The structure of 4-pyridoxolactonase from M. loti was determined.
Keywords: zinc-dependent hydrolase, docking simulation, catalytic mechanisms, 4-pyridoxolactonase, Mesorhizobium loti, vitamin B6
Abstract
4-Pyridoxolactonase from Mesorhizobium loti catalyzes the zinc-dependent lactone-ring hydrolysis of 4-pyridoxolactone (4PAL) to 4-pyridoxic acid (4PA) in vitamin B6 degradation pathway I. The crystal structures of 4-pyridoxolactonase and its complex with 5-pyridoxolactone (5PAL; the competitive inhibitor) were determined. The overall structure was an αβ/βα sandwich fold, and two zinc ions were coordinated. This strongly suggested that the enzyme belongs to subclass B3 of the class B β-lactamases. In the complex structure, the carbonyl group of 5PAL pointed away from the active site, revealing why it acts as a competitive inhibitor. Based on docking simulation with 4PAL, 4PA and a reaction intermediate, 4-pyridoxolactonase probably catalyzes the reaction through a subclass B2-like mechanism, not the subclass B3 mechanism.
1. Introduction
4-Pyridoxolactonase (EC 3.1.1.27) is involved in vitamin B6 degradation pathway I, which is found in a limited number of soil microorganisms (Burg & Snell, 1969 ▶; Jong & Snell, 1986 ▶). The enzyme catalyzes the zinc-dependent lactone-ring hydrolysis of 4-pyridoxolactone (4PAL) to 4-pyridoxic acid (4PA) in the pathway (Fig. 1 ▶ a). The enzymatic properties of the 4-pyridoxolactonases in Pseudomonas sp. MA-1 (Jong & Snell, 1986 ▶) and Mesorhizobium loti (Funami et al., 2005 ▶) have been reported; the two enzymes have nearly identical enzymatic properties, although it is unknown whether zinc ions are present in the Pseudomonas sp. MA-1 enzyme. 5-Pyridoxolactone (5PAL) acts as a competitive inhibitor (Fig. 1 ▶ b), and the K i values for the Pseudomonas sp. MA-1 and M. loti enzymes were 48 and 150 µM, respectively. The primary structure of the enzyme from M. loti is ∼30% identical to members of the class B β-lactamases (also known as the metallo-β-lactamase superfamily), indicating that it belongs to this superfamily. The class B β-lactamases are divided into three subclasses according to their manner of coordination and the affinities of their active sites for zinc ions (Wang et al., 1999 ▶; Garau et al., 2004 ▶; Palzkill, 2013 ▶). Subclass B1 enzymes have two zinc-binding sites, and require one or two zinc ions for their activity. The affinity of one binding site is higher than the other, and the dizinc enzyme shows a higher activity than the monozinc enzyme. The B1 enzymes share a consensus sequence, HXHXD…H…C…H, for zinc coordination. Although subclass B2 enzymes require only one zinc ion for their activity, they have two zinc-binding sites. Binding of the second zinc ion inactivates the B2 enzymes. The B2 enzymes share a consensus sequence, NXHXD…H…C…H. Subclass B3 enzymes require two zinc ions for their activity. Both binding sites have high affinities for zinc, and they are inactive in the monozinc form. The B3 enzymes share a consensus sequence, HXHXDH…H…H.
Figure 1.
(a) Reaction catalyzed by 4-pyridoxolactonase and (b) the chemical structure of 5-pyridoxolactone (5PAL).
Several catalytic mechanisms have been proposed for the subclasses (Palzkill, 2013 ▶). For the subclass B1 and B3 enzymes coordinated to two zinc ions (Zn1 and Zn2), the following mechanism has been suggested. (i) A β-lactam substrate binds to an active site, where the carbonyl O atom of the substrate interacts with Zn1. This interaction further polarizes the carbonyl group of the substrate. (ii) A hydroxide ion (an activated water molecule) between the two zinc ions attacks the positively polarized carbonyl C atom of the substrate. This nucleophilic attack by the hydroxide ion creates a tetrahedral intermediate. (iii) The C—N bond of the tetrahedral intermediate is cleaved to form an anionic nitrogen intermediate and the anionic nitrogen interacts with Zn2. This interaction stabilizes the anionic intermediate. (iv) The anionic nitrogen intermediate readily forms a ring-opened product.
