Abstract
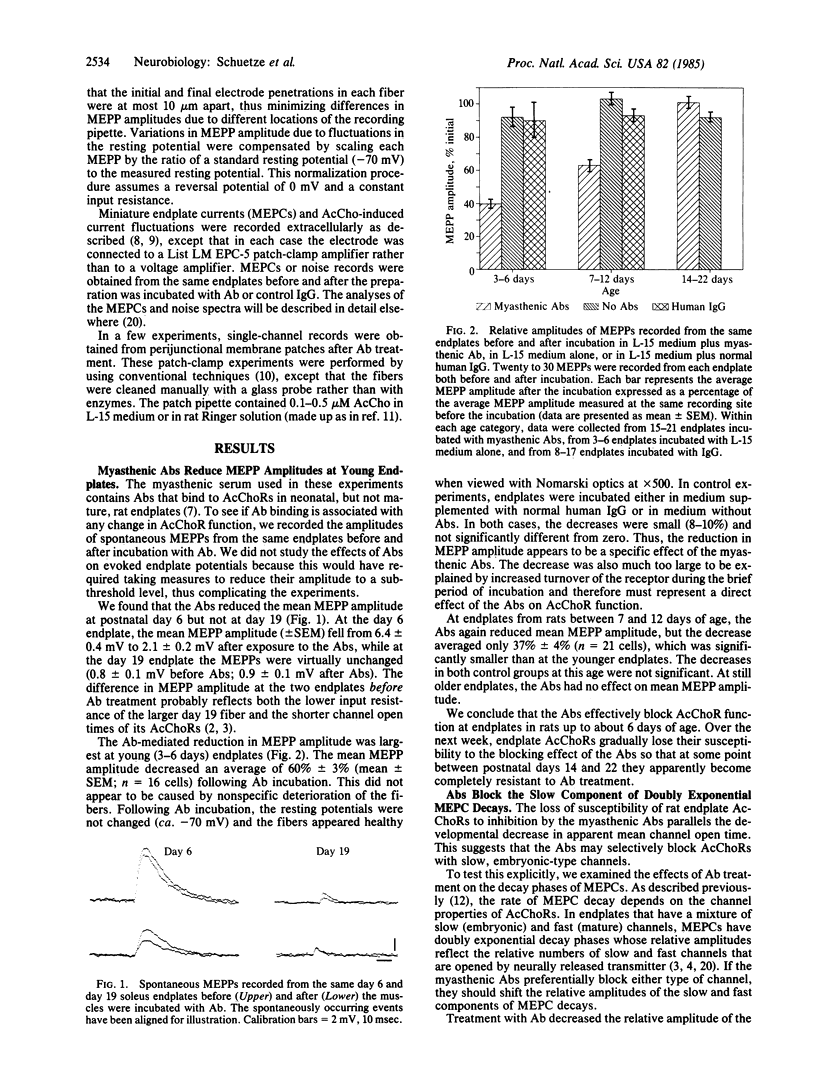
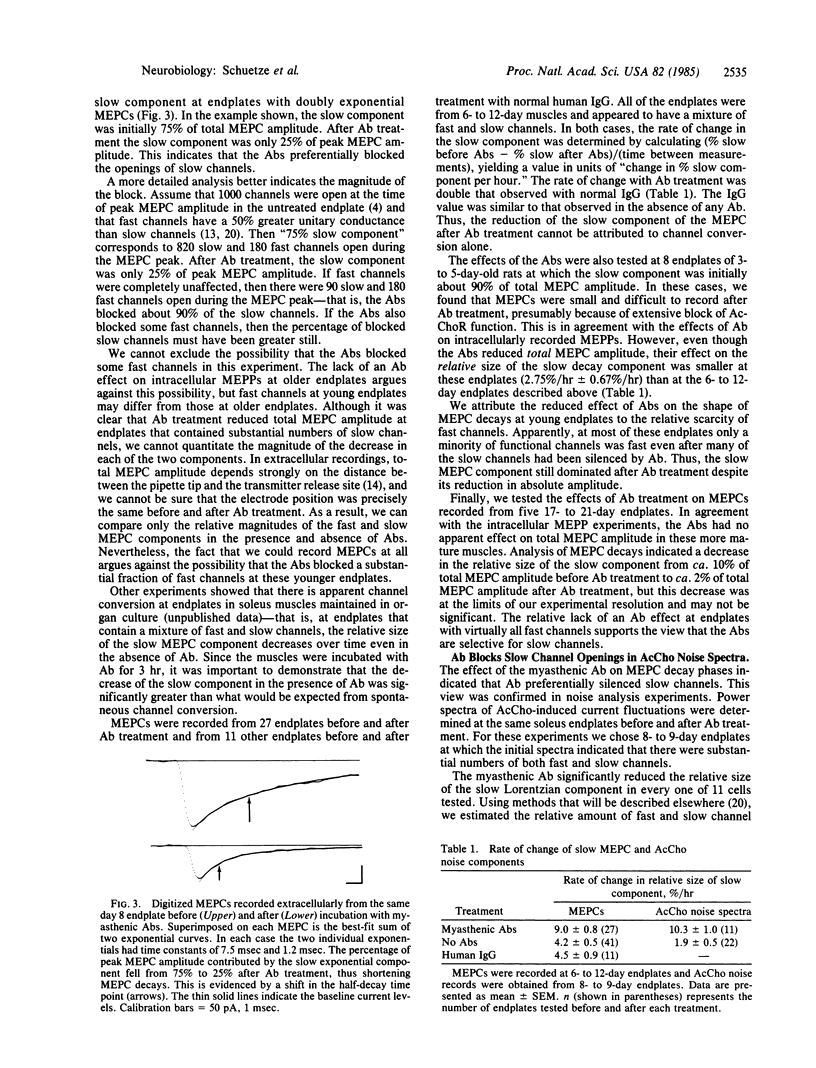
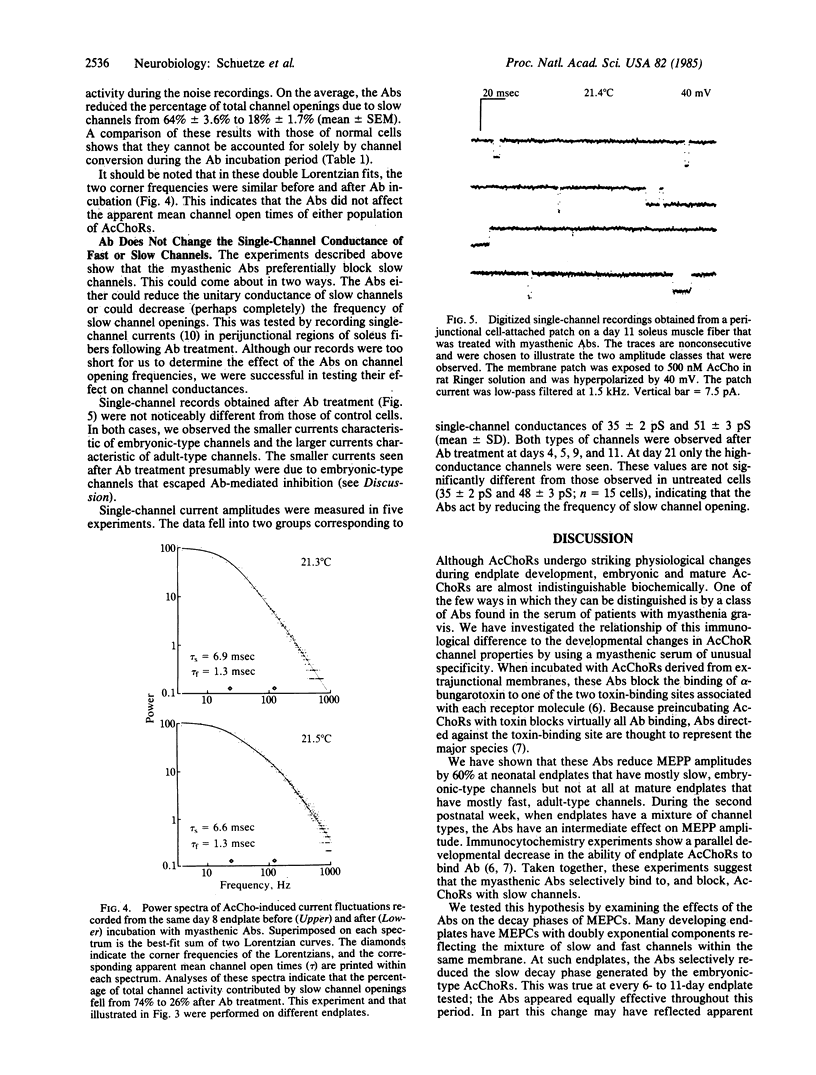
We have examined the physiological effects of antibodies from a highly specific myasthenic serum on acetylcholine receptors at developing rat endplates. The antibodies reduced the amplitude of miniature endplate potentials by 60% in 3- to 6-day-old animals but had no effect after day 14. Between days 7 and 12 the antibodies had an intermediate effect. This is the same period during which acetylcholine receptors with long channel open times (slow channels) disappear and receptors with short open times (fast channels) increase in number. Therefore, we examined the effect of the antibodies at endplates with a mixture of channel types more carefully. At all times tested, both noise analysis and analysis of miniature endplate currents showed that the antibodies reduced slow channel activity selectively. Single-channel recordings indicated that acetylcholine receptors that remained active after antibody treatment had normal gating properties. Thus, the antibodies appeared to silence slow channels selectively.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albuquerque E. X., Rash J. E., Mayer R. F., Satterfield J. R. An electrophysiological and morphological study of the neuromuscular junction in patients with myasthenia gravis. Exp Neurol. 1976 Jun;51(3):536–563. doi: 10.1016/0014-4886(76)90179-5. [DOI] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fluctuations in the microsecond time range of the current through single acetylcholine receptor ion channels. Nature. 