Abstract
Incidental 18F-fluorodeoxyglucose (18F-FDG) uptake in the prostate is often experienced in clinical practice; however, it is difficult to determine whether incidental uptake is indicative of a malignancy or benign state based on the maximum standardized uptake value (SUVmax). In the present study, we investigated the clinical significance of incidental prostate uptake by 18F-FDG positron emission tomography (PET)/CT, and examined the differences between malignant and benign uptake from a clinicopathological viewpoint. We reviewed 3,236 male subjects who underwent 18F-FDG PET/CT scans at Hirosaki University Hospital (Japan) from 2008 to 2012 in order to identify cases of incidental prostate FDG uptake. The final diagnosis was made by serum prostate-specific antigen (PSA) levels, biopsy, imaging studies and clinical follow-up with PET findings. Incidental FDG uptake of the prostate was observed in 53 cases (2%). Four cases were excluded due to insufficient clinical data, and 49 cases were included in the present study. Of the 49 cases, 8 (16%) had prostate cancer, while 41 (84%) were benign. All 8 malignant cases had high uptake areas, e.g. in the prostate peripheral zone, where there was no coexistence of calcification or FDG uptake. Of the 41 benign cases, 19 had high uptake in the inner zone, 17 in the peripheral zone, and 5 in both the inner and peripheral zones. Of the 41 cases, 18 (44%) showed FDG uptake coexisting with prostatic calcification. Incidental prostate 18F-FDG uptake infrequently signifies prostate cancer; however, FDG uptake not coexisting with calcification indicates the possibility of prostate cancer and should be included in the differential diagnosis for performing other clinical examinations.
Keywords: 18F-FDG PET/CT, prostate cancer, prostate calcification, incidental prostate FDG uptake
Introduction
Positron emission tomography (PET) is a method for determining biochemical and physiological processes by using radiopharmaceuticals labeled with positron-emitting radionuclides. FDG-PET has been widely applied to assess brain function, heart muscle metabolism and for the diagnosis of various tumors (1,2); however, the positive predictive value of prostate cancer has been considered to be low. A previous study reported that the maximum standardized uptake value (SUVmax) is not essential for differential diagnostic criteria of prostatic lesions (3). Prostate cancer shows no or mild FDG uptake because of its low glucose metabolism (4,6). In addition, FDG-PET is able to detect some prostate cancers due to urinary excretion of the radiotracer (5,6). FDG uptake is known to be unspecific to the cancer and may result from an inflammatory condition such as prostatitis (7). A previous study reported that FDG-PET shows 4.0% of histopathology-confirmed prostate cancer (8). On the other hand, cancer surveillance has revealed that the incidence of second primary cancers is 1.53–8.5% in patients with known first malignancies, and this incidence has risen due to the increase in the number of elderly patients and the prolonged cancer survival rate (9,10). Detection of second primary cancers is important, since they have a significant influence on patient management, particularly early cancers that require radical treatment (11).
Incidental uptake in the prostate is often experienced in clinical practice, but it is difficult to determine whether the uptake indicates a malignancy or a benign state based on the SUV alone. Only a few reports have described incidental prostate uptake on FDG-PET/CT (3). In the present study, we investigated the frequency and clinical significance of incidental prostate uptake on FDG-PET/CT and examined the relationship between the location of FDG uptake and prostate calcification.
Materials and methods
Patients
From May 2008 to August 2012, 3,236 male patients underwent 18F-fluorodeoxyglucose (18F-FDG)-PET/CT scans for various types of cancers at our hospital. Of the 3,236 PET/CT scan cases, 53 cases demonstrated incidental FDG uptake in the prostate. Of the 53 PET/CT scans, we analyzed the 49 cases demonstrating incidental FDG uptake with sufficient follow-up in this retrospective study, while 4 cases were excluded from the study due to insufficient follow-up.