For the subclass B2 enzymes coordinated to one zinc ion (this zinc ion corresponds to Zn2 in the subclasses B1 and B3), a similar mechanism involving His and Asp residues instead of Zn1 has been proposed (Palzkill, 2013 ▶). In addition, the following alternative catalytic mechanism has also been proposed for subclass B2 enzymes (Bounaga et al., 1998 ▶). The same tetrahedral intermediate is formed through a pathway similar to (i) and (ii) above by Zn2 and a hydroxide ion. (iii) A dianionic tetrahedral intermediate is formed by transfer of a proton between the tetrahedral intermediate and the Asp residue in the active site. Zn2 stabilizes the dianionic intermediate by interacting with both anions. (iv) Finally, the same product is readily formed. Although 4-pyridoxolactonase from M. loti belongs to the class B β-lactamases (Funami et al., 2005 ▶), the manner of coordination of the zinc ions and the catalytic mechanism of the enzyme have not been elucidated.
A preliminary X-ray crystallographic analysis of 4-pyridoxolactonase from M. loti has been reported (Matsuda et al., 2009 ▶). However, the crystal structure has not been elucidated owing to the phase problem. In this report, we determined the crystal structure of 4-pyridoxolactonase from M. loti by the single-wavelength anomalous dispersion method using selenium (the Se-SAD method). We also determined the structures of the native 4-pyridoxolactonase and the native 4-pyridoxolactonase–5PAL complex. Finally, we obtained the binding models of 4PAL, 4PA and the dianionic intermediate by docking simulation. Based on these results, we discuss the structural features, competitive inhibition mechanism by 5PAL and catalytic mechanism of 4-pyridoxolactonase.
2. Materials and methods
2.1. Plasmid construction
The expression plasmid vector of 4-pyridoxolactonase, pET21a6805, has already been constructed (Funami et al., 2005 ▶). It was engineered in order to add a His6 tag to the C-terminus of the enzyme. The plasmid of 4-pyridoxolactonase-His6, pET21a6805-H6, was prepared by using a KOD -Plus- mutagenesis kit (Toyobo) according to the instruction manual. The primers were 5′-CATATGTCGGATACAAAGGTCTACCTGCTCGAC-3′ and 5′-AAGCTTTCAGTGGTGGTGGTGGTGGTGGCCGTAGAATTGCGTGCCGGTC-3′.
2.2. Expression
The method used to induce the expression of the native 4-pyridoxolactonase-His6 was the same as the previously reported method for the native enzyme without a His6 tag (Funami et al., 2005 ▶), but the culture time was set to 48 h. The selenomethionine-derivatized (SeMet) 4-pyridoxolactonase-His6 was expressed using Escherichia coli B834 (DE3) cells transformed by the two vectors pET21a6805-H6 and pKY206 containing the GroEL/ES chaperonin genes (Machida et al., 1998 ▶). The cell colony was cultured with 5 ml LB medium containing two antibiotics (50 µg ml−1 ampicillin and 12.5 µg ml−1 tetracycline) for 12 h at 310 K, and was then washed twice using SeMet core medium (Wako). The washed cells were cultured with 200 ml SeMet core medium containing 1%(w/v) glucose, 0.0025%(w/v) MgSO4.7H2O, 0.0004175%(w/v) FeSO4.7H2O, 50 mg l−1 seleno-l-methionine and the antibiotics at 296 K. When the OD500 reached 0.5, 0.5 mM IPTG was added. The cultured cells were collected after 36 h at 296 K.
2.3. Enzyme and protein assay
The activity of 4-pyridoxolactonase was assayed by measuring the decrease in the absorbance of 4PAL (356 nm) as reported previously (Funami et al., 2005 ▶). The protein concentration was measured by a Bio-Rad protein assay (Bio-Rad) using bovine serum albumin as a standard. The concentration of purified 4-pyridoxolactonase was determined from the molecular absorption coefficient (∊ = 51 990 M −1 cm−1) at 280 nm.
