572. doi: 10.1128/aem.63.11.4567-4572.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars AE, Kasberg T, Kaschabek SR, van Agteren MH, Janssen DB, Reineke W. Microbial degradation of chloroaromatics: use of the meta-cleavage pathway for mineralization of chlorobenzene. J Bacteriol. 1997;179:4530–4537. doi: 10.1128/jb.179.14.4530-4537.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordin K, Unell M, Jansson JK. Novel 4-chlorophenol degradation gene clusterand degradation route via hydroxyquinol in Arthrobacter chlorophenolicus A6. Appl Environ Microbiol. 2005;71(11):6538–6544. doi: 10.1128/AEM.71.11.6538-6544.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xun L, Topp E, Orser CS. Purification and characterization of a tetrachloro-p-hydroquinone reductive dehalogenase from a Flavobacterium sp. J Bacteriol. 1992;174:8003–8007. doi: 10.1128/jb.174.24.8003-8007.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora PK, Jain RK. Metabolism of 2-chloro-4-nitrophenol in a gram negative bacterium, Burkholderia sp. RKJ 800. PLOS One. 2012;7(6):e38676. doi: 10.1371/journal.pone.0038676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey J, Heipieper HJ, Chauhan A, Arora PK, Prakash D, Takeo M, Jain RK. Reductive dehalogenation mediated initiation of aerobic degradation of 2-chloro-4-nitrophenol (2C4NP) by Burkholderia sp. strain SJ98. Appl Microbiol Biotechnol. 2011;92:597–607. doi: 10.1007/s00253-011-3254-y. [DOI] [PubMed] [Google Scholar]
- Arora PK, Sharma A, Mehta R, Shenoy BD, Srivastava A, Singh VP. Metabolism of 4-chloro-2-nitrophenol in a gram-positive bacterium, Exiguobacterium sp. PMA. Microb Cell Fact. 2012;11:150. doi: 10.1186/1475-2859-11-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora PK, Srivastava A, Singh VP. Novel degradation pathway of 4-chloro-2-aminophenol via 4-chlorocatechol in Burkholderia sp. RKJ 800. Environ Sci Pollut Res Int. 2013. Doi: 10.1007/s11356-013-2167-y. [DOI] [PubMed]
- Arora PK, Srivastava A, Singh V. Novel degradation pathway of 2-chloro-4-aminophenol in Arthrobacter sp. SPG. PeerJ PrePrints. 2014;2:e194v1. doi: 10.1186/s12934-014-0164-6. http://dx.doi.org/10.7287/peerj.preprints.194v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikodem P, Hecht V, Schlömann M, Pieper DH. New bacterial pathway for 4- and 5-chlorosalicylate degradation via 4-chlorocatechol and maleylacetate in Pseudomonas sp. strain MT1. J Bacteriol. 2003;185(23):6790–6800. doi: 10.1128/JB.185.23.6790-6800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn E, Hellwig M, Reineke W, Knackmuss HJ. Isolation and characterization of a 3-chlorobenzoate degrading pseudomonad. Arch Microbiol. 1974;99(1):61–70. doi: 10.1007/BF00696222. [DOI] [PubMed] [Google Scholar]
- Weisshaar MP, Franklin FC, Reineke W. Molecular cloning and expression of the 3-chlorobenzoate-degrading genes from Pseudomonas sp. strain B13. J Bacteriol. 1987;169(1):394–402. doi: 10.1128/jb.169.1.394-402.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajithkumar PV, Kunhi AA. Pathways for 3-chloro- and 4-chlorobenzoate degradation in Pseudomonas aeruginosa 3mT. Biodegradation. 2000;11(4):247–261. doi: 10.1023/a:1011124220003. [DOI] [PubMed] [Google Scholar]
- Arensdorf JJ, Focht DD. Formation of chlorocatechol meta cleavage products by a pseudomonad during metabolism of monochlorobiphenyls. Appl Environ Microbiol. 1994;60(8):2884–2889. doi: 10.1128/aem.60.8.2884-2889.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz A, Busse HJ, Kämpfer P. Pseudomonas knackmussii sp. nov. Int J Syst Evol Microbiol. 2007;57(Pt 3):572–576. doi: 10.1099/ijs.0.64761-0. [DOI] [PubMed] [Google Scholar]
- Knackmuss HJ, Hellwig M. Utilization and cooxidation of chlorinated phenols by Pseudomonas sp B-13. Arch Microbiol. 1978;117:1–7. doi: 10.1007/BF00689343. [DOI] [PubMed] [Google Scholar]
- Fava F, Armenante PM, Kafkewitz D. Aerobic degradation and dechlorination of 2-chlorophenol, 3-chlorophenol and 4-chlorophenol by a Pseudomonas pickettii strain. Lett Appl Microbiol. 1995;21(5):307–312. doi: 10.1111/j.1472-765x.1995.tb01066.x. [DOI] [PubMed] [Google Scholar]