18F-FDG PET/CT
In preparation for PET/CT, all patients fasted for at least 4 h, while water intake was encouraged. Delivered 18F-FDG (FDG scan injectable, 185 MBq on assay date; Nihon Medi-Physics, Co., Ltd., Tokyo, Japan) was injected intravenously and scanning was initiated 60 min later. During the 60-min uptake period, the patients drank a sufficient amount of water. A PET/CT system (Discovery ST Elite 16; GE Healthcare, Buckinghamshire, UK) was used to acquire all data in 7–8 bed positions, with an acquisition time of 2.5–3.0 min per bed position. CT was performed first (30–80 mA, 120 kV, 3.75–3.27 mm slice thickness). CT data were used for attenuation correction of PET data, as well as for co-registration with attenuation-corrected PET images. Then, PET data were acquired immediately from the same body region. PET, CT and fused PET/CT images were available for review and were displayed in axial, coronal and sagittal planes on a viewer system.
18F-FDG PET analysis
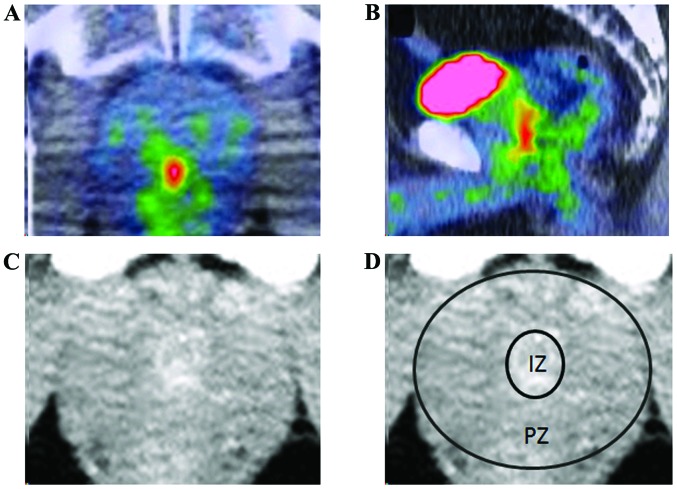
FDG uptake in the prostate was visually defined as positive or negative. In the present study, physiological uptake in the prostate urethra on coronal, sagittal and axial views was excluded. The SUVmax for the prostate was obtained from transaxial views, and analyzed by the Mann-Whitney test (P<0.05, statistical significance). Prostate sites of FDG uptake (inner or peripheral zone) and patterns of FDG uptake (focal or diffuse) were evaluated on the axial view. The CT portion of the FDG-PET/CT was used to recognize the inner (prostate central gland: central + transition zones) and peripheral zones according to the contrast difference (Fig. 1).
Figure 1.
Image analysis. (A and B) Physiological uptake in the prostate urethra on coronal, sagittal and axial views was excluded. (C and D) To segment the inner zone and peripheral zone, we used the density difference in the CT portion of FDG-PET/CT. IZ, inner zone. PZ, peripheral zone.
Prostate calcification analysis
When prostate calcification and the FDG uptake area overlapped in 40% or more of the areas, the cases were defined as ‘uptake coexisting with calcification’. The other cases, i.e. <40% of overlapped areas, were defined as ‘uptake not coexisting with calcification’ (Fig. 2). In the present study, the number and position of the prostate calcifications were not evaluated.
Figure 2.

Prostate calcification analysis. The number and position of the prostate calcifications were not evaluated in the present study. (A) When prostate calcification and the FDG uptake area overlapped in less than 40% of the areas, the cases were defined as ‘uptake not coexisting with calcification’. (B) The other cases, i.e. 40% or more of overlapped areas, were defined as ‘uptake coexisting with calcification’.
Clinicopathological examinations
Final clinical diagnoses of the patients were determined based on the summarized results of the biopsy, serum prostate-specific antigen (PSA) levels, imaging studies (CT, MRI, follow-up PET/CT) and urological examinations. Urologists performed a biopsy for suspicious cases, and 12 patients underwent biopsy. In 28 cases, PSA levels were calculated after the PET/CT scan.
Results
Of the 3,236 PET/CT images, incidental FDG uptake of the prostate was observed in 53 cases (2%), while 4 cases were excluded. These 49 cases were analyzed in the present study. Of the 49 cases, 8 (16%) had prostate cancer, and 41 (84%) were benign, i.e., the prostatic cancer discovery rate was 0.25% for all PET/CT scans. The SUVmax was not significantly different between the two groups.