2.4. Purification
The native and SeMet 4-pyridoxolactonase-His6 were purified by the same method. The harvested cells were suspended in buffer A [20 mM potassium phosphate buffer (KPB) pH 7.5, 14 mM β-mercaptoethanol (βME), 10%(w/v) glycerol, 20 mM imidazole, 300 mM NaCl] and then sonicated for 5 min on ice. A crude extract was obtained by centrifugation at 8000g for 10 min at 277 K. The crude extract was immediately applied onto an Ni–NTA agarose (Qiagen) column. The column was equilibrated with buffer A and the crude extract was then applied. The column was washed with buffer A containing 50 mM imidazole, and the proteins bound to the column were then eluted with a linear gradient of 50–500 mM imidazole. The fractions showing 4-pyridoxolactonase activity eluted at around 200 mM imidazole. The fractions were collected, the buffer was changed to buffer B (20 mM Tris–HCl pH 7.5, 14 mM βME, 10% glycerol) and the solution was concentrated using a Vivaspin 20 (10 000 MWCO; Saltorius Stedim Biotech). The sample was also applied onto a hydroxyapatite column. The column was equilibrated with buffer B and the sample was then applied. The column was washed with buffer B and the bound proteins were eluted with a linear gradient of 0–50 mM KPB. The fractions with 4-pyridoxolactonase activity were eluted at around 20 mM KPB. The fractions were further applied onto a Shodex protein KW-803 (Showa Denko) gel-filtration column using crystallization buffer [10 mM HEPES–Na pH 7.5, 10% glycerol, 3 mM tris(2-carboxyethyl)phosphine–HCl]. Fractions containing proteins with a molecular mass of around 60 000 Da, which corresponds to the 4-pyridoxolactonase dimer, were collected and used for crystallization.
2.5. Crystallization and data collection
The purified native-His6 and SeMet-His6 enzymes were concentrated to 8 mg ml−1 by ultrafiltration using a Vivaspin 20. The native enzyme without the His6 tag has already been crystallized using reservoir solution consisting of 30%(w/v) PEG 4000, 200 mM ammonium acetate, 100 mM sodium acetate (Matsuda et al., 2009 ▶). After optimization, both the native-His6 and SeMet-His6 enzymes were crystallized by mixing equal volumes (2 µl:2 µl) of enzyme solution (8 mg ml−1 in crystallization buffer) and reservoir solution [30%(w/v) PEG 4000, 150 mM ammonium acetate, 100 mM sodium acetate] by the sitting-drop method for one month at 277 K. The native-His6–5PAL complex was not crystallized under this condition. After screening with Crystal Screen and Crystal Screen 2 (Hampton Research) and Wizard I and II (Emerald Bio) and further optimization, a crystal was obtained by mixing equal volumes (1 µl:1 µl) of enzyme solution (8 mg ml−1 and 5 mM 5PAL in the crystallization buffer) and reservoir solution [20%(w/v) PEG 3350, 50 mM ammonium citrate pH 5.1] by the sitting-drop method for three months at 277 K.
All diffraction data were collected at SPring-8 (Hyogo, Japan). The crystals were flash-cooled directly in a liquid-nitrogen stream. The data for the SeMet crystal were collected using a sagittal light collection system at BL38B1. The wavelength was set to 0.97800 Å from X-ray absorption fine structure (XAFS) measurements, and the crystal-to-detector distance was set to 150.0 mm. Oscillation images of 0.7° were collected with an exposure time of 6 s. Data for the native-His6 and native-His6–5PAL complex crystals were collected using a normal light collection system. For the native-His6 and its 5PAL complex crystals, the wavelength was set to 1.000 and 0.9000 Å, respectively, and the crystal-to-detector distance was set to 250 and 270 mm, respectively. Oscillation images of 1° and 1° were collected with exposure times of 4 and 2.5 s, respectively. All data were processed by HKL-2000 (Otwinowski & Minor, 1997 ▶). The data-collection statistics are shown in Table 1 ▶.
Table 1. Data-collection and refinement statistics.
Values in parentheses are for the highest resolution shell.
