Abstract
Objective
To investigate the association of behavioral and psychological symptoms of dementia (BPSD) in Alzheimer’s disease (AD) and MRI measures of brain atrophy and white matter hyperintensities (WMH).
Methods
Thirty-seven patients with probable AD received the Neuropsychiatric Inventory (NPI), the Mini Mental Status Exam (MMSE), and an MRI scan as part of their initial evaluation at the Outpatient Memory Diagnostic Clinic at McLean Hospital. MRI-based volumetric measurements of whole brain atrophy, hippocampal volumes and WMH were obtained. Analysis of covariance models, using age as a covariate and the presence of specific BPSD as independent variables, were used to test for differences in whole brain volumes, hippocampal volumes and WMH volumes.
Results
Increased WMH were associated with symptoms of anxiety, aberrant motor behavior, and nighttime disturbance, while symptoms of disinhibition were linked to lower WMH volume. No associations were found for of whole brain or hippocampal volumes and BPSD.
Conclusions
These findings suggest that white matter changes are associated with the presence of BPSD in AD.
Keywords: Alzheimer’s disease, behavioral and psychological symptoms of dementia, white matter hyperintensities, magnetic resonance imaging, Neuropsychiatric Inventory
1. Introduction
Approximately 4.5 million Americans suffer from Alzheimer’s Disease (AD), the most prevalent neurodegenerative dementia (Hebert, Scherr, Bienias, Bennett, & Evans, 2003). These behavioral and psychological symptoms of dementia (BPSD) affect as many as 66%–88% of patients with AD (Assal & Cummings, 2002) and are associated with reduced quality of life (Shin, Carter, Masterman, Fairbanks, & Cummings, 2005), and an increase in functional impairment (Mok, Chu, Chung, Chan, & Hui, 2004; Rapoport, et al., 2001), caregiver burden (Berger, et al., 2005), cost of care (Beeri, Werner, Davidson, & Noy, 2002; Murman, et al., 2002), and likelihood of institutionalization (Scarmeas, et al., 2005; Steele, Rovner, Chase, & Folstein, 1990). Even though the BPSD can be the most debilitating aspects of a patient’s illness, relatively little is known about their relationship to AD and its underlying pathophysiology.
Brain atrophy as assessed by magnetic resonance imaging (MRI) based volumetric analysis offers an in-vivo index of disease severity in patients with AD. While the relationship between cognitive decline in patients with AD and volumetric reduction of the whole brain and hippocampus has been well established through MRI studies (Bigler, et al., 2004; Murphy, et al., 1993; Petersen, et al., 2000; Stout, Jernigan, Archibald, & Salmon, 1996), the possible association between BPSD and MRI measures of brain atrophy merits further exploration. An association, if present, would be consistent with findings from other studies, which have demonstrated that many BPSD become more prevalent in patients with AD as disease severity progresses (Fuh, Wang, & Cummings, 2005; Shimabukuro, Awata, & Matsuoka, 2005). Some BPSD, however, follow a clinical course that is relatively independent of cognitive changes (Spalletta, et al., 2004; Tractenberg, Weiner, Cummings, Patterson, & Thal, 2005), and the development of these symptoms may be associated with other neurodegenerative changes that are less closely tied to cognitive deterioration.
The measurement and characterization of white matter changes manifested as hyperintense signals on T2-weighted MRI scans may provide another useful target for exploring the nature of BPSD in AD. White matter hyperintensities (WMH) are commonly seen on the MRI scans of demented and nondemented elderly subjects and have been linked to aging, cerebrovascular risk factors and late life depression (Bigler, et al., 2003; Firbank, et al., 2005; Gunning-Dixon & Raz, 2000). Several studies have failed to detect a relationship between WMH and cognitive impairment in patients with AD (Bigler, et al., 2003; Hirono, Yasuda, Tanimukai, Kitagaki, & Mori, 2000; Starkstein, et al., 1997), suggesting that WMH may reflect aspects of neurodegeneration that are independent of the progression of cognitive symptoms. Neuropathological evidence indicates that the processes associated with WMH involve a variety of changes to the white matter including axonal demyelination, gliosis and degeneration of the ventricular lining (Scheltens, et al., 1995). Increased prevalence of WMH in AD patients has been reported in association with symptoms of depression (Clark, et al., 1998), apathy (Starkstein, et al., 1997), and aberrant motor behaviors (Hirono, et al., 2000), raising the possibility that these changes to the white matter may be associated with the presence and course of BPSD in AD.
The present study explores the relationships between volumetric measures of brain atrophy, WMH, and BPSD in a population of subjects with probable AD. We tested the hypothesis that the presence of specific BPSD in AD would be associated with accelerated neurodegeneration as assessed by volumetric reduction of the whole brain and hippocampus and an increase in white matter changes identified through the quantification of WMH.
2. Methods
2.1 Participants
Seventy-two patients who had completed the Neuropsychiatric Inventory (NPI) (Cummings, 1997) and obtained an MRI scan at the McLean Hospital Neuroimaging Center as part of their clinical assessment were identified using an IRB approved research database of consecutive admissions to the McLean Hospital Memory Diagnostic Clinic. Of these patients, 37 met the National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer's Disease and Related Disorders Association’s (NINCDS-ADRDA) criteria for probable Alzheimer’s disease (AD) (McKhann, et al., 1984), and had a Mini Mental Status Exam (MMSE) (Folstein, Folstein, & McHugh, 1975) score less than or equal to 27. This MMSE cutoff score was used to objectively document cognitive performance at or below the median scores of age and education matched normative populations (Crum, Anthony, Bassett, & Folstein, 1993). These subjects were comprised of 19 women and 18 men, aged 56 to 90+ years (mean age 77.6±8.5 years), with a mean MMSE score of 19.5 (±7.2) as shown in Table 1. No significant gender differences were found for age (F(1, 35) = 1.64, p =0.21) or MMSE score (Z = 0.473, p = 0.64). The McLean Hospital Institutional Review Board approved this retrospective study.
Table 1.
Subject Demographics and MMSE Scores
| N | 37 |
| % Female | 51% |
| Mean Age (±SD) | 77.6 (±8.5) |
| Mean MMSE Score (±SD) | 19.5 (±7.2) |
2.2 Neuropsychiatric Inventory (NPI)
Behavioral symptoms were measured with the Neuropsychiatric Inventory (NPI) (Cummings, 1997), an informant-based questionnaire that assesses 12 neuropsychiatric domains in patients with dementia. The NPI was administered to the caregiver of each patient admitted to the Memory Diagnostic Clinic. The NPI domains assessed were delusions, hallucinations, agitation/aggression, depression/dysphoria, anxiety, apathy/indifference, irritability/lability, elation/euphoria, disinhibition, aberrant motor behavior, nighttime behaviors and appetite/eating change (Cummings, 1997).