- Finkel’shtein ZI, Baskunov BP, Golovlev EL, Moiseeva OV, Vervoort J, Rietjens I, Golovleva LA. Dependence of the conversion of chlorophenols by rhodococci on the number and position of chlorine atoms in the aromatic ring. Microbiology. 2000;69:40–47. [PubMed] [Google Scholar]
- Menke B, Rehm HJ. Degradation of mixtures of monochlorophenols and phenol as substrates for free and immobilized cells of Alcaligenes sp. A7–2. Appl Microbiol Biotechnol. 1992;37:655–661. [Google Scholar]
- Hollender J, Hopp J, Dott W. Cooxidation of chloro- and methylphenols by Alcaligenes xylosoxidans JH1. World J Microbiol Biotechnol. 2000;16:445–450. [Google Scholar]
- Bae HS, Rhee SK, Cho YG, Hong JK, Lee ST. Two different pathways (a chlorocatechol and a hydroquinone pathway) for the 4-chlorophenol degradation in two isolated bacterial strains. J Microbiol Biotechnol. 1997;7:237–241. [Google Scholar]
- Westerberg K, Elvang AM, Stackebrandt E, Jansson JK. Arthrobacter chlorophenolicus sp nov., a new species capable of degrading high concentrations of 4-chlorophenol. Int J Syst Evol Microbiol. 2000;50:2083–2092. doi: 10.1099/00207713-50-6-2083. [DOI] [PubMed] [Google Scholar]
- Bae HS, Lee JM, Kim YB, Lee ST. Biodegradation of the mixtures of 4-chlorophenol and phenol by Comamonas testosteroni CPW301. Biodegradation. 1997;7:463–469. doi: 10.1007/BF00115293. [DOI] [PubMed] [Google Scholar]
- Im WT, Bae HS, Yokota A, Lee ST. Herbaspirillum chlorophenolicum sp. nov., a 4-chlorophenol-degrading bacterium. Int J Syst Evol Microbiol. 2004;54(Pt3):851–855. doi: 10.1099/ijs.0.02812-0. [DOI] [PubMed] [Google Scholar]
- Konovalova EI, Solyanikova IP, Golovleva LA. Degradation of 4-chlorophenol by the bacterium Rhodococcus opacus 6a. Microbiol (Moscow, Engl Transl) 2009;78(6):805–807. [Google Scholar]
- Bae HS, Lee JM, Lee ST. Biodegradation of 4-chlorophenol via a hydroquinone pathway by Arthrobacter ureafaciens CPR706. FEMS Microbiol Lett. 1996;145(1):125–129. doi: 10.1111/j.1574-6968.1996.tb08566.x. [DOI] [PubMed] [Google Scholar]
- Solyanikova IP, Golovleva LA. Biochemical Features of the degradation of pollutants by Rhodococcus as a basis for contaminated wastewater and soil cleanup. Microbiology. 2011;80(5):591–607. [PubMed] [Google Scholar]
- Cho YG, Yoon JH, Park YH, Lee ST. Simultaneous degradation of pnitrophenol and phenol by a newly isolated Nocardioides sp. J Gen Appl Microbiol. 1998;44:303–309. doi: 10.2323/jgam.44.303. [DOI] [PubMed] [Google Scholar]
- Golovleva LA, Zaborina O, Pertsova R, Baskunov B, Schurukhin Y, Kuzmin S. Degradation of polychlorinated phenols by Streptomyces rochei 303. Biodegradation. 1992;2:201–208. doi: 10.1007/BF00124494. [DOI] [PubMed] [Google Scholar]
- Farrell A, Quilty B. Degradation of mono-chlorophenols by a mixed microbial community via a meta- cleavage pathway. Biodegradation. 1999;10(5):353–362. doi: 10.1023/a:1008323811433. [DOI] [PubMed] [Google Scholar]
- Moiseeva OV, Belova OV, Solyanikova IP, Schlömann M, Golovleva LA. Enzymes of a new modified ortho-pathway utilizing 2-chlorophenol in Rhodococcus opacus 1CP. Biochemistry (Mosc) 2001;66(5):548–555. doi: 10.1023/a:1010267104238. [DOI] [PubMed] [Google Scholar]
- Moiseeva OV, Solyanikova IP, Kaschabek SR, Gröning J, Thiel M, Golovleva LA, Schlömann MA. New modified ortho cleavage pathway of 3-chlorocatechol degradation by Rhodococcus opacus 1CP: genetic and biochemical evidence. J Bacteriol. 2002;184(19):5282–5292. doi: 10.1128/JB.184.19.5282-5292.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels I, Knackmuss HJ, Reineke W. Suicide inactivation of catechol 2,3-dioxygenase from Pseudomonas putida mt-2 by 3-halocatechols. Appl Environ Microbiol. 1984;47(3):500–505. doi: 10.1128/aem.47.3.500-505.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze M, Zerlin KF, Retzlaff A, Pohl JO, Schmidt E, Janssen DB, Vilchez-Vargas R, Pieper DH, Reineke W. Degradation of chloroaromatics by Pseudomonas putida GJ31: assembled route for chlorobenzene degradation encoded by clusters on plasmid pKW1 and the chromosome. Microbiology. 2009;155(Pt12):4069–4083. doi: 10.1099/mic.0.032110-0. [DOI] [PubMed] [Google Scholar]