| SeMet-His6 (3aj3) | Native-His6 (3aj0) | Native-His6 + 5PAL (4keq) | |
|---|---|---|---|
| Data collection | |||
| X-ray source | BL38B1, SPring-8 | BL38B1, SPring-8 | BL44XU, SPring-8 |
| Wavelength (Å) | 0.97800 | 1.0000 | 0.9000 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| Space group | C2 | C2 | C2 |
| Unit-cell parameters | |||
| a (Å) | 82.777 | 84.751 | 84.737 |
| b (Å) | 39.214 | 39.198 | 39.557 |
| c (Å) | 84.191 | 84.200 | 84.564 |
| β (°) | 117.92 | 117.68 | 117.69 |
| Resolution limit (Å) | 28.99–1.58 (1.64–1.58) | 50–1.83 (1.86–1.83) | 50–2.28 (2.32–2.28) |
| Measured reflections | 217995 (16621) | 133238 (6678) | 47918 (2124) |
| Unique reflections | 32428 (3078) | 21861 (1113) | 11493 (559) |
| Multiplicity | 6.7 (5.4) | 6.1 (6.0) | 4.2 (3.8) |
| Completeness (%) | 97.8 (93.0) | 99.8 (99.7) | 99.6 (98.8) |
| 〈I/σ(I)〉 | 22.6 (7.90) | 19.7 (5.65) | 30.3 (12.7) |
| R merge (%) | 5.1 (28.4) | 5.3 (40.5) | 7.8 (22.8) |
| Refinement | |||
| Resolution range (Å) | 28.99–1.58 (1.63–1.58) | 36.1–1.83 (1.91–1.83) | 36.2–2.28 (2.51–2.28) |
| R work (%) | 16.3 (18.9) | 17.5 (19.5) | 18.8 (20.4) |
| R free (%) | 19.0 (22.0) | 20.9 (25.8) | 24.6 (29.2) |
| Amino-acid residues | 273 | 273 | 269 |
| Zn | 2 | 2 | 2 |
| 5PAL | 2 | ||
| Acetate | 1 | ||
| Water | 325 | 90 | 41 |
| B factors (Å2) | |||
| Protein | 16.49 | 29.03 | 22.42 |
| Water | 25.36 | 29.23 | 19.75 |
| Zn1/Zn2 | 30.21/14.32 | 20.76/21.62 | 20.00/19.67 |
| Acetate | 29.01 | ||
| 5PAL in the active site | 21.47 | ||
| Ramachandran plot (%) | |||
| Most favoured region | 87.7 | 88.4 | 84.7 |
| Allowed region | 11.4 | 11.2 | 14.4 |
| Disallowed region | 0.9 | 0.4 | 0.9 |
2.6. Structure determination
The crystal structure of the SeMet-His6 enzyme was determined by the Se-SAD method. Four selenium sites were identified by SHELXD and the initial phase was determined by SHELXE (Sheldrick, 2010 ▶). Density modification (Terwilliger, 2000 ▶) and model building (Terwilliger, 2003 ▶) were performed using RESOLVE. The structure was refined to 1.58 Å resolution by PHENIX (Afonine et al., 2012 ▶) and Coot (Emsley et al., 2010 ▶). The initial phases of the native-His6 and native-His6–5PAL complex structures were determined by a molecular-replacement method with MOLREP (Vagin & Teplyakov, 2010 ▶) in CCP4 (Winn et al., 2011 ▶) using the SeMet structure as a template. Refinement and modelling of both structures were performed using REFMAC5 (Murshudov et al., 2011 ▶), PHENIX and Coot. The model of 5PAL was generated by PRODRG (Schüttelkopf & van Aalten, 2004 ▶). All final structures and structure factors were evaluated by PROCHECK (Laskowski et al., 1993 ▶) and SFCHECK (Vaguine et al., 1999 ▶). These refinement statistics are shown in Table 1 ▶. The structural similarity search was conducted using DALI (Holm & Rosenström, 2010 ▶). Interaction areas of the enzymes in crystal form were calculated using PISA (Krissinel & Henrick, 2007 ▶). The crystal structures and structure factors have been deposited in the Protein Data Bank (PDB) as entries 3aj0 (native-His6), 3aj3 (SeMet-His6) and 4keq (native-His6–5PAL complex).
2.7. Docking simulation
Docking simulations of 4-pyridoxolactonase with 4PAL (substrate), 4PA (product) or a dianion intermediate (a reaction intermediate) were performed using AutoDock 4.2 (Morris et al., 2009 ▶). The models of 4PAL, 4PA and the dianion intermediate were generated by PRODRG (Schüttelkopf & van Aalten, 2004 ▶). The model of 4-pyridoxolactonase was prepared from the structure of the 4-pyridoxolactonase–5PAL complex. All water molecules and ligands in the 4-pyridoxolactonase model were removed except for the catalytic water and two zinc ions in the active site. H atoms and partial charges were added by AutoDockTools 1.5.6. Glu65, Phe99, Leu132 and Phe223 in the active site were set to be flexible. The docking site was defined by a grid box of 40 × 40 × 40 points and a spacing of 0.375 Å with the 5PAL binding site as the centre. The docking was performed with the Lamarckian genetic algorithm with a population size of 150. 30 independent docking trials were performed for each compound.