2.3 Magnetic Resonance Imaging (MRI)
All patients evaluated by the Memory Diagnostic Clinic were scanned on a 1.5 T GE Signa LX (GE, Milwaukee) unless they had a sufficiently current pre-existing scan or had a contraindication to MRI scanning. The standard clinical scan series included a 3D spoiled gradient recalled acquisition in steady state (3D SPGR) sequence that provides a high resolution, T1 weighted, structural scan with 124 coronal slices (slice thickness 1.5 mm; repetition time (TR) 35 ms, echo time (TE) 5 ms; flip angle 45°); and an axial fluid attenuated inversion recovery (FLAIR) scan (slice thickness 5 mm; TR 9002 ms, TE 133 ms, inversion time 2200 ms, flip angle 90°). The 3D SPGR images of 2 subjects and the FLAIR images of 4 subjects were excluded from analysis due to motion artifacts or scanning parameters that differed from those described above.
2.4 Semi Automated Volumetric Analysis
The 3D SPGR files were converted into Analyze format with the software program MRIcro (Rorden & Brett, 2000), which removed protected health information from the files. Each de-identified image file was then coded with a study identification number, reoriented and cropped to remove portions of the neck. Modified Analyze images were run through the semi automated image analysis program, Structural Image Evaluation using Normalization of Atrophy for cross sectional measurements (SIENAX) (Smith, 2002) (see Figure 1). Using a deformable model, brain and skull surfaces were identified based on a user defined intensity threshold and were used to produce an image containing only brain tissue (Smith, 2002). Two scans were removed during this step due to misclassification of brain tissue. The skull surface was then registered onto a standard atlas to create a normalization-scaling factor (Jenkinson & Smith, 2001; Smith, 2002; Smith, et al., 2002). Using a process based on a hidden Markov random field model and an expectation-maximization algorithm to correct for bias field inhomogeneities, the extracted brain image was segmented into brain tissue and CSF (Zhang, Brady, & Smith, 2001). Whole brain volumes were quantified using estimates of partial volume effects at each voxel (Smith, 2002). These volumes were then multiplied by the previously calculated scaling factor to produce normalized measures of whole brain volume (Smith, et al., 2002). A reliability study of the impact of rater variability on intensity threshold adjustments and initial image cropping with 10 scans and two trained raters, each taking two attempts per scan, yielded high intraclass correlation coefficients (ICC (A,1)) (McGraw & Wong, 1996) for both intra- (scaling factor = 0.996; whole brain = 0.967) and inter- (scaling factor = 0.996; whole brain = 0.923) rater reliability.
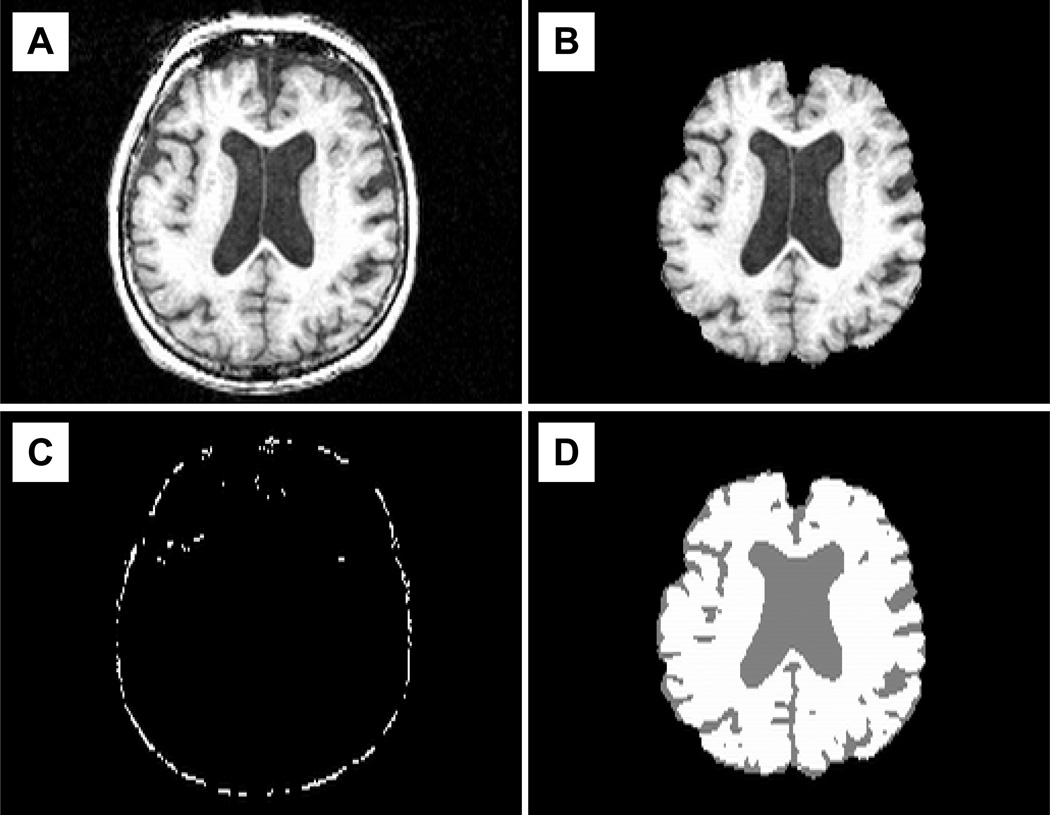
Figure 1. Automated measurement and normalization of whole brain volumes.
1A) T1-weighted images were loaded into the SIENAX algorithm. 1B) This program identifies brain and skull surfaces using a deformable model and produces images containing only brain tissue. 1C) The skull surface is registered to a standard atlas to create a normalization scaling factor. 1D) Voxels representing brain tissue are segmented from those representing cerebrospinal fluid and used to calculate raw whole brain volumes. This raw volume is multiplied by the scaling factor to produce normalized whole brain volumes.