Malignant lesions
Of the 8 prostate cancer cases, 7 were ordinary adenocarcinomas and the other case was signet-ring cell carcinoma. All 7 cases of adenocarcinoma showed increased serum PSA levels, but the case of signet-ring cell carcinoma exhibited serum PSA levels within the normal range. The mean SUVmax of the 8 malignant lesions was 7.2±2.0. Prostate calcifications were present near the FDG uptake in 2 cases. All 8 cancer cases showed FDG uptake not coexisting with calcification, while the cancer lesions were located in the peripheral zone in 7 cases, and in both inner and peripheral zones in one case (Table I).
Table I.
Malignant cases (n=8).
| Patients | Age (years) | Indication for PET/CT | Gleason score | PSA (ng/ml) | SUVmax | Site | Calcification |
|---|---|---|---|---|---|---|---|
| Patient 1 | 57 | Lung cancer | 3+4 | 30.55 | 6.7 | Peripheral | Absent |
| Patient 2 | 67 | Bone tumor | 4+5 | 85.20 | 9.0 | Peripheral | Absent |
| Patient 3 | 73 | Cholangiocarcinoma | 4+4 | 8.23 | 5.7 | Peripheral | Absent |
| Patient 4 | 76 | Head and neck cancer | 4+5 | 49.10 | 7.9 | Peripheral | Presenta |
| Patient 5 | 80 | Head and neck cancer | 4+5 | 31.00 | 7.3 | Peripheral | Presenta |
| Patient 6 | 61 | Cancer screening | 5+4 | 0.19 | 7.0 | Peripheral | Absent |
| Patient 7 | 78 | Lung cancer | 4+5 | 19.71 | 3.7 | Peripheral | Absent |
| Patient 8 | 66 | Cancer screening | 4+5 | 79.60 | 10.9 | Peripheral-inner | Absent |
Prostatic calcification not coexisting with FDG uptake.
Benign lesions
Of the 41 benign cases, 8 showed increased serum PSA levels, and 12 exhibited serum PSA levels within the normal range. PSA levels of the other 21 cases were not evaluated after PET/CT, but they were diagnosed as benign lesions by follow-up imaging studies or medical examination by urologists. The mean SUVmax of the 41 cases was 6.0±1.8. Eighteen cases (44%) exhibited FDG uptake coexisting with prostate calcifications, i.e. 13 of the 18 cases showed uptake in the inner zone and 5 in the peripheral zone. Of the 41 benign cases, 23 did not coexist with prostate calcification; there was FDG uptake in the inner zone in 6 cases, peripheral zone in 12 cases, and the inner to peripheral zone in 5 cases (Table II).
Table II.
Benign cases: Uptake with or without calcification.
| A, Uptake with calcification (n=18) | |||
|---|---|---|---|
|
| |||
| Site | PSA unmeasured | PSA normal | PSA high |
| Inner zone (n=13) | 10 | 2 | 1 |
| Peripheral zone (n=5) | 3 | 1 | 1 |
|
| |||
| B, Uptake without calcification (n=23) | |||
|
| |||
| Site | PSA unmeasured | PSA normal | PSA high |
|
| |||
| Inner zone (n=6) | 4 | 1 | 1 |
| Peripheral zone (n=12) | 1 | 8 | 3 |
| Both lobes (n=4) | 3 | 0 | 1 |
| Right lobes (n=1) | 0 | 0 | 1 |
Discussion
Our results demonstrated that abnormal hypermetabolic lesions in the prostate glands are not common (2%), and most were benign lesions (84%). The results are similar to those of previous reports (Table III); however, the incidence of prostate cancer in the present study was much different from previous studies. Cho et al (3) described incidental prostate uptake in 148 of 14,854 scans (1.0%), and prostate cancer in 9 of 67 subjects (13.4%) with further evaluations. Han et al (12) observed incidental prostate uptake in 63 of 5,119 scans (1.2%), and prostate cancer in 3 of 55 subjects (5.4%) with further evaluation. Hwang et al (13) observed incidental prostate uptake in 184 of 1,2037 scans (1.5%), and prostate cancer was found in 23 of 120 subjects (19.2%) with further evaluation. The different frequencies of incidental prostate cancer resulted from not only the large number of enrolled patients, but also the different confirmation methods. They also speculated that the incidence of prostate cancer detected by PET/CT might depend on the age and characteristics of the population, such as healthy people or patients with other cancers.