1981 Dec 3;294(5840):464–466. doi: 10.1038/294464a0. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Miledi R., Trautmann A. End-plate currents and acetylcholine noise at normal and myasthenic human end-plates. J Physiol. 1979 Feb;287:247–265. doi: 10.1113/jphysiol.1979.sp012657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Ziskind-Conhaim L., Harris A. J. Development of neuromuscular junctions in rat embryos. Dev Biol. 1981 Jan 30;81(2):266–279. doi: 10.1016/0012-1606(81)90290-6. [DOI] [PubMed] [Google Scholar]
- Dwyer D. S., Bradley R. J., Furner R. L., Kemp G. E. Immunochemical properties of junctional and extrajunctional acetylcholine receptor. Brain Res. 1981 Jul 27;217(1):23–40. doi: 10.1016/0006-8993(81)90182-7. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M. Control of acetylcholine receptors in skeletal muscle. Physiol Rev. 1979 Jan;59(1):165–227. doi: 10.1152/physrev.1979.59.1.165. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M., Drachman D. B., Satyamurti S. Neuromuscular junction in myasthenia gravis: decreased acetylcholine receptors. Science. 1973 Oct 19;182(4109):293–295. doi: 10.1126/science.182.4109.293. [DOI] [PubMed] [Google Scholar]
- Fischbach G. D., Schuetze S. M. A post-natal decrease in acetylcholine channel open time at rat end-plates. J Physiol. 1980 Jun;303:125–137. doi: 10.1113/jphysiol.1980.sp013275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E., Fischbach G. D. Early events in neuromuscular junction formation in vitro: induction of acetylcholine receptor clusters in the postsynaptic membrane and morphology of newly formed synapses. J Cell Biol. 1979 Oct;83(1):143–158. doi: 10.1083/jcb.83.