2.5 Hippocampal Measurements
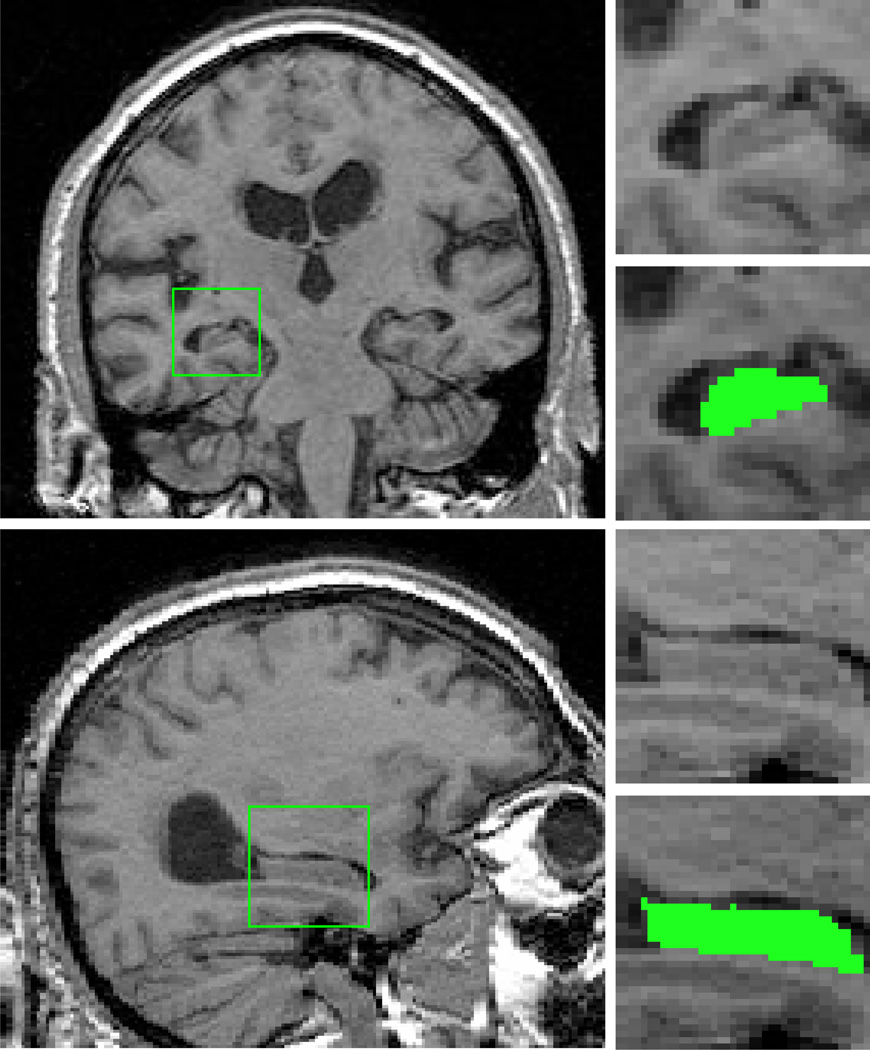
Hippocampal volumes were measured by a trained rater (YB) using MRIcro’s (Rorden & Brett, 2000) region of interest tools (see Figure 2). We defined the boundaries of the hippocampal regions of interest using a neuroanatomical atlas (Duvernoy, 1999) and previously established guidelines for MRI-based, volumetric analysis of the hippocampus (Watson, et al., 1992). These boundaries included the Cornu Ammonis sectors, subiculum, dentate gyrus, alveus, and fimbria and excluded the parahippocampal gyrus and the portion of the hippocampal tail within and posterior to the coronal slice containing the crus of the fornix (Watson, et al., 1992). The identified volumes were then normalized for differences in skull size using the scaling factor calculated by SIENAX. A reliability study of this method was conducted with four trained raters each measuring left and right hippocampal volumes from eight scans, yielding an ICC of 0.926 for inter-rater reliability (McGraw & Wong, 1996).
Figure 2. Coronal and sagittal views of the hippocampus.
2.6 Hyperintensity Quantification
To quantify the volume of FLAIR hyperintensities, we devised a 2-step thresholding process using the software program MRIcro (Rorden & Brett, 2000). In this procedure, threshold parameters were set to include all voxels containing white matter hyperintensities by visual inspection. These settings also identified non-brain voxels, such as the skull and eyes, and therefore manual editing was used to exclude these non-brain voxels (See Figure 3). The volume in milliliters was obtained by multiplying the remaining identified voxels by the voxel dimensions. These volumes were normalized for skull size using the scaling factor obtained from SIENAX. We conducted a reliability study of this method with three trained raters each measuring 23 FLAIR images and inter-rater reliability was high (ICC (A,1) (McGraw & Wong, 1996) = 0.980).
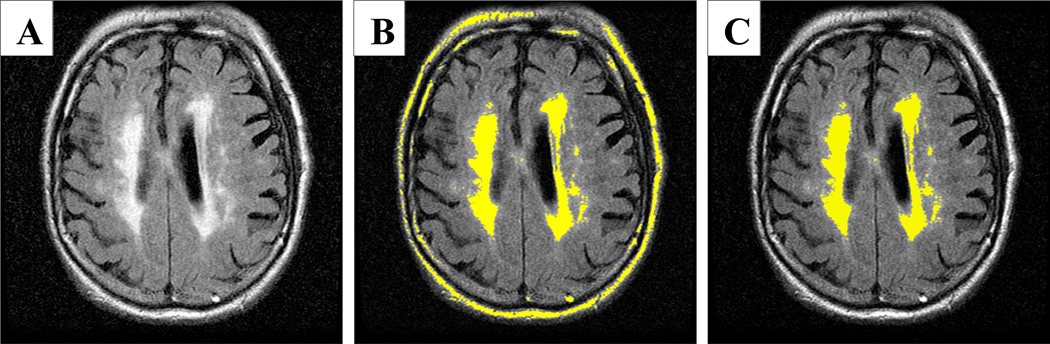
Figure 3. Quantification of FLAIR hyperintensities.
A.) Original fluid attenuated inversion recovery (FLAIR) image. B) Initial threshold parameters set to include all voxels containing hyperintensities. C) Final hyperintensity region of interest with non-brain voxels excluded.
2.7 Statistical Analysis
Subjects were grouped based on the presence of behavioral and psychological symptoms, as assessed by a positive response to the screening questions on the NPI that addressed each symptom domain. Hierarchical clustering using Ward’s minimum variance method was employed to measure associations between the behavioral domains.