- Gobel M, Kranz OH, Kaschabek SR, Schmidt E, Pieper DH, Reineke W. Microorganisms degrading chlorobenzene via a meta-cleavage pathway harbor highly similar chlorocatechol 2,3-dioxygenase-encoding gene clusters. Arch Microbiol. 2004;182:147–156. doi: 10.1007/s00203-004-0681-5. [DOI] [PubMed] [Google Scholar]
- Tarao M, Seto M. Estimation of the yield coefficient of Pseudomonas sp. strain DP-4 with a low substrate (2,4-dichlorophenol [DCP]) concentration in a mineral medium from which uncharacterized organic compounds were eliminated by a non-DCP-degrading organism. Appl Environ Microbiol. 2000;66:566–570. doi: 10.1128/aem.66.2.566-570.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami M, Shivaraman N, Singh RP. Kinetics of chlorophenol degradation by benzoate-induced culture of Rhodococcus erythropolis M1. World J Microbiol Biotechnol. 2002;18:779–783. [Google Scholar]
- Tyler JE, Finn RK. Growth rates of a Pseudomonad on 2,4-D and 2,4 dichlorophenol. Appl Microbiol. 1974;28:181–184. doi: 10.1128/am.28.2.181-184.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CC, Lee CM, Kuan CH. Removal of 2,4-dichlorophenol by suspended and immobilized Bacillus insolitus. Chemosphere. 2000;41(3):447–452. doi: 10.1016/s0045-6535(99)00263-5. [DOI] [PubMed] [Google Scholar]
- Fukumori F, Hausinger RP. Purification and characterization of 2,4-dichlorophenoxyacetate/alpha-ketoglutarate dioxygenase. J Biol Chem. 1993;268(32):24311–243117. [PubMed] [Google Scholar]
- Perkins EJ, Gordon MP, Caceres O, Lurquin PF. Organization and sequence analysis of the 2,4-dichlorophenol hydroxylase and dichlorocatechol oxidative operons of plasmid pJP4. J Bacteriol. 1990;172(5):2351–2359. doi: 10.1128/jb.172.5.2351-2359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh S, McCullar MV, Focht DD. Biodegradation of 2,4-dichlorophenol through a distal meta-fission pathway. Appl Environ Microbiol. 1997;63(5):2054–2057. doi: 10.1128/aem.63.5.2054-2057.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DY, Eberspacher J, Wagner B, Kuntzer J, Lingens F. Degradation of 2,4,6-trichlorophenol by Azotobacter sp. strain GP1. Appl Environ Microbiol. 1991;57:1920–1928. doi: 10.1128/aem.57.7.1920-1928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyohara H, Hatta T, Ogawa Y, Kakuda T, Yokoyama H, Takizawa N. Isolation of Pseudomonas pickettii strains that degrade 2,4,6-trichlorophenol and their dechlorination of chlorophenols. Appl Environ Microbiol. 1992;58:1276–1283. doi: 10.1128/aem.58.4.1276-1283.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus V, Sanchez MA, Martinez M, Gonzalez B. Efficient degradation of 2,4,6-trichlorophenol requires a set of catabolic genes related to tcp genes from Ralstonia eutropha JMP134(pJP4) Appl Environ Microbiol. 2003;69:7108–7115. doi: 10.1128/AEM.69.12.7108-7115.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xun LY, Webster CM. A monooxygenase catalyzes sequential dechlorinations of 2,4,6-trichlorophenol by oxidative and hydrolytic reactions. J Biol Chem. 2004;279:6696–6700. doi: 10.1074/jbc.M312072200. [DOI] [PubMed] [Google Scholar]
- Mannisto MK, Tiirola MA, Salkinoja-Salonen MS, Kulomaa MS, Puhakka JA. Diversity of chlorophenol degrading bacteria isolated from contaminated borealgroundwater. Arch Microbiol. 1999;171:189–197. doi: 10.1007/s002030050698. [DOI] [PubMed] [Google Scholar]
- Tiirola MA, Busse HJ, Kampfer P, Mannisto MK. Novosphingobium lentum sp nov., a psychrotolerant bacterium from a polychlorophenol bioremediation process. Int J Syst Evol Microbiol. 2005;55:583–588. doi: 10.1099/ijs.0.63386-0. [DOI] [PubMed] [Google Scholar]