Table III.
Summary of incidental FDG uptake in previously reported cases.
The incidence of prostatic cancer has recently increased in elderly people aged 60 and over. Tumor growth is gradual and it occurs frequently as multiple lesions. More than 95% of prostate cancer consists of adenocarcinoma histologically, and most prostate cancers arise from the peripheral zone (14). All of our carcinoma cases existed in the peripheral zone, whereas benign lesions existed in the inner zone.
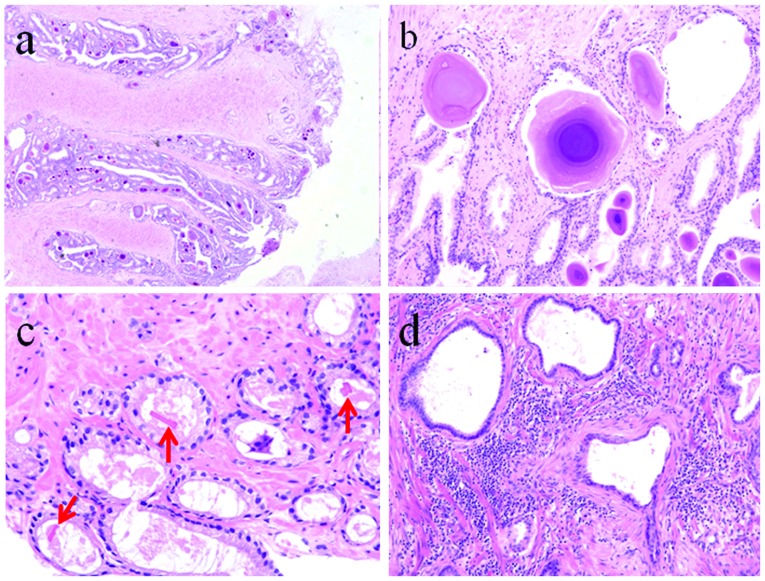
In elderly male patients, corpora amylacea, dense accumulations of calcified proteinaceous material, are frequently found in the prostatic ducts of elderly men (Fig. 3) and gradually form prostatic calcification, often without related clinical symptoms. Prostatic calcifications are associated with benign hyperplasia, chronic granulomatous prostatitis, and a history of chronic obstruction or stasis, such as in patients with urethral strictures and secondary intraprostatic reflux of urine (15–18). Bock et al (19) reported that prostatic calcifications were noted in 47.2% of men under 50 years of age and in 86% of men over 50 years of age. Histopathologically, corpora amylacea are frequently observed in benign glands but are rarely noted in carcinomas (20). On the other hand, prostatic crystalloid (intraluminal eosinophilic structures), intraluminal acidic mucin and intraluminal amorphous eosinophilic materials are less frequently found in both benign glands and adenocarcinomas (21–23). It is extremely important to determine corpora amylacea as criteria of benign lesions. Prostatic crystalloid (intraluminal eosinophilic structures), intraluminal acidic mucin, or intraluminal amorphous eosinophilic materials are invisible to CT because of their low density of calcification, while corpora amylacea are visualized by CT (20); therefore, FDG uptake coexisting with calcification visualized on CT is almost always consistent with benign findings (Fig. 4). On the other hand, FDG uptake without calcification in the peripheral zone is thought to possibly indicate a malignant lesion, but the differential diagnosis of benign/malignant prostatic lesions is not easy; and further clinical examinations such as serum PSA, MRI and biopsy are necessary to verify the diagnosis (24,25).
Figure 3.
Histopathology of prostatic lesions. (a) H&E staining (magnification, ×12.5) shows multiple corpora amylacea at the prostatic urethra. (b) H&E staining (magnification, ×100) shows corpora amylacea of the layer structure. They are frequently observed in benign glands but are rarely noted in carcinomas. (c) H&E staining (magnification, ×100) shows prostatic crystalloids (arrows). They are often observed in malignant or atypical glands. (d) H&E staining (magnification, ×40) shows prostatitis with moderate lymphocyte infiltration.
Figure 4.