Only one subject was reported to have symptoms of elation and therefore this behavior was excluded from the analysis. Thirty three 3D SPGR and 33 FLAIR images were included in the final analysis. Normality assumptions were verified using the Shapiro-Wilk test (W). FLAIR hyperintensity volumes were not normally distributed (W=0.62, p<0.0001) and therefore we used a log transformation to bring the values within the limits of a normal distribution (W=0.97, p=0.64). MMSE scores were not normally distributed (W=0.84, p<0.0001) and therefore nonparametric methods were used for each test that examined this measure. We found no significant differences between left and right hippocampal volumes and therefore combined these volumes into a single measure for analysis.
Pearson Product Moment (Rp) and Spearman Rank (Rs) correlations were used to identify relationships between normalized volumetric measurements of the whole brain, hippocampus and log transformed hyperintensities, age, and MMSE score. Three analysis of covariance (ANCOVA) models were constructed that simultaneously tested the 11 behavioral domains against (1) whole brain volumes, (2) hippocampal volumes and (3) log transformed hyperintensity volumes. Age and MMSE score were applied to the models as covariates in a hierarchical nested design. Age was a significant predictor in all 3 models and was included as a covariate in the final design. MMSE score was not a significant predictor in any of the resulting models and was therefore dropped from further use as a covariate.
3. Results
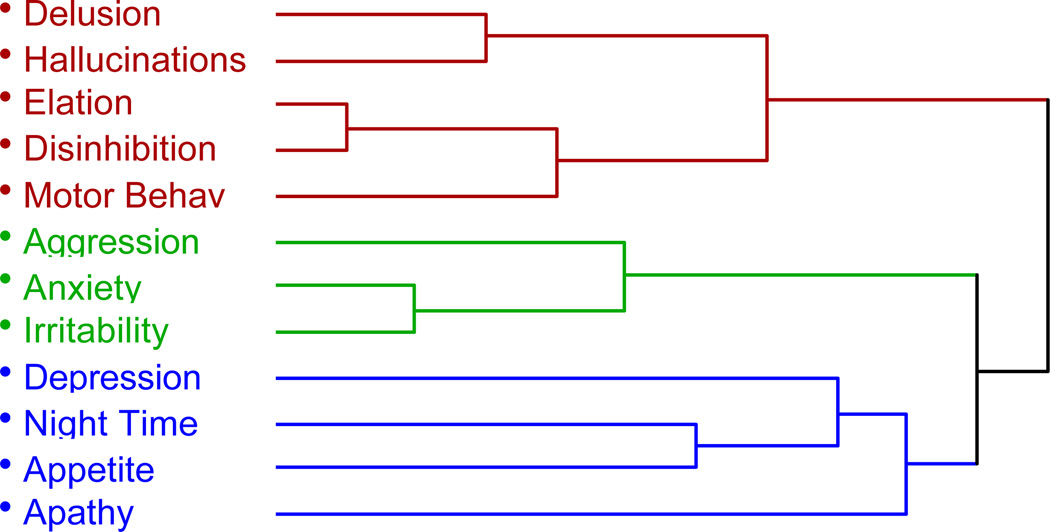
The percentages of subjects with specific BPSD are presented in Table 2. Cluster analysis of the 11 behavioral domains of the NPI suggested a 3-cluster solution (Figure 4). There was strong relatedness between the delusion, hallucination, elation, disinhibition and motor behavior domains suggesting a single cluster of psychotic/disinhibited symptoms. There was also strong relatedness between aggression, anxiety and irritability suggesting an independent cluster of anxious/aggressive symptoms. Finally, there was a weak cluster of depression, night-time behaviors, appetite and possibly apathy suggesting a potential domain of affective/apathetic symptoms.
Table 2.
Frequency of Behavioral and Psychological Symptom of Dementia (BPSD)
| n (%) | |
|---|---|
| Delusions | 12 (32%) |
| Hallucinations | 10 (27%) |
| Aggression | 18 (49%) |
| Depression | 27 (73%) |
| Anxiety | 15 (41%) |
| Apathy | 29 (78%) |
| Disinhibition | 9 (24%) |
| Irritability | 20 (54%) |
| Aberrant Motor Behaviors | 8 (22%) |
| Nightime Behaviors | 23 (62%) |
| Appetite Changes | 17 (46%) |
Figure 4. Dendrogram derived from cluster analysis showing the relatedness between the 11 behavioral symptoms of the NPI based on their presence or absence.
MMSE scores were positively correlated with whole brain volumes (Rs = 0.39, p = 0.025) and hippocampal volumes (Rs = 0.38, p = 0.031). Associations between MMSE scores and decreased log hyperintensity volumes (Rs = −0.31, p = 0.080) and age (Rs = −0.32, p = 0.051) approached but did not reach statistical significance.
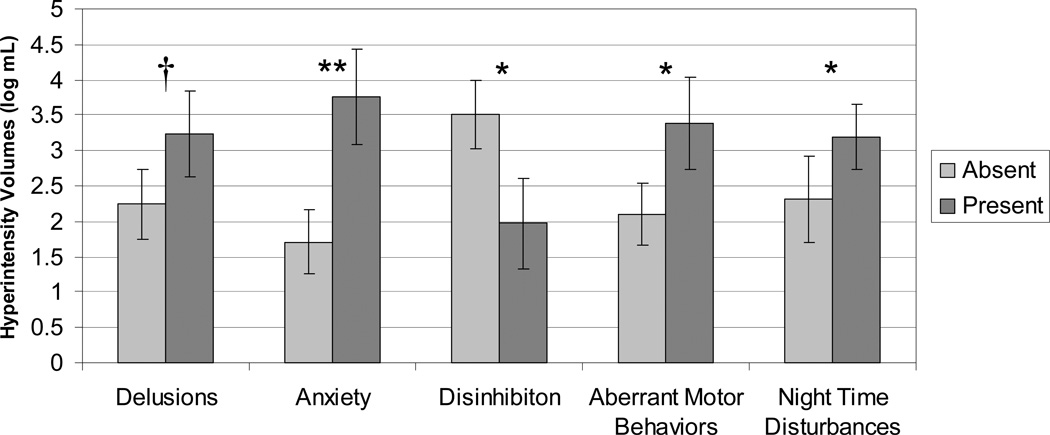
The ANCOVA model testing for differences in hyperintensity volumes using the presence or absence of behavioral and psychological symptoms as independent factors and age as a covariate, yielded a significant overall model (F(12, 20) = 3.14, p = 0.011). Significant main effects were found for the presence of symptoms of anxiety (F(1, 20) = 13.08, p = 0.0017), aberrant motor behavior (F(1, 20) = 6.74, p = 0.017) and sleep disturbance (F(1, 20) = 4.60, p = 0.045) and a trend for the presence of delusions (F(1, 20) = 3.89, p = 0.063), with increased hyperintensity volumes associated with the presence of these symptoms. The main effect for disinhibition (F(1, 20) = 7.48, p = 0.013) revealed reduced hyperintensity volumes for subjects with this behavior (Figure 5).