- Louie TM, Webster CM, Xun L. Genetic and biochemical characterization of a 2,4,6-trichlorophenol degradation pathway in Ralstonia eutropha JMP134. J Bacteriol. 2002;184(13):3492–3500. doi: 10.1128/JB.184.13.3492-3500.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenye T, Henry D, Speert DP, Vandamme P. Burkholderia phenoliruptrix sp. nov., to accommodate the 2,4,5-trichlorophenoxyacetic acid and halophenol-degrading strain AC1100. Syst Appl Microbiol. 2004;27(6):623–627. doi: 10.1078/0723202042369992. [DOI] [PubMed] [Google Scholar]
- Webb BN, Ballinger JW, Kim E, Belchik SM, Lam KS, Youn B, Nissen MS, Xun L, Kang C. Characterization of chlorophenol 4-monooxygenase (TftD) and NADH:FAD oxidoreductase (TftC) of Burkholderia cepacia AC1100. J Biol Chem. 2010;285(3):2014–2027. doi: 10.1074/jbc.M109.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisi MR, Xun L. Characterization of chlorophenol 4-monooxygenase (TftD) and NADH: flavin adenine dinucleotide oxidoreductase (TftC) of Burkholderia cepacia AC1100. J Bacteriol. 2009;185(9):2786–2792. doi: 10.1128/JB.185.9.2786-2792.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborina O, Daubaras DL, Zago A, Xun L, Saido K, Klem T, Nikolic D, Chakrabarty AM. Novel pathway for conversion of chlorohydroxyquinol to maleylacetate in Burkholderia cepacia AC1100. J Bacteriol. 1998;180(17):4667–4675. doi: 10.1128/jb.180.17.4667-4675.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo Y, Miyauchi K, Kanda K, Hatta T, Kiyohara H, Senda T, Nagata Y, Mitsui Y, Takagi M. PcpA, which is involved in the degradation of pentachlorophenol in Sphingomonas chlorophenolica ATCC39723, is a novel type of ring-cleavage dioxygenase. FEBS Lett. 1999;459(3):395–398. doi: 10.1016/s0014-5793(99)01305-8. [DOI] [PubMed] [Google Scholar]
- Lange CC, Schneider BJ, Orser CS. Verification of the role of PCP 4-monooxygenase in chlorine elimination from pentachlorophenol by Flavobacterium sp. strain ATCC 39723. Biochem Biophys Res Commun. 1996;219(1):146–149. doi: 10.1006/bbrc.1996.0196. [DOI] [PubMed] [Google Scholar]
- Orser CS, Lange CC. Molecular analysis of pentachlorophenol degradation. Biodegradation. 1994;5:277–288. doi: 10.1007/BF00696465. [DOI] [PubMed] [Google Scholar]
- Uotila JS, Salkinoja-Salonen MS, Apajalahti JHA. Dechlorination of pentachlorophenol by membrane boundenzymes of Rhodococcus chlorophenolicus PCP-I. Biodegradation. 1991;2:25–31. doi: 10.1007/BF00122422. [DOI] [PubMed] [Google Scholar]
- Uotila JS, Kitunen VH, Saastamoinen T, Coote T, Haggblom MM, Salkinoja-Salonen MS. Characterization of aromatic dehalogenases of Mycobacterium fortuitum CG-2. J Bacteriol. 1992;174:5669–5675. doi: 10.1128/jb.174.17.5669-5675.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apajalahti JHA, Salkinoja-Salonen MS. Complete dechlorination of tetrachlorohydroquinone by cell-extracts of pentachlorophenol-induced Rhodococcus chlorophenolicus. J Bacteriol. 1987;169:5125–5130. doi: 10.1128/jb.169.11.5125-5130.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora PK, Jain RK. Pathway for degradation of 2-chloro-4-nitrophenol by Arthrobacter sp. SJCon. Curr Microbiol. 2011;63:568–573. doi: 10.1007/s00284-011-0022-2. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Khurana M, Chauhan A, Takeo M, Chakraborti AK, Jain RK. Degradation of 4-nitrophenol, 2-chloro-4-nitrophenol, and 2,4-dinitrophenol by Rhodococcus imtechensis strain RKJ300. Environ Sci Technol. 2010;44:1069–1077. doi: 10.1021/es9034123. [DOI] [PubMed] [Google Scholar]
- Bruhn C, Bayly RC, Knackmuss HJ. The Invivo construction of 4-chloro-2-nitrophenol assimilatory bacteria. Arch Microbiol. 1988;150:171–177. [Google Scholar]
- Beunink J, Rehm HJ. Coupled reductive and oxidative degradation of 4-chloro-2-nitrophenol by a co-immobilized mixed culture system. Appl Microbiol Biotechnol. 1990;34:108–115. doi: 10.1007/BF00170933. [DOI] [PubMed] [Google Scholar]
- Arora PK, Jain RK. Biotransformation of 4-chloro-2-nitrophenol into 5-chloro-2. methylbenzoxazole by a marine Bacillus sp. strain MW-1. Biodegradation. 2012;23:325–331. doi: 10.1007/s10532-011-9512-y. [DOI] [PubMed] [Google Scholar]