Benign uptake. All cases of FDG uptake coexisting with calcification were benign lesions irrespective of the site. (A–D) Calcification may be influenced by attenuation correction; therefore, changing window levels is needed for interpretation. Arrow shows calcification (E).
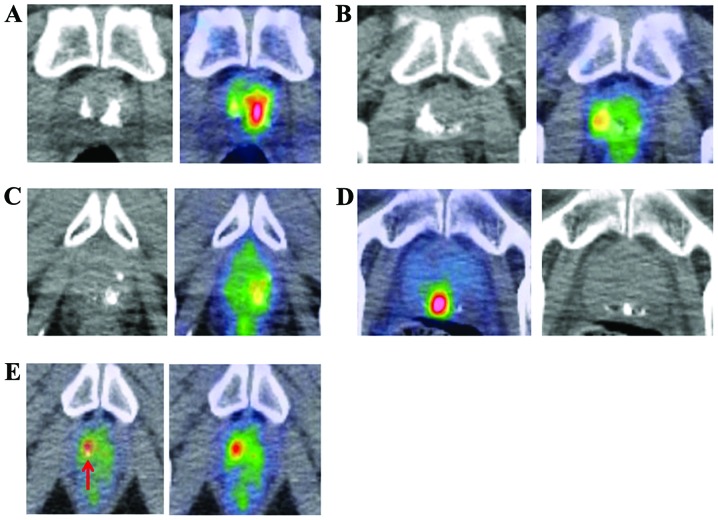
FDG uptake is not only specific to cancer, but is also positive in inflammatory conditions such as prostatitis. Previous studies have reported that inflammation is associated with prostate cancer (26,27); however, reproducible FDG uptake in the same area is thought to be a potential malignant lesion, and careful observation is recommended (Fig. 5). In the present study, 4 patients showed reproducible FDG uptake in the same area and 1 patient had adenocarcinoma. In addition, distribution of the FDG uptake is important (15,28); FDG uptake in the inner area probably indicates benign uptake, since prostatic cancer frequently occurs in the peripheral zone, but not in the inner glands.
Figure 5.
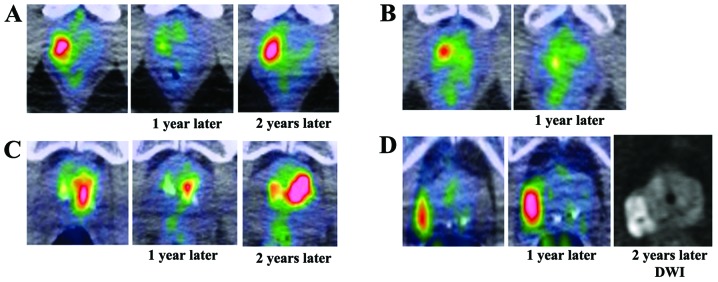
Reproducible FDG uptake. (A–C) All 3 cases were diagnosed as chronic inflammatory change such as prostatitis. Although SUVmax was different as assessed each year, the FDG uptake tended to exist in the same area. (D) One case was diagnosed as adenocarcinoma 2 years following the initial PET/CT scan. During those 2 years, biopsies were performed 3 times. DWI, diffusion weighted image.
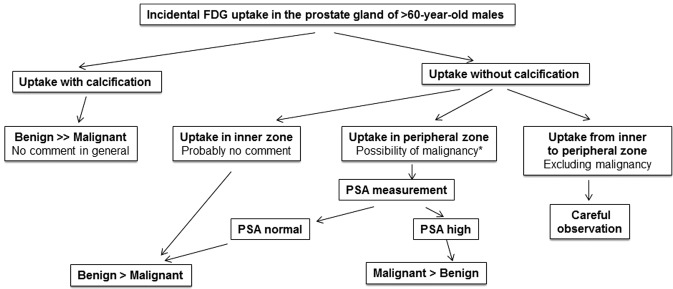
Fig. 6 is a diagnostic flow chart of incidental FDG uptake in the prostate.
Figure 6.
Diagnostic flow chart. Incidental FDG uptake in the prostate gland of males 60 years of age or older. *, Strong FDG uptake that occurs within a short period of time indicates probable inflammatory change.