Figure 5. White matter hyperintensity volumes and the presence of behavioral and psychological symptoms of dementia (BPSD) in probable Alzheimer’s disease.
Least squares means and standard error estimates after covarying for age and other BPSD. † p < 0.10, * p < 0.05, ** p <0.005
The overall ANCOVA model for measures of whole brain volumes and hippocampal volumes were not significant, therefore no further analysis was performed for these measures.
4. Discussion
In this cross sectional and retrospective study, we investigated the relationships between the presence of specific BPSD in patients with AD as assessed by the NPI and MRI-based volumetric measures of white matter changes and brain atrophy. Increased WMH were found to be associated with symptoms of anxiety, aberrant motor behavior, and nighttime disturbance, while symptoms of disinhibition were linked to reduced WMH volume. These findings suggest that white matter changes are associated with the presence of BPSD in patients with AD. We did not find evidence to support the association of BPSD with changes in whole brain or hippocampal volumes.
The prevalence rates of BPSD in this sample of patients with AD were comparable to those reported in other studies (Hart, et al., 2003). Similarly, our findings that normalized measures of whole brain volumes and hippocampal volumes correlated with performance on the MMSE are consistent with previous studies (Bigler, et al., 2004; Horinek, et al., 2006). Although, we were unable to detect a significant correlation between decreasing MMSE scores and WMH load, we did observe a trend in this direction, highlighting the possible cognitive impact of white matter changes in patients with AD.
We were not able to detect any significant relationships between hippocampal volumes and BPSD. This negative finding suggests that degeneration of the hippocampus may not be closely correlated with the emergence of BPSD in AD as it is with global cognitive decline and memory impairment (Petersen, et al., 2000). Other researchers have also failed to demonstrate a significant association between decreasing volume of limbic structures, including the hippocampus and amygdala, and BPSD in AD (Horinek, et al., 2006). Taken together, these negative findings are suggestive that the pathophysiological processes responsible for cognitive decline in AD may be independent of the processes underlying the emergence of BPSD.
Our hypothesis that the presence of BPSD would be associated with increased WMH in patients with AD was supported in the case of aberrant motor behaviors, sleep disturbance and anxiety. These findings are consistent with a previous study that reported a positive correlation of aberrant motor behavior scores with WMH volumes in patients with AD (Hirono, et al., 2000).
Increased WMH were also associated with symptoms of anxiety and sleep disturbance in our patients with AD, suggesting that white matter changes observed as WMH are important correlates of these symptoms. Anxiety and sleep disturbance are common and often co-occur in patients with AD (McCurry, Gibbons, Logsdon, & Teri, 2004; Moran, et al., 2005) and the present findings suggest these two symptoms may be linked to common pathological processes occurring in the white matter. Patterns of extensive WMH have been linked to ischemic damage, suggesting that the processes involved in these findings may include vascular pathology of the white matter (DeCarli, Fletcher, Ramey, Harvey, & Jagust, 2005). This explanation would be consistent with previous reports, which have associated symptoms of anxiety and sleep disturbance with vascular pathology in demented and non-demented patients.
Anxiety has been reported to be more prevalent and severe in demented patients with vascular pathology compared to those without (Ballard, et al., 2000; Kim, Lyons, Shin, & Yoon, 2003; Porter, et al., 2003; Sultzer, Levin, Mahler, High, & Cummings, 1993). In studies examining the incidence of BPSD in patients with vascular dementia (VaD) and AD, anxiety symptoms were reported to be about twice as common in patients with VaD compared to patients with AD (Ballard, et al., 2000; Porter, et al., 2003). Similarly, the severity of anxious symptoms in dementia patients with VaD has been reported to be greater (Kim, et al., 2003; Sultzer, et al., 1993). Anxiety has also been shown to be a common symptom in patients after cerebrovascular lesions (Chemerinski & Levine, 2006; Morrison, Pollard, Johnston, & MacWalter, 2005).
Similarly, sleep disturbance has also been associated with vascular pathology in studies examining the incidence and severity of BPSD. BPSD profiles of dementia patients meeting DSM-IV criteria (Association, 1994) for VaD have more severe sleep disturbance than AD patients who had no evidence of “silent” lacunes or cortical infarcts (Fuh, et al., 2005). In another study of individuals with AD, which used pathological diagnosis to separate subjects into groups with and without ischemic brain lesions, AD patients with ischemic brain lesions were reported to have more sleep disturbances (Del Ser, Hachinski, Merskey, & Munoz, 2005). Similarly, other studies have shown that patients with multiinfarct dementia, as assessed by CT and DSM III criteria, have more sleep disturbed breathing, and disorganized sleep-wake cycles than AD patients without vascular brain lesions (Aharon-Peretz, et al., 1991; Erkinjuntti, et al., 1987; Mishima, et al., 1997). Sleep disordered breathing syndromes are associated with vascular risk factors (McArdle, Hillman, Beilin, & Watts, 2007), and may play a causative role in the development of WMH. Sleep disturbance is also strongly associated with strokes (Bassetti, Milanova, & Gugger, 2006; Good, Henkle, Gelber, Welsh, & Verhulst, 1996). In a study examining the relationship between CT white matter lesions and sleep patterns in elderly patients with dementia, the presence of periventricular white matter lesions was associated with increased hours of sleep during the day and decreased sleep at night (Meguro, et al., 1995). Consistent with these findings, our current results provide additional evidence that vascular pathology is associated with the emergence of symptoms of anxiety and nighttime disturbance in AD. Further studies are needed, however, to determine the nature of this relationship.
