Opportunistic infections occur at an increased rate in patients with inflammatory bowel disease (IBD), largely due to the use of immunosuppressants. One study found that the odds ratio for opportunistic infections in immunosuppressed patients compared to nonimmunosuppressed patients was 2.6 for patients on 1 drug and increased to 12.9 when 2 or more drugs were used.1 Varicella zoster virus (VZV) is the causative agent of chickenpox and herpes zoster, and case studies have reported disseminated infections caused by VZV in patients on tumor necrosis factor α (TNFα) inhibitors.2-4 Although the incidence of infection can be decreased through vaccination, the VZV vaccine utilizes a live attenuated virus and is contraindicated in patients who are immunosuppressed.5 This report presents a case of disseminated VZV infection with encephalitis in an unvaccinated patient with ulcerative colitis (UC) who was receiving infliximab (Remicade, Janssen Biotech).
Case Report
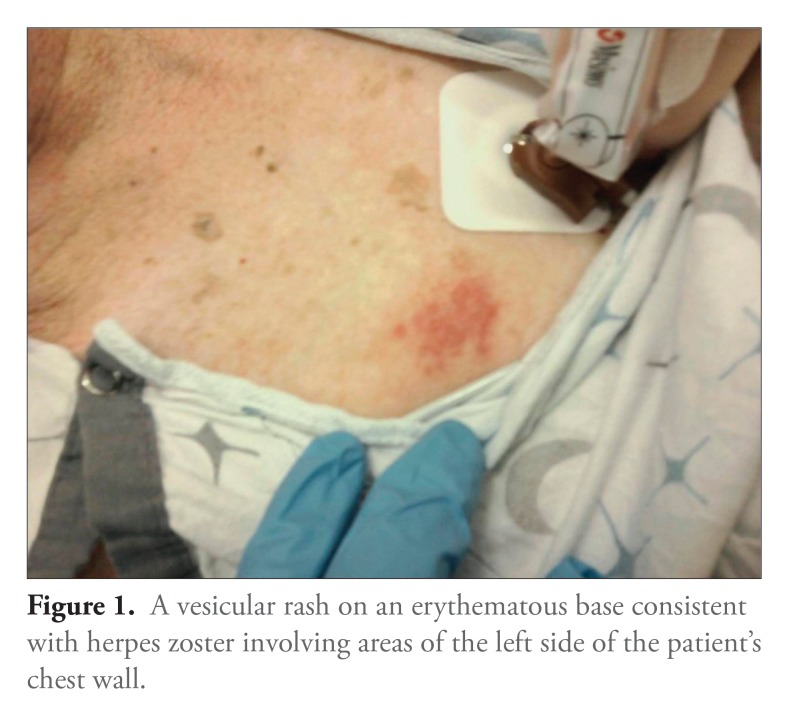
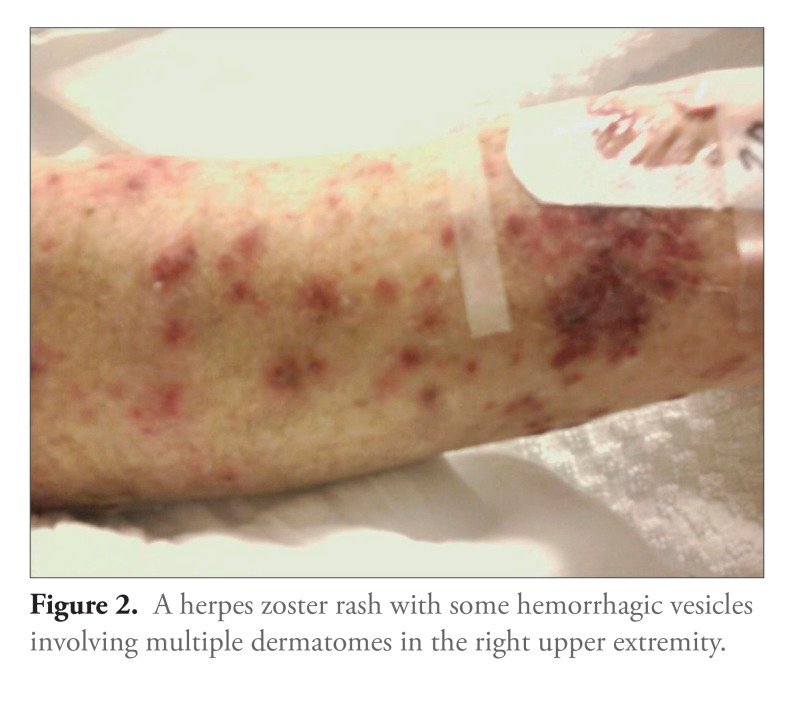
A 71-year-old man with long-standing UC who had been maintained for years on infliximab presented to the emergency department with altered mental status, gait instability, and progressive weakness over several days. His last infliximab infusion had been 2 weeks prior to presentation. The patients vital signs were notable for a temperature of 38.6°C and tachycardia. His physical examination revealed a vesicular rash with erythematous bases, including some hemorrhagic vesicles, that affected multiple dermatomes on the right upper extremity and left chest (Figures 1 and 2). His mental status waxed and waned during the initial evaluation. His neurologic examination was nonfocal, and meningeal signs were negative. Blood and urine samples were cultured, and the patient was started on broad-spectrum antibiotics and acyclovir prior to neuroimaging and lumbar puncture.
Figure 1.
A vesicular rash on an erythematous base consistent with herpes zoster involving areas of the left side of the patient’s chest wall.
Figure 2.
A herpes zoster rash with some hemorrhagic vesicles involving multiple dermatomes in the right upper extremity.
Magnetic resonance imaging showed chronic small vessel disease that was unchanged from a previous examination in 2008. An analysis of the patient's cerebrospinal fluid (CSF) was significant for a nucleated cell count of 140 cells/µL (90% lymphocytes), a protein level of 125 mg/dL, and a glucose level of 106 mg/dL (plasma glucose level of 191 mg/dL). The patient's chemistries, liver function test results, and complete blood cell count were otherwise unremarkable. CSF culture and Gram staining; herpes simplex virus 1 and 2, cytomegalovirus, and Epstein-Barr virus titers; and cryptococcal antigen testing were all negative. VZV DNA was detected by polymerase chain reaction in the CSF, and the patient was diagnosed with disseminated VZV infection, including