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Sakmann B. Multiple conductance states of single acetylcholine receptor channels in embryonic muscle cells. Nature. 1981 Dec 3;294(5840):462–464. doi: 10.1038/294462a0. [DOI] [PubMed] [Google Scholar]
- Michler A., Sakmann B. Receptor stability and channel conversion in the subsynaptic membrane of the developing mammalian neuromuscular junction. Dev Biol. 1980 Nov;80(1):1–17. doi: 10.1016/0012-1606(80)90494-7. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Brenner H. R. Change in synaptic channel gating during neuromuscular development. Nature. 1978 Nov 23;276(5686):401–402. doi: 10.1038/276401a0. [DOI] [PubMed] [Google Scholar]
- Schuetze S. M., Frank E. F., Fischbach G. D. Channel open time and metabolic stability of synaptic and extrasynaptic acetylcholine receptors on cultured chick myotubes. Proc Natl Acad Sci U S A. 1978 Jan;75(1):520–523. doi: 10.1073/pnas.75.1.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetze S. M. The acetylcholine channel open time in chick muscle is not decreased following innervation. J Physiol. 1980 Jun;303:111–124. doi: 10.1113/jphysiol.1980.sp013274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg C. B., Hall Z. W. Antibodies from patients with myasthenia gravis recognize determinants unique to extrajunctional acetylcholine receptors. Proc Natl Acad Sci U S A. 1979 Jan;76(1):504–508. doi: 10.1073/pnas.76.1.504. [DOI] [PMC free article] [PubMed] [Google Scholar]