Contrary to our hypothesis, symptoms of disinhibition were found to be associated with reduced WMH load, suggesting that AD patients with increased WMH were less likely to exhibit these symptoms. This unexpected finding may reflect the importance of alternate neurodegenerative processes in the development of disinhibited behavior that occur independently of white matter changes, such as frontal gray matter degeneration (Rosen, et al., 2005). This finding may also indicate that the constellation of BPSD in AD associated with WMH does not typically include disinhibition. Symptoms of disinhibition are relatively rare in AD, but still occur in 24–3% of cases (Hart, et al., 2003) as was seen in the current sample (24%). These symptoms have been associated with advanced dementia severity (Shimabukuro, et al., 2005), and reduced brain volume (Hirono, et al., 2000). In the present study, however, we were unable to associate the presence or absence of disinhibition on NPI with differences in MMSE scores or brain volumes.
In this study, we were unable to replicate previously reported associations of WMH with symptoms of depression (Barber, et al., 1999; O'Brien, et al., 2002) apathy (Starkstein, et al., 1997) and delusions (Lee, et al., 2006) in patients with AD. This may be due in part to our methods of classifying BPSD based on the presence or absence of symptoms rather than using a graded scale of severity in our analysis. Subclinical symptoms of apathy and depression are highly prevalent in AD and occurred in about 75% of our sample, suggesting that our methods of BPSD classification may have emphasized sensitivity at the expense of specificity for common symptoms. Our findings regarding symptoms of anxiety and sleep disturbance may relate to previous studies of depressive symptoms as these symptoms often overlap and many depression-rating scales include insomnia and anxiety dimensions in the total depression scores. Some of the most robust and repeatable findings in these three BPSD dimensions involve hypometabolism of specific regions measured by FDG-PET (Hirono, et al., 1998), (Sultzer, et al., 2003), (Holthoff, et al., 2005). We did detect a trend linking increased WMH with symptoms of delusions and this lends partial support for the association of WMH and delusions in patients with AD (Lee, et al., 2006).
Another possible source for discrepancies with previous studies (Barber, et al., 1999; Lee, et al., 2006; Starkstein, et al., 1997) may be due to our use of volumetric measures instead of visual rating scales to quantify WMH load (Scheltens, et al., 1993). Volumetric measuring techniques have the advantage of being unbiased and robust in their reliability and have been reported to be more sensitive than visual rating scales at detecting relationships between WMH and clinical measures (van Straaten, et al., 2006). These measures, however, did not discriminate between types of WMH based on location or distribution and this lack of specificity may have been a limitation of this study.
Conclusion
Changes to the white matter as measured by increased WMH volume were associated with symptoms of aberrant motor behaviors, anxiety and sleep disturbance in patients with AD. These finding indicate a potential role of superimposed vascular pathology in AD contributing to the etiology of these symptoms. Our study failed to detect associations of any BPSD with hippocampal atrophy and whole brain volume, suggesting that BPSD and cognitive decline in AD may progress independently. Additional studies investigating these relationships may provide insight to future directions for the diagnosis and treatment of BPSD in Alzheimer’s disease.
Acknowledgments
Supported by NIH R01 AG020654 and the Harvard Center for Neurodegeneration and Repair.
Footnotes
No disclosures to report.
References
- Aharon-Peretz J, Masiah A, Pillar T, Epstein R, Tzischinsky O, Lavie P. Sleep-wake cycles in multi-infarct dementia and dementia of the Alzheimer type. Neurology. 1991;41(10):1616–1619. doi: 10.1212/wnl.41.10.1616. lost in the final stages of Alzheimer's disease. [DOI] [PubMed] [Google Scholar]
- Assal F, Cummings JL. Neuropsychiatric symptoms in the dementias. Curr Opin Neurol. 2002;15(4):445–450. doi: 10.1097/00019052-200208000-00007. [DOI] [PubMed] [Google Scholar]
- Association AP. Diagnostic and Statistical Manual of Mental Disorders. (4th ed.) Washington D.C: American Psychiatric Association; 1994. [Google Scholar]
- Ballard C, Neill D, O'Brien J, McKeith IG, Ince P, Perry R. Anxiety, depression and psychosis in vascular dementia: prevalence and associations. J Affect Disord. 2000;59(2):97–106. doi: 10.1016/s0165-0327(99)00057-9. [DOI] [PubMed] [Google Scholar]
- Barber R, Scheltens P, Gholkar A, Ballard C, McKeith I, Ince P, et al. White matter lesions on magnetic resonance imaging in dementia with Lewy bodies, Alzheimer's disease, vascular dementia, and normal aging. J Neurol Neurosurg Psychiatry. 1999;67(1):66–72. doi: 10.1136/jnnp.67.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassetti CL, Milanova M, Gugger M. Sleep-disordered breathing and acute ischemic stroke: diagnosis, risk factors, treatment, evolution, and long-term clinical outcome. Stroke. 2006;37(4):967–972. doi: 10.1161/01.STR.0000208215.49243.c3. [DOI] [PubMed] [Google Scholar]
- Beeri MS, Werner P, Davidson M, Noy S. The cost of behavioral and psychological symptoms of dementia (BPSD) in community dwelling Alzheimer's disease patients. Int J Geriatr Psychiatry. 2002;17(5):403–408. doi: 10.1002/gps.490. [DOI] [PubMed] [Google Scholar]
- Berger G, Bernhardt T, Weimer E, Peters J, Kratzsch T, Frolich L. Longitudinal study on the relationship between symptomatology of dementia and levels of subjective burden and depression among family caregivers in memory clinic patients. J Geriatr Psychiatry Neurol. 2005;18(3):119–128. doi: 10.1177/0891988704273375. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Lowry CM, Kerr B, Tate DF, Hessel CD, Earl HD, et al. Role of white matter lesions, cerebral atrophy, and APOE on cognition in older persons with and without dementia: the Cache County, Utah, study of memory and aging. Neuropsychology. 2003;17(3):339–352. doi: 10.1037/0894-4105.17.3.339. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Neeley ES, Miller MJ, Tate DF, Rice SA, Cleavinger H, et al. Cerebral volume loss, cognitive deficit and neuropsychological performance: comparative measures of brain atrophy: I Dementia. J Int Neuropsychol Soc. 2004;10(3):442–452. doi: 10.1017/S1355617704103111. [DOI] [PubMed] [Google Scholar]