encephalitis. Although the patient remembered having varicella in childhood, the current presentation was the patient’s first episode of shingles, and he denied receiving a VZV vaccination prior to initiation of infliximab therapy. The patients mentation improved over several days, and he was discharged home to complete a 3-week course of intravenous acyclovir therapy. After completion of this therapy, his only residual symptom was postherpetic neuralgia.
Discussion
VZV is a member of the human herpesvirus family and is designated HHV-3. VZV infection initially presents as varicella (chickenpox), often during childhood. The virus usually remains dormant in cranial nerve and dorsal root ganglia.2 The virus may reactivate as herpes zoster (shingles), often in older or immunocompromised individuals. When the infection is limited, herpes zoster affects 1 or a few adjacent dermatomes, presenting as a painful vesicular rash that may be complicated by postherpetic neuralgia.2
VZV infection of the central nervous system (CNS) can present along a spectrum of meningoencephalitis. Meningitis is the infection and inflammation of the meninges, and this condition is classically seen in HIV-infected hosts.6 Encephalitis is the result of a VZV CNS vasculitis, which causes 1 of 3 morphologic syndromes based on the location of the vasculitis. When large cerebral vessels are affected, the result is an acute infarct manifesting as a focal neurologic deficit consistent with stroke.6 Large vessel disease is more common in immunocompetent hosts. Conversely, small vessel disease is almost always seen in immunocompromised individuals.7 Small vessel disease presents as the subacute onset of symptoms such as headache, fever, nausea, seizures, and mental status changes, with findings of aphasia, hemiplegia, and visual field deficits.7 The third and least common presentation of VZV encephalitis is due to infection of periventricular ependymal cells; this type of disease presents with gait disorder and hydrocephalus.6
The risk of herpes zoster is increased in patients with rheumatoid arthritis who are receiving TNFα inhibitors.8 In addition, the presentation of herpes zoster is usually more severe in these patients, and they have an increased risk of hospitalization.9 In the RATIO study, 24 cases of herpes zoster were reported in patients receiving TNFα inhibitors, and 8 of these cases were classified as severe (4 multidermatomal infections, 3 ophthalmic infections, and 1 case of meningitis).4
Two cases of VZV encephalitis have been reported in patients receiving TNFα inhibitors. The first case occurred in a patient with psoriatic arthritis who was receiving adalimumab (Humira, Abbott).10 The patient received a 3-week course of acyclovir and had no residual symptoms. The second patient had rheumatoid arthritis and was receiving a combination of adalimumab and methotrexate.11 This patient recovered but had persistent bilateral lower extremity weakness.