- Arora PK. Decolourization of 4-chloro-2-nitrophenol by a soil bacterium, Bacillus subtilis RKJ 700. PLoS One. 2012;7:e52012. doi: 10.1371/journal.pone.0052012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenzle A, Lenke H, Spain JC, Knackmuss HJ. Chemoselective nitro group reduction and reductive dechlorination initiate degradation of 2-chloro-5-nitrophenol by Ralstonia eutropha JMP134. Appl Environ Microbiol. 1999;65:2317–2323. doi: 10.1128/aem.65.6.2317-2323.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey J, Sharma NK, Khan F, Ghosh A, Oakeshott JG, Jain RK, Pandey G. Chemotaxis of Burkholderia sp. strain SJ98 towards chloronitroaromatic compounds that it can metabolise. BMC Microbiol. 2010;12:19. doi: 10.1186/1471-2180-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner U, Baumbach R, Becker D, Kitunen V, Auling G, Salkinoja-Salonen M. Degradation of 4-chloro-2-methylphenol by an activated sludge isolate and its taxonomic description. Biodegradation. 1995;6(2):83–92. doi: 10.1007/BF00695339. [DOI] [PubMed] [Google Scholar]
- Mikesell MD, Boyd SA. Complete reductive dechlorination and mineralization of pentachlorophenol by anaerobic microorganisms. Appl Environ Microbiol. 1986;52:861–865. doi: 10.1128/aem.52.4.861-865.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londry KL, Fedorak PM. Benzoic-acid intermediates in the anaerobic biodegradation of phenols. Can J Microbiol. 1992;38:1–11. doi: 10.1139/m92-001. [DOI] [PubMed] [Google Scholar]
- Yang S, Shibata A, Yoshida N, Katayama A. Anaerobic mineralization of pentachlorophenol (PCP) by combining PCP-dechlorinating and phenol-degrading cultures. Biotechnol Bioeng. 2009;102(1):81–90. doi: 10.1002/bit.22032. [DOI] [PubMed] [Google Scholar]
- Becker JG, Stahl DA, Rittmann BE. Reductive dehalogenation and conversion of 2-chlorophenol to 3-chlorobenzoate in a methanogenic sediment community:Implications for predicting the environmental fate of chlorinated pollutants. Appl Environ Microbiol. 1999;65:5169–5172. doi: 10.1128/aem.65.11.5169-5172.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd SA, Shelton DR. Anaerobic biodegradation of chlorophenols in fresh and acclimated sludge. Appl Environ Microbiol. 1984;47:272–277. doi: 10.1128/aem.47.2.272-277.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson DK, Woods SL, Istok JD, Peek DC. Reductive dechlorination of chlorophenols by a pentachlorophenolacclimated methanogenic consortium. Appl Environ Microbiol. 1992;58:2280–2286. doi: 10.1128/aem.58.7.2280-2286.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen T, Aamand H. Anaerobic transformation and toxicity of trichlorophenols in a stable enrichment culture. Appl Environ Microbiol. 1992;58(2):557–561. doi: 10.1128/aem.58.2.557-561.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YT, Muthukrishnan S, Wang ZM. Reductive dechlorination of chlorophenols in methanogenic cultures. J Environ Eng-ASCE. 1998;124:231–238. [Google Scholar]
- Takeuchi R, Suwa Y, Yamagishi T, Yonezawa Y. Anaerobic transformation of chlorophenols in methanogenic sludge unexposed to chlorophenols. Chemosphere. 2000;41:1457–1462. doi: 10.1016/s0045-6535(99)00521-4. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wiegel J. Sequential anaerobic degradation of 2 4 dichlorophenol in freshwater sediments. Appl Environ Microbiol. 1990;56:1119–1127. doi: 10.1128/aem.56.4.1119-1127.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häggblom MM, Young LY. Chlorophenol degradation coupled to sulfate reduction. Appl Environ Microbiol. 1990;56(11):3255–3260. doi: 10.1128/aem.56.11.3255-3260.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggblom MM, Young LY. Anaerobic degradation of halogenated phenols by sulfate-reducing consortia. Appl Environ Microbiol. 1995;61:1546–1550. doi: 10.1128/aem.61.4.1546-1550.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häggblom MM, Rivera MD, Young LY. Influence of alternative electron acceptors on the anaerobic biodegradability of chlorinated phenols and benzoic acids. Appl Environ Microbiol. 1993;59(4):1162–1167. doi: 10.1128/aem.59.4.1162-1167.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae HS, Yamagishi T, Suwa Y. Evidence for degradation of 2-chlorophenol by enrichment cultures under denitrifying conditions. Microbiology. 2002;148:221–227. doi: 10.1099/00221287-148-1-221. [DOI] [PubMed] [Google Scholar]