- Chemerinski E, Levine SR. Neuropsychiatric disorders following vascular brain injury. Mt Sinai J Med. 2006;73(7):1006–1014. [PubMed] [Google Scholar]
- Clark LM, McDonald WM, Welsh-Bohmer KA, Siegler IC, Dawson DV, Tupler LA, et al. Magnetic resonance imaging correlates of depression in early- and late-onset Alzheimer's disease. Biol Psychiatry. 1998;44(7):592–599. doi: 10.1016/s0006-3223(98)00106-1. [DOI] [PubMed] [Google Scholar]
- Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. Jama. 1993;269(18):2386–2391. [PubMed] [Google Scholar]
- Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology. 1997;48(5) Suppl 6:S10–S16. doi: 10.1212/wnl.48.5_suppl_6.10s. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ. Anatomical mapping of white matter hyperintensities (WMH): exploring the relationships between periventricular, WMH deep WMH, total WMH burden. Stroke. 2005;36(1):50–55. doi: 10.1161/01.STR.0000150668.58689.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Ser T, Hachinski V, Merskey H, Munoz DG. Alzheimer's disease with and without cerebral infarcts. J Neurol Sci. 2005;231(1–2):3–11. doi: 10.1016/j.jns.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. The Human Brain: Surface, Three-Dimensional Sectional Anatomy with MRI, Blood Supply. New York: Springer; 1999. [Google Scholar]
- Erkinjuntti T, Partinen M, Sulkava R, Telakivi T, Salmi T, Tilvis R. Sleep apnea in multiinfarct dementia and Alzheimer's disease. Sleep. 1987;10(5):419–425. doi: 10.1093/sleep/10.5.419. [DOI] [PubMed] [Google Scholar]
- Firbank MJ, O'Brien JT, Pakrasi S, Pantoni L, Simoni M, Erkinjuntti T, et al. White matter hyperintensities and depression--preliminary results from the LADIS study. Int J Geriatr Psychiatry. 2005;20(7):674–679. doi: 10.1002/gps.1342. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fuh JL, Wang SJ, Cummings JL. Neuropsychiatric profiles in patients with Alzheimer's disease and vascular dementia. J Neurol Neurosurg Psychiatry. 2005;76(10):1337–1341. doi: 10.1136/jnnp.2004.056408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good DC, Henkle JQ, Gelber D, Welsh J, Verhulst S. Sleep-disordered breathing and poor functional outcome after stroke. Stroke. 1996;27(2):252–259. doi: 10.1161/01.str.27.2.252. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14(2):224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Hart DJ, Craig D, Compton SA, Critchlow S, Kerrigan BM, McIlroy SP, et al. A retrospective study of the behavioural and psychological symptoms of mid and late phase Alzheimer's disease. Int J Geriatr Psychiatry. 2003;18(11):1037–1042. doi: 10.1002/gps.1013. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60(8):1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- Hirono N, Mori E, Ishii K, Ikejiri Y, Imamura T, Shimomura T, et al. Frontal lobe hypometabolism and depression in Alzheimer's disease. Neurology. 1998;50(2):380–383. doi: 10.1212/wnl.50.2.380. [DOI] [PubMed] [Google Scholar]
- Hirono N, Yasuda M, Tanimukai S, Kitagaki H, Mori E. Effect of the apolipoprotein E epsilon4 allele on white matter hyperintensities in dementia. Stroke. 2000;31(6):1263–1268. doi: 10.1161/01.str.31.6.1263. [DOI] [PubMed] [Google Scholar]
- Holthoff VA, Beuthien-Baumann B, Kalbe E, Ludecke S, Lenz O, Zundorf G, et al. Regional cerebral metabolism in early Alzheimer's disease with clinically significant apathy or depression. Biol Psychiatry. 2005;57(4):412–421. doi: 10.1016/j.biopsych.2004.11.035. [DOI] [PubMed] [Google Scholar]
- Horinek D, Petrovicky P, Hort J, Krasensky J, Brabec J, Bojar M, et al. Amygdalar volume and psychiatric symptoms in Alzheimer's disease: an MRI analysis. Acta Neurol Scand. 2006;113(1):40–45. doi: 10.1111/j.1600-0404.2006.00540.x. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kim JM, Lyons D, Shin IS, Yoon JS. Differences in the behavioral and psychological symptoms between Alzheimer's disease and vascular dementia: are the different pharmacologic treatment strategies justifiable? Hum Psychopharmacol. 2003;18(3):215–220. doi: 10.1002/hup.466. [DOI] [PubMed] [Google Scholar]
- Lee DY, Choo IH, Kim KW, Jhoo JH, Youn JC, Lee UY, et al. White matter changes associated with psychotic symptoms in Alzheimer's disease patients. J Neuropsychiatry Clin Neurosci. 2006;18(2):191–198. doi: 10.1176/jnp.2006.18.2.191. [DOI] [PubMed] [Google Scholar]
- McArdle N, Hillman D, Beilin L, Watts G. Metabolic risk factors for vascular disease in obstructive sleep apnea: a matched controlled study. Am J Respir Crit Care Med. 2007;175(2):190–195. doi: 10.1164/rccm.200602-270OC. [DOI] [PubMed] [Google Scholar]
- McCurry SM, Gibbons LE, Logsdon RG, Teri L. Anxiety and nighttime behavioral disturbances. Awakenings in patients with Alzheimer's disease. J Gerontol Nurs. 2004;30(1):12–20. doi: 10.3928/0098-9134-20040101-05. [DOI] [PubMed] [Google Scholar]
- McGraw KO, Wong SO. Forming inferences about some intraclass correlation coefficients. Psychological Methods. 1996;1:30–46. [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Meguro K, Ueda M, Kobayashi I, Yamaguchi S, Yamazaki H, Oikawa Y, et al. Sleep disturbance in elderly patients with cognitive impairment, decreased daily activity and periventricular white matter lesions. Sleep. 1995;18(2):109–114. doi: 10.1093/sleep/18.2.109. [DOI] [PubMed] [Google Scholar]
- Mishima K, Okawa M, Satoh K, Shimizu T, Hozumi S, Hishikawa Y. Different manifestations of circadian rhythms in senile dementia of Alzheimer's type and multi-infarct dementia. Neurobiol Aging. 1997;18(1):105–109. doi: 10.1016/s0197-4580(96)00167-4. [DOI] [PubMed] [Google Scholar]