In conclusion, incidental FDG uptake in the prostate is an extremely rare finding in patients who undergo FDG-PET/CT. FDG uptake coexisting with calcification is indicative of a benign lesion; however, FDG uptake without calcification in the peripheral zone can indicate prostate cancer, and further examinations such as PSA, MRI and biopsy are necessary to exclude malignancy. Reproducible FDG uptake should be observed carefully since it can indicate malignancy or probable malignant potential.
References
- 1.Phelps ME. PET: the merging of biology and imaging into molecular imaging. J Nucl Med. 2000;41:661–681. [PubMed] [Google Scholar]
- 2.Gambhir SS. Molecular imaging of cancer with position emission tomography. Nat Rev Cancer. 2002;2:683–693. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 3.Cho SK, Choi JY, Yoo J, Cheon M, Lee JY, Hyun SH, Lee EJ, Lee KH, Kim BT. Incidental focal 18F-FDG uptake in the prostate: clinical significance and differential diagnostic criteria. Nucl Med Mol Imaging. 2011;45:192–196. doi: 10.1007/s13139-011-0092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Effert PJ, Bares H, Handt S, Wolff JM, Büll U, Janse G. Metabolic imaging of untreated prostate cancer by PET with 18fluorine labeled deoxyglucose. J Urol. 1996;155:994–998. [PubMed] [Google Scholar]
- 5.Hofer C, Laubenbacher C, Block T, Breul J, Hartung R, Schwaiger M. Fluorine-18-fluorodeoxyglucose positron emission tomography is useless for the detection of local recurrence after radical prostatectomy. Eur Urol. 1999;36:31–35. doi: 10.1159/000019923. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi N, Inoue T, Lee J, Yamaguchi T, Shizukuishi K. The roles of PET and PET/CT in the diagnosis and management of prostate cancer. Oncology. 2007;72:226–233. doi: 10.1159/000112946. [DOI] [PubMed] [Google Scholar]
- 7.Kao PF, Chou YH, Lai CW. Diffuse FDG uptake in acute prostatitis. Clin Nucl Med. 2008;33:308–310. doi: 10.1097/RLU.0b013e3181662f8b. [DOI] [PubMed] [Google Scholar]
- 8.Liu IJ, Zafar MB, Lai YH, Segall GM, Terris MK. Fluorodeoxyglucose positron emission tomography studies in diagnosis and staging of clinically organ-confined prostate cancer. Urology. 2001;57:108–111. doi: 10.1016/s0090-4295(00)00896-7. [DOI] [PubMed] [Google Scholar]
- 9.Dong C, Hemminki K. Second primary neoplasms among 53 159 haemotolymphoproliferative malignancy patients in Sweden, 1958–1996 a search for common mechanisms. Br J Cancer. 2001;85:997–1005. doi: 10.1054/bjoc.2001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ueno M, Muto T, Oya M, Ota H, Azekura K, Yamaguchi T. Multiple primary cancer: an experience at the Cancer Institute Hospital with special reference to colorectal cancer. Int J Clin Oncol. 2003;8:162–167. doi: 10.1007/s10147-003-0322-z. [DOI] [PubMed] [Google Scholar]
- 11.Leön X, Ferlito A, Myer CM, III, Saffiotti U, Shaha AR, Bradley PJ, Brandwein MS, Anniko M, Elluru RG, Rinaldo A. Second primary tumors in head and neck cancer patients. Acta Otolaryngol. 2002;122:765–778. [PubMed] [Google Scholar]
- 12.Han EJ, HOJ, Choi WH, Yoo IR, Chung SK. Significance of incidental focal uptake in prostate on 18-fluro-2-deoxyglucose positron emission tomography CT images. Br J Radiol. 2010;83:915–920. doi: 10.1259/bjr/19887771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang I, Chong A, Jung SI, Hwang EC, Kim SO, Kang TW, Kwon DD, Park K, Ryu SB. Is further evaluation needed for incidental focal uptake in the prostate in 18-fluori-2-deoxyglucose positron emission tomography-computed tomography images? Ann Nucl Med. 2013;27:140–145. doi: 10.1007/s12149-012-0663-7. [DOI] [PubMed] [Google Scholar]