- Sanford RA, Tiedje JM. Chlorophenol dechlorination and subsequent degradation in denitrifying microcosms fed low concentrations of nitrate. Biodegradation. 1997;7:425–434. [Google Scholar]
- Kazumi J, Haggblom MM, Young LY. Degradation of monochlorinated and nonchlorinated aromatic-compounds under iron-reducing conditions. Appl Environ Microbiol. 1995;61:4069–4073. doi: 10.1128/aem.61.11.4069-4073.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HY, Wang YK, Chen PC, Li FB, Chen MJ, Hu M, Ouyang X. Effect of nitrate addition on reductive transformation of pentachlorophenol in paddy soil in relation to iron(III) reduction. J Environ Manage. 2013;132C:42–48. doi: 10.1016/j.jenvman.2013.10.020. [DOI] [PubMed] [Google Scholar]
- Itoh K, Mihara Y, Tanimoto N, Shimada T, Suyama K. Reductive dechlorination of chlorophenols in estuarine sediments of Lake Shinji and Lake Nakaumi. J Environ Sci Health B. 2010;45(5):399–407. doi: 10.1080/03601231003800016. [DOI] [PubMed] [Google Scholar]
- Itoh K, Mihara Y, Toshima Y, Suyama K. Characterization of microbial consortia that reductively dechlorinate 4-chlorophenol and transform phenol to benzoate enriched from estuarine sediment of Lake Shinji. J Environ Sci Health B. 2011;46(2):181–190. doi: 10.1080/03601234.2011.539147. [DOI] [PubMed] [Google Scholar]
- Li Z, Inoue Y, Suzuki D, Ye L, Katayama A. Long-term anaerobic mineralization of pentachlorophenol in a continuous-flow system using only lactate as an external nutrient. Environ Sci Technol. 2013;47(3):1534–1541. doi: 10.1021/es303784f. [DOI] [PubMed] [Google Scholar]
- Villemur R. The pentachlorophenol-dehalogenating Desulfitobacterium hafniense strain PCP-1. Philos Trans R Soc B. 2013;368:20120319. doi: 10.1098/rstb.2012.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen N, Ahring BK. Desulfitobacterium hafniense sp nov, an anaerobic, reductively dechlorinating bacterium. Int J Syst Bacteriol. 1996;46:442–448. [Google Scholar]
- Mohn WW, Kennedy KJ. Reductive dehalogenation of chlorophenols by Desulfomonile tiedjei DCB-1. Appl Environ Microbiol. 1992;58(4):1367–1370. doi: 10.1128/aem.58.4.1367-1370.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford RA, Cole JR, Tiedje JM. Characterization and description of Anaeromyxobacter dehalogenans gen. nov., sp. nov., an aryl-halorespiring facultative anaerobic myxobacterium. Appl Environ Microbiol. 2002;68(2):893–900. doi: 10.1128/AEM.68.2.893-900.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utkin I, Woese C, Wiegel J. Isolation and characterization of Desulfitobacterium dehalogenans gen. nov, sp. nov, an anaerobic bacterium which reductively dechlorinates chlorophenolic compounds. Int J Syst Bacteriol. 1994;44:612–619. doi: 10.1099/00207713-44-4-612. [DOI] [PubMed] [Google Scholar]
- Sanford RA, Cole JR, Loffler FE, Tiedje JN. Characterization of Desulfitobacterium chlororespirans sp nov, which grows by coupling the oxidation of lactate to the reductive dechlorination of 3-chloro-4-hydroxybenzoate. Appl Environ Microbiol. 1996;62:3800–3808. doi: 10.1128/aem.62.10.3800-3808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Sanford RA. Induction characteristics of reductive dehalogenation in the ortho-halophenol-respiring bacterium. Anaeromyxobacter dehalogenans. Biodegradation. 2002;13(5):307–316. doi: 10.1023/a:1022342421909. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhang W, Yang KL, He J. Isolation and characterization of a novel Dehalobacter species strain TCP1 that reductively dechlorinates 2,4,6-trichlorophenol. Biodegradation. 2013. doi:10.1007/s10532-013-9662-1. [DOI] [PubMed]