- Mok WY, Chu LW, Chung CP, Chan NY, Hui SL. The relationship between non-cognitive symptoms and functional impairment in Alzheimer's disease. Int J Geriatr Psychiatry. 2004;19(11):1040–1046. doi: 10.1002/gps.1207. [DOI] [PubMed] [Google Scholar]
- Moran M, Lynch CA, Walsh C, Coen R, Coakley D, Lawlor BA. Sleep disturbance in mild to moderate Alzheimer's disease. Sleep Med. 2005;6(4):347–352. doi: 10.1016/j.sleep.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Morrison V, Pollard B, Johnston M, MacWalter R. Anxiety and depression 3 years following stroke: demographic, clinical, and psychological predictors. J Psychosom Res. 2005;59(4):209–213. doi: 10.1016/j.jpsychores.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Murman DL, Chen Q, Powell MC, Kuo SB, Bradley CJ, Colenda CC. The incremental direct costs associated with behavioral symptoms in AD. Neurology. 2002;59(11):1721–1729. doi: 10.1212/01.wnl.0000036904.73393.e4. [DOI] [PubMed] [Google Scholar]
- Murphy DG, DeCarli CD, Daly E, Gillette JA, McIntosh AR, Haxby JV, et al. Volumetric magnetic resonance imaging in men with dementia of the Alzheimer type: correlations with disease severity. Biol Psychiatry. 1993;34(9):612–621. doi: 10.1016/0006-3223(93)90153-5. [DOI] [PubMed] [Google Scholar]
- O'Brien JT, Wiseman R, Burton EJ, Barber B, Wesnes K, Saxby B, et al. Cognitive associations of subcortical white matter lesions in older people. Ann N Y Acad Sci. 2002;977:436–444. doi: 10.1111/j.1749-6632.2002.tb04849.x. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Jack CR, Jr, Xu YC, Waring SC, O'Brien PC, Smith GE, et al. Memory and MRI-based hippocampal volumes in aging and AD. Neurology. 2000;54(3):581–587. doi: 10.1212/wnl.54.3.581. [DOI] [PubMed] [Google Scholar]
- Porter VR, Buxton WG, Fairbanks LA, Strickland T, O'Connor SM, Rosenberg-Thompson S, et al. Frequency and characteristics of anxiety among patients with Alzheimer's disease and related dementias. J Neuropsychiatry Clin Neurosci. 2003;15(2):180–186. doi: 10.1176/jnp.15.2.180. [DOI] [PubMed] [Google Scholar]
- Rapoport MJ, van Reekum R, Freedman M, Streiner D, Simard M, Clarke D, et al. Relationship of psychosis to aggression, apathy and function in dementia. Int J Geriatr Psychiatry. 2001;16(2):123–130. doi: 10.1002/1099-1166(200102)16:2<123::aid-gps260>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12(4):191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128(Pt 11):2612–2625. doi: 10.1093/brain/awh628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Brandt J, Albert M, Hadjigeorgiou G, Papadimitriou A, Dubois B, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62(10):1601–1608. doi: 10.1001/archneur.62.10.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheltens P, Barkhof F, Leys D, Pruvo JP, Nauta JJ, Vermersch P, et al. A semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J Neurol Sci. 1993;114(1):7–12. doi: 10.1016/0022-510x(93)90041-v. [DOI] [PubMed] [Google Scholar]
- Scheltens P, Barkhof F, Leys D, Wolters EC, Ravid R, Kamphorst W. Histopathologic correlates of white matter changes on MRI in Alzheimer's disease and normal aging. Neurology. 1995;45(5):883–888. doi: 10.1212/wnl.45.5.883. [DOI] [PubMed] [Google Scholar]
- Shimabukuro J, Awata S, Matsuoka H. Behavioral and psychological symptoms of dementia characteristic of mild Alzheimer patients. Psychiatry Clin Neurosci. 2005;59(3):274–279. doi: 10.1111/j.1440-1819.2005.01371.x. [DOI] [PubMed] [Google Scholar]
- Shin IS, Carter M, Masterman D, Fairbanks L, Cummings JL. Neuropsychiatric symptoms and quality of life in Alzheimer disease. Am J Geriatr Psychiatry. 2005;13(6):469–474. doi: 10.1176/appi.ajgp.13.6.469. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17(1):479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- Spalletta G, Baldinetti F, Buccione I, Fadda L, Perri R, Scalmana S, et al. Cognition and behaviour are independent and heterogeneous dimensions in Alzheimer's disease. J Neurol. 2004;251(6):688–695. doi: 10.1007/s00415-004-0403-6. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Sabe L, Vazquez S, Di Lorenzo G, Martinez A, Petracca G, et al. Neuropsychological, psychiatric, and cerebral perfusion correlates of leukoaraiosis in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1997;63(1):66–73. doi: 10.1136/jnnp.63.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele C, Rovner B, Chase GA, Folstein M. Psychiatric symptoms and nursing home placement of patients with Alzheimer's disease. Am J Psychiatry. 1990;147(8):1049–1051. doi: 10.1176/ajp.147.8.1049. [DOI] [PubMed] [Google Scholar]
- Stout JC, Jernigan TL, Archibald SL, Salmon DP. Association of dementia severity with cortical gray matter and abnormal white matter volumes in dementia of the Alzheimer type. Arch Neurol. 1996;53(8):742–749. doi: 10.1001/archneur.1996.00550080056013. [DOI] [PubMed] [Google Scholar]
- Sultzer DL, Brown CV, Mandelkern MA, Mahler ME, Mendez MF, Chen ST, et al. Delusional thoughts and regional frontal/temporal cortex metabolism in Alzheimer's disease. Am J Psychiatry. 2003;160(2):341–349. doi: 10.1176/appi.ajp.160.2.341. [DOI] [PubMed] [Google Scholar]
- Sultzer DL, Levin HS, Mahler ME, High WM, Cummings JL. A comparison of psychiatric symptoms in vascular dementia and Alzheimer's disease. Am J Psychiatry. 1993;150(12):1806–1812. doi: 10.1176/ajp.150.12.1806. [DOI] [PubMed] [Google Scholar]
- Tractenberg RE, Weiner MF, Cummings JL, Patterson MB, Thal LJ. Independence of changes in behavior from cognition and function in community-dwelling persons with Alzheimer's disease: a factor analytic approach. J Neuropsychiatry Clin Neurosci. 2005;17(1):51–60. doi: 10.1176/appi.neuropsych.17.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Straaten EC, Fazekas F, Rostrup E, Scheltens P, Schmidt R, Pantoni L, et al. Impact of white matter hyperintensities scoring method on correlations with clinical data: the LADIS study. Stroke. 2006;37(3):836–840. doi: 10.1161/01.STR.0000202585.26325.74. [DOI] [PubMed] [Google Scholar]
- Watson C, Andermann F, Gloor P, Jones-Gotman M, Peters T, Evans A, et al. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology. 1992;42(9):1743–1750. doi: 10.1212/wnl.42.9.1743. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]