3. Results and discussion
3.1. Expression and purification
The native-His6 and SeMet-His6 enzymes were expressed in E. coli. The expression levels were almost the same (∼10% in the crude extracts by SDS–PAGE densitogram). Both enzymes were purified to homogeneity by Ni–NTA, hydroxyapatite and gel-filtration column chromatography. The native-His6 and SeMet-His6 enzymes showed the same retention time on gel-filtration column chromatography. The purified native-His6 enzyme showed almost the same activity (∼120%) as the purified native enzyme without a His6 tag, but the SeMet-His6 enzyme showed an extremely low activity (0.24% of that of the native enzyme without a His6 tag).
3.2. Dimeric structure
Although 4-pyridoxolactonase from M. loti exists as a dimer in solution (Funami et al., 2005 ▶), a monomer was found in the asymmetric unit. The monomer interacted with a monomer related by a crystallographic twofold axis through residues 17–28, 127–129 and 217–235 to form a possible dimer (Figs. 2 ▶ a and 2 ▶ b). This dimer formed C–C contacts between Trp23 and Phe129* and between Phe129 and Trp23* (4.0 Å). The dimer also formed four intermolecular hydrogen bonds between His20 N∊2 and Asn24* O (3.0 Å), between Trp23 N∊1 and Ala222* O (2.8 Å), between Asn24 O and His20* N∊2, and between Ala222 O and Trp23 N∊1. The buried solvent-accessible surface area per monomer by this dimerization was ∼790 Å2 (6.6% of the whole area). 368–4746 Å2 is considered to be reasonable for formation of a functional dimer (Jones & Thornton, 1996 ▶). Although further mutational analysis is needed, this dimer (∼1580 Å2) seems to be a functional dimer in the solution phase.
Figure 2.
The crystal structure of 4-pyridoxolactonase. (a) A crystallographic dimer. The monomers are coloured green and cyan. The N- and C-termini are labelled N and C, respectively. Zinc ions are shown as grey spheres. (b) The dimeric interface shown in stereo. The interface is enlarged from the same direction as in (a). Amino-acid residues involved in the dimerization are shown as stick models. The main chains of Asn24 and Ala222 are also shown as sticks. Hydrogen bonds are shown as yellow broken lines with their distances. (c) The overall structure of the native 4-pyridoxolactonase monomer. α-Helices, β-strands and loops are coloured red, yellow and green, respectively. α-Helices (H1–H13) and β-strands (S1–S11) are numbered from the N-terminus. (d) The topology diagram of the monomer. The secondary-structural elements are shown in the same manner as in (c). The numbers shown above and below the elements correspond to the number of amino-acid residues from the N-terminus.
3.3. Monomeric structure
The overall structure of the 4-pyridoxolactonase monomer was an αβ/βα sandwich fold (Figs. 2 ▶ c and 2 ▶ d): it consisted of central six-stranded and five-stranded mixed β-sheets, and 13 α-helices which sandwiched the two β-sheets. No major structural difference was observed among the structures of the SeMet-His6, native-His6 and native-His6–5PAL complex (r.m.s.d. < 0.2 Å). A similarity search with the DALI server indicated that 4-pyridoxolactonase showed high similarities to zinc-dependent N-acylhomoserine lactone (AHL) lactonase AiiB from Agrobacterium tumefaciens (PDB entry 2r2d, Z-score = 32.6, r.m.s.d. = 1.7 Å; Liu et al., 2007 ▶), zinc-dependent AHL lactonase AiiA from Bacillus thuringiensis (PDB entry 3dhb, Z-score = 31.8, r.m.s.d. = 1.8 Å; Liu et al., 2008 ▶) and methyl parathion hydrolase from Pseudomonas sp. WBC-3 (PDB entry 1p9e, Z-score = 22.8, r.m.s.d. = 2.4 Å; Y. Dong, L. Sun, M. Bartlam, Z. Rao & Z. X. Zhang, unpublished work). The enzyme also showed slight similarities to the IMP-1 (which belongs to subclass B1), VIM-4 (B1), VIM-2 (B1), AIM-1 (B3), FEZ-1 (B3), NDM-1 (B1) and L1 (B3) β-lactamases (Z-score of ∼15, r.m.s.d. of ∼3 Å). All enzymes belonged to the class B β-lactamase, and their fold type was the αβ/βα sandwich fold.