A live attenuated vaccine for herpes zoster is currently available and can decrease the rate of herpes zoster and the severity of postherpetic neuralgia.12 In 2008, the Advisory Committee on Immunization Practices (ACIP) recommended vaccination of nonimmunosuppressed adults above the age of 60 years.5 The safety and efficacy of the herpes zoster vaccine in patients receiving TNFα inhibitors is unknown. Previously published case studies have reported immunocompromised patients (patients with hematologic malignancies, patients receiving corticosteroids or chemotherapy, and transplant recipients) who developed disseminated disease following vaccination.13,14 A recent retrospective study identified 47 patients with rheumatoid arthritis or IBD who received the herpes zoster vaccine while receiving TNFα inhibitors. None of the 47 subjects developed herpes zoster in the 30 days after vaccination; however, the depressed immune system in these patients may not allow for generation of a protective immune response.15 Patients who are receiving immunosuppressive therapy have been shown to mount a diminished response to influenza and pneumococcal vaccines.16 The ACIP recommends that the herpes zoster vaccine be avoided in patients receiving TNFα inhibitors and that vaccination be deferred for at least 1 month after discontinuation of such therapy.5 Administering this vaccine prior to initiation of immunosuppressive therapy is ideal, and patients should wait 1—3 months after vaccination before starting TNFα inhibitors.17
Data indicate that IBD patients are underimmunized. Melmed and colleagues reported that only 9% of surveyed IBD patients were vaccinated against pneumococcus, 28% against influenza, and 45% against tetanus.18 No data exist on rates of vaccination against VZV in this population. According to a recent study, only 14% of gastroenterologists inquired about vaccination history19
The current case highlights the risk of the severe, potentially disabling infections that can occur when clinicians fail to vaccinate patients prior to initiating chronic immunosuppressive therapy. Although primary care providers coordinate patient care among specialists, immunosuppressive medications are often initiated by gastroenterologists or rheumatologists, and these specialists share the responsibility for assessing patients’ vaccination status and providing patients with the appropriate vaccines. Protecting patients prior to initiating immunosuppressive therapy will help to ensure that the cure is not worse than the disease.