- Don RH, Weightman AJ, Knackmuss HJ, Timmis KN. Transposon mutagenesis and cloning analysis of the pathways for degradation of 2,4-dichlorophenoxyacetic acid and 3-chlorobenzoate in Alcaligenes eutrophus JMP134(pJP4) J Bacteriol. 1995;161(1):85–90. doi: 10.1128/jb.161.1.85-90.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez MA, González B. Genetic characterization of 2, 4, 6-trichlorophenol degradation in Cupriavidus necator JMP134. Appl Environ Microbiol. 2007;73(9):2769–2776. doi: 10.1128/AEM.02584-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee DK, Kellogg ST, Hamada S, Chakrabarty AM. Plasmid specifying total degradation of 3-chlorobenzoate by a modified ortho pathway. J Bacteriol. 1981;146(2):639–646. doi: 10.1128/jb.146.2.639-646.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer JR, van Neerven AR, de Vries EJ, de Vos WM, Zehnder AJ. Cloning and characterization of plasmid-encoded genes for the degradation of 1,2-dichloro-, 1,4-dichloro-, and 1,2,4-trichlorobenzene of Pseudomonas sp. strain P51. J Bacteriol. 1991;173(1):6–15. doi: 10.1128/jb.173.1.6-15.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatta T, Fujii E, Takizawa N. Analysis of two gene clusters involved in 2,4,6-trichlorophenol degradation by Ralstonia pickettii DTP0602. Biosci Biotechnol Biochem. 2012;76:892–899. doi: 10.1271/bbb.110843. [DOI] [PubMed] [Google Scholar]
- Torii H, Machida A, Hara H, Hatta T, Takizawa N. The regulatory mechanism of 2,4,6-trichlorophenol catabolic operon expression by HadR in Ralstonia pickettii DTP0602. Microbiology. 2013;159:665–677. doi: 10.1099/mic.0.063396-0. [DOI] [PubMed] [Google Scholar]
- Cámara B, Nikodem P, Bielecki P, Bobadilla R, Junca H, Pieper DH. Characterization of a gene cluster involved in 4-chlorocatechol degradation by Pseudomonas reinekei MT1. J Bacteriol. 2009;191(15):4905–4915. doi: 10.1128/JB.00331-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner A, Danganan CE, Xun L, Chakrabarty AM, Hendrickson W. Genes for 2,4,5-trichlorophenoxyacetic acid metabolism in Burkholderia cepacia AC1100: characterization of the tftC and tftD genes and locations of the tft operons on multiple replicons. Appl Environ Microbiol. 1998;64:2086–2093. doi: 10.1128/aem.64.6.2086-2093.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danganan CE, Ye RW, Daubaras DL, Xun L, Chakrabarty AM. Nucleotide sequence and functional analysis of the genes encoding 2,4,5-trichlorophenoxyacetic acid oxygenase in Pseudomonas cepacia AC1100. Appl Environ Microbiol. 1994;60:4100–4106. doi: 10.1128/aem.60.11.4100-4106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubaras DL, Hershberger CD, Kitano K, Chakrabarty AM. Sequence analysis of a gene cluster involved in metabolism of 2,4,5-trichlorophenoxyacetic acid by Burkholderia cepacia AC1100. Appl Environ Microbiol. 1995;61:1279–1289. doi: 10.1128/aem.61.4.1279-1289.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai M, Xun L. Organization and regulation of pentachlorophenol-degrading genes in Sphingobium chlorophenolicum ATCC 39723. J Bacteriol. 2002;184(17):4672–4680. doi: 10.1128/JB.184.17.4672-4680.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xun L, Topp E, Orser CS. Diverse substrate range of a Flavobacterium pentachlorophenol hydroxylase and reaction stoichiometries. J Bacteriol. 1992;174:2898–2902. doi: 10.1128/jb.174.9.2898-2902.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlson U, Rojo F, vanElsas JD, Moore E. Genetic and serological evidence for the recognition of four pentachlorophenol degrading bacterial strains as a species of the genus Sphingomonas. Syst Appl Microbiol. 1996;18:539–548. [Google Scholar]
- Ederer MM, Crawford RL, Herwig RP, Orser CS. PCP degradation is mediated by closely related strains of the genus Sphingomonas. Mol Ecol. 1997;6:39–49. doi: 10.1046/j.1365-294x.1997.00151.x. [DOI] [PubMed] [Google Scholar]
- Cassidy MB, Lee H, Trevors JT, Zablotowicz RB. Chlorophenol and nitrophenol metabolism by Sphingomonas sp. UG30. J Ind Microbiol Biotechnol. 1999;23:232–241. doi: 10.1038/sj.jim.2900749. [DOI] [PubMed] [Google Scholar]