- 14.McNeal JE. The zonal anatomy of the prostate. Prostate. 1981;2:35–49. doi: 10.1002/pros.2990020105. [DOI] [PubMed] [Google Scholar]
- 15.Kilmas R, Bennett B, Gardner WA., Jr Prostate calculi: a review. Prostate. 1985;7:91–96. doi: 10.1002/pros.2990070110. [DOI] [PubMed] [Google Scholar]
- 16.Eykyn S, Bultitude MI, Mayo ME, Lloyd-Davies RW. Prostatic calculi as a source of recurrent bacteriuria in the male. Br J Urol. 1974;46:527–532. doi: 10.1111/j.1464-410x.1974.tb03851.x. [DOI] [PubMed] [Google Scholar]
- 17.Meares EM. Infection stones of prostate gland. Laboratory diagnosis and clinical management. Urology. 1974;4:560–566. doi: 10.1016/0090-4295(74)90490-7. [DOI] [PubMed] [Google Scholar]
- 18.Cross PA, Bartley CJ, McClure J. Amyloid in prostatic corpora amylacea. J Clin Pathol. 1992;45:894–897. doi: 10.1136/jcp.45.10.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bock E, Calugi V, Stolfi V, Rossi P, D’Ascenzo R, Solivetti FM. Calcifications of the prostate: a transrectal echographic study. Radiol Med. 1989;77:501–503. (In Italian) [PubMed] [Google Scholar]
- 20.Cohen RJ, McNeal JE, Redmond SL, Meehan K, Thomas R, Wilce M, Dawkins HJ. Luminal contents of benign and malignant prostatic glands: correspondence to altered secretory mechanisms. Hum Pathol. 2000;31:94–100. doi: 10.1016/s0046-8177(00)80204-x. [DOI] [PubMed] [Google Scholar]
- 21.Luna More S. Argyrophil crystalloids in adenocarcinoma of the prostate. Prostate. 1993;24:309–315. doi: 10.1002/pros.2990220405. [DOI] [PubMed] [Google Scholar]
- 22.Luna More S, Florez P, Ayala A, Diaz F, Santos A. Neutral and acid mucins and eosinophilic and argyrophil crystalloids in carcinoma and atypical adenomatous hyperplasia of the prostate. Pathol Res Pract. 1997;193:291–298. doi: 10.1016/S0344-0338(97)80006-4. [DOI] [PubMed] [Google Scholar]
- 23.Epstein JI. Diagnostic criteria of limited adenocarcinoma of the prostate on needle biopsy. Hum Pathol. 1995;26:223–229. doi: 10.1016/0046-8177(95)90041-1. [DOI] [PubMed] [Google Scholar]
- 24.Lee R, Localio AR, Armstrong K, Malkowicz SB, Schwartz JS. A meta-analysis of the performance characteristics of the free prostate-specific antigen test. Urology. 2006;67:762–768. doi: 10.1016/j.urology.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 25.Wefer AE, Hricak H, Vigneron DB, Coakley FV, Lu Y, Wefer J, Meller-Lisse U, Carroll PR, Kurhanewicz J. Sextant localization of prostate cancer: comparison of sextant biopsy, magnetic resonance imaging and magnetic resonance spectroscopic imaging with step section histology. J Urol. 2000;164:400–404. [PubMed] [Google Scholar]
- 26.De Marzo AM, Platz EA, Sutcliffe S, Xu J, Grönberg H, Drake CG, Nakai Y, Issacs WB, Nelson WG. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2004;7:256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vral A, Magri V, Montanari E, Gazzano G, Gourvas V, Marras E, Perletti G. Topographic and quantitative relationship between prostate inflammation, proliferative inflammatory atrophy and low-grade prostate intraepithelial neoplasia: a biopsy study in chronic prostatitis patients. Int J Oncol. 2012;41:1950–1958. doi: 10.3892/ijo.2012.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McNeal JE, Redwine EA, Freiha FS, Stamey TA. Zonal distribution of prostatic adenocarcinoma. Correlation with histologic pattern and direction of spread. Am J Surg Pathol. 1988;12:897–906. doi: 10.1097/00000478-198812000-00001. [DOI] [PubMed] [Google Scholar]