References
- 1.Toruner M, Loftus EV, Jr, Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134:929–936. doi: 10.1053/j.gastro.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 2.Mueller NH, Gilden DH, Cohrs RJ, Mahalingam R, Nagel MA. Varicella zoster virus infection: clinical features, molecular pathogenesis of disease, and latenc. Neurol Clin. 2008;26:675–697, viii.. doi: 10.1016/j.ncl.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumgart DC, Dignass AU. Shingles following infliximab infusion. Ann Rheum Dis. 2002;61:661. doi: 10.1136/ard.61.7.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serac G, Tubach R, Mariette X, et al. Risk of herpes zoster in patients receiving anti-TNF-alpha in the prospective French RATIO registry. J Invest Dermatol. 2012;132:726–729. doi: 10.1038/jid.2011.383. [DOI] [PubMed] [Google Scholar]
- 5.Harpaz R, Ortega-Sanchez IR, Seward JF. Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2008;57(RR-5):l–30. quiz CE2-CE4. [PubMed] [Google Scholar]
- 6.Gilden DH, Kleinschmidt-DeMasters BK, LaGuardia JJ, Mahalingam R, Cohrs RJ. Neurologic complications of the reactivation of varicella-zoster virus. N Engl J Med. 2000;342:635–645. doi: 10.1056/NEJM200003023420906. [DOI] [PubMed] [Google Scholar]
- 7.Gilden DH. Varicella zoster virus vasculopathy and disseminated encephalomyelitis. J Neurol Sci. 2002;195:99–101. doi: 10.1016/s0022-510x(02)00021-7. [DOI] [PubMed] [Google Scholar]
- 8.Strangfeld A, Listing J, Herzer P, et al. Risk of herpes zoster in patients with rheumatoid arthritis treated with anti-TNF-alpha agents. JAMA. 2009;301:737–744. doi: 10.1001/jama.2009.146. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Doval I, Perez-Zafrilla B, Descalzo MA, et al. BIOBADASER2.0 Study Group. Incidence and risk of hospitalisation due to shingles and chickenpox in patients with rheumatic diseases treated with TNF antagonists. Ann Rheum Dis. 2010;69:1751–1755. doi: 10.1136/ard.2009.125658. [DOI] [PubMed] [Google Scholar]
- 10.Buccoliero G, Lonero G, Romanelli C, Loperfido P, Resta F. Varicella zoster virus encephalitis during treatment with anti-tumor necrosis factor-alpha agent in a psoriatic arthritis patient. New Microbiol. 2010;33:271–274. [PubMed] [Google Scholar]
- 11.Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50:1400–1411. doi: 10.1002/art.20217. [DOI] [PubMed] [Google Scholar]
- 12.Oxman MN, Levin MJ, Johnson GR, et al. Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 13.Curtis KK, Connolly MK, Northfelt DW. Live, attenuated varicella zoster vaccination of an immunocompromised patient. J Gen Intern Med. 2008;23:648–649. doi: 10.1007/s11606-008-0558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galea SA, Sweet A, Beninger P, et al. The safety profile of varicella vaccine: a 10-year review. J Infect Dis. 2008;197(suppl 2):S165–S169. doi: 10.1086/522125. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Delzell E, Xie F, et al. The use, safety, and effectiveness of herpes zoster vaccination in individuals with inflammatory and autoimmune diseases: a longitudinal observational study. Arthritis Res Ther. 2011;13 doi: 10.1186/ar3497. R174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agarwal N, Ollington K, Kaneshiro M, Frenck R, Melmed GY. Are immunosuppressive medications associated with decreased responses to routine immunizations? A systematic review. Vaccine. 2012;30:1413–1424. doi: 10.1016/j.vaccine.2011.11.109. [DOI] [PubMed] [Google Scholar]
- 17.Wasan SK, Baker SE, Skolnik PR, Farraye FA. A practical guide to vaccinating the inflammatory bowel disease patient. Am J Gastroenterol. 2010;105:1231–1238. doi: 10.1038/ajg.2009.733. [DOI] [PubMed] [Google Scholar]
- 18.Melmed GY, Ippoliti AF, Papadakis KA, et al. Patients with inflammatory bowel disease are at risk for vaccine-preventable illnesses. Am J Gastroenterol. 2006;101:1834–1840. doi: 10.1111/j.1572-0241.2006.00646.x. [DOI] [PubMed] [Google Scholar]
- 19.Yeung JH, Goodman KJ, Fedorak RN. Inadequate knowledge of immunization guidelines: a missed opportunity for preventing infection in immunocompromised IBD patients. Inflamm Bowel Dis. 2012;18:34–40. doi: 10.1002/ibd.21668. [DOI] [PubMed] [Google Scholar]