- Leung KT, Campbell S, Gan Y, White DC, Lee H, Trevors JT. The role of the Sphingomonas species UG30 pentachlorophenol-4-monooxygenase in p-nitrophenol degradation. FEMS Microbiol Lett. 1999;173:247–253. doi: 10.1111/j.1574-6968.1999.tb13509.x. [DOI] [PubMed] [Google Scholar]
- Leung KT, Cassidy MB, Shaw KW, Lee H, Trevors JT, Lohmeier-Vogel EM, Vogel HJ. Pentachlorophenol biodegradation by Pseudomonas spp. UG25 and UG30. World J Microbiol Biotechnol. 1997;13:305–313. [Google Scholar]
- Tiirola MA, Mannisto MK, Puhakka JA, Kulomaa MS. Isolation and characterization of Novosphingobium sp. strain MT1, a dominant polychlorophenol-degrading strain in a groundwater bioremediation system. Appl Environ Microbiol. 2002;68:173–180. doi: 10.1128/AEM.68.1.173-180.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saboo VM, Gealt MA. Gene sequences of the pcpB gene of pentachlorophenol-degrading Sphingomonas chlorophenolica found in non degrading bacteria. Can J Microbiol. 1998;44:667–675. [PubMed] [Google Scholar]
- Smidt H, van Leest M, van der Oost J, de Vos WM. Transcriptional regulation of the cpr gene cluster in ortho-chlorophenol-respiring Desulfitobacterium dehalogenans. J Bacteriol. 2000;182:5683–5691. doi: 10.1128/jb.182.20.5683-5691.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemur R, Lanthier M, Beaudet R, Le’pine F. The Desulfitobacterium genus. FEMS Microbiol Rev. 2006;30:706–733. doi: 10.1111/j.1574-6976.2006.00029.x. [DOI] [PubMed] [Google Scholar]
- Smidt H, van Leest M, van der Oost J, de Vos WM. Transcriptional regulation of the cpr gene cluster in ortho-chlorophenol-respiring Desulfitobacterium dehalogenans. J Bacteriol. 2000;182(20):5683–5691. doi: 10.1128/jb.182.20.5683-5691.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gábor K, Veríssimo CS, Cyran BC, Ter Horst P, Meijer NP, Smidt H, de Vos WM, van der Oost J. Characterization of CprK1, a CRP/FNR-type transcriptional regulator of halorespiration from Desulfitobacterium hafniense. J Bacteriol. 2006;188(7):2604–2613. doi: 10.1128/JB.188.7.2604-2613.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier A, Beaudet R, Le’pine F, Juteau P, Villemur R. Occurrence and expression of crdA and cprA5 encoding chloroaromatic reductive dehalogenases in Desulfitobacterium strains. Can J Microbiol. 2006;52:47–55. doi: 10.1139/w05-111. [DOI] [PubMed] [Google Scholar]
- van de Pas BA, Smidt H, Hagen WR, van der Oost J, Schraa G, Stams AJM, de Vos WM. Purification and molecular characterization of ortho-chlorophenol reductive dehalogenase, a key enzyme of halorespiration in Desulfitobacterium dehalogenans. J Biol Chem. 1999;274:20287–20292. doi: 10.1074/jbc.274.29.20287. [DOI] [PubMed] [Google Scholar]
- Bisaillon A, Beaudet R, Le’pine F, Deziel E, Villemur R. Identification and characterization of a novel CprA reductive dehalogenase specific to highly chlorinated phenols from Desulfitobacterium hafniense strain PCP-1. Appl Environ Microbiol. 2010;76:7536–7540. doi: 10.1128/AEM.01362-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeau J, Gauthier A, Duguay M, Villemur R, Le’pine F, Juteau P, Beaudet R. Purification, cloning, and sequencing of a 3,5-dichlorophenol reductive dehalogenase from Desulfitobacterium frappieri PCP-1. Appl Environ Microbiol. 2004;70:4532–4537. doi: 10.1128/AEM.70.8.4532-4537.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaillon A, Beaudet R, Le’pine F, Villemur R. Quantitative analysis of the relative transcript levels of four chlorophenol reductive dehalogenase genes in Desulfitobacterium hafniense PCP-exposed to chlorophenols. Appl Environ Microbiol. 2011;77:6261–6264. doi: 10.1128/AEM.00390-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer A, Page’-Be’langer R, Saucier M, Villemur R, Le’pine F, Juteau P, Beaudet R. Purification, cloning and sequencing of an enzyme mediating the reductive dechlorination of 2,4,6-trichlorophenol from Desulfitobacterium frappieri PCP-1. Biochem J. 2003;373:297–303. doi: 10.1042/BJ20021837. [DOI] [PMC free article] [PubMed] [Google Scholar]