3.4. Active site
The active site of 4-pyridoxolactonase was located at the top of the central β-sheets, and two zinc ions were coordinated (Fig. 3 ▶). One zinc ion (Zn1) was coordinated by His96 N∊2, His98 Nδ1, His185 N∊2, Asp207 Oδ2 and an acetate molecule that was probably from the crystallization solution. The other (Zn2) was coordinated by Asp100 Oδ2, His101 N∊2, Asp207 Oδ2, His252 N∊2 and the same acetate molecule. Although one acetate molecule was the ligand of the zinc ions in the native structure (Figs. 3 ▶ a and 3 ▶ b), two water molecules were the ligands in the SeMet structure (Fig. 3 ▶ c). In other dinuclear zinc enzymes, the distance between Zn1 and Zn2 ranged from 3.5 to 4.4 Å (Wang et al., 1999 ▶; Auld, 2001 ▶). In the 4-pyridoxolactonases with the native-His6, native-His6–5PAL or SeMet-His6 structures, the distances between the two ions was also 3.5 Å. In the SeMet-His6 enzyme, the electron density of Zn1 was too weak to distinguish the nature of the molecule. When the density was modelled as Zn1, the occupancy was low (occupancy = 0.23). Therefore, the low activity of the SeMet-His6 enzyme (0.24% of that of the native enzyme) was probably attributable to the low occupancy of Zn1 in the active site. This low occupancy was probably due to a limited amount of zinc in the SeMet core medium. Thus, active 4-pyridoxolactonase coordinated and required two zinc ions, and the manner of coordination was H96FHFDH101…H185…H252, with the additional coordination of Asp207. These results strongly suggested that 4-pyridoxolactonase belongs to the subclass B3 of the class B β-lactamase (Palzkill, 2013 ▶).
Figure 3.
The active site of 4-pyridoxolactonase shown in stereo. (a) The active site of the native 4-pyridoxolactonase. Amino-acid side chains are shown as green stick models. Zinc ions are shown as grey spheres. Coordination bonds are shown as yellow broken lines with their distances. (b) 2F o − F c maps around the zinc ions of the native 4-pyridoxolactonase contoured at 2.0σ. (c) 2F o − F c maps around the zinc ions of the SeMet 4-pyridoxolactonase contoured at 2.0σ.
3.5. PAL complex
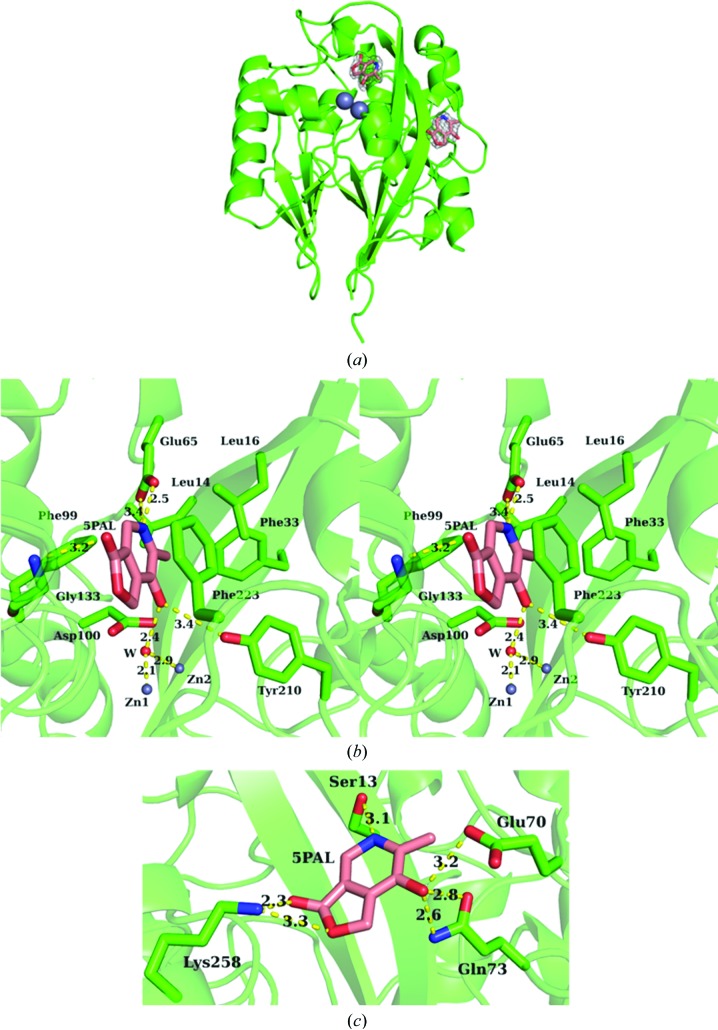
5PAL bound to two sites of the monomer (Fig. 4 ▶ a). One 5PAL bound to the hydrophobic active site consisting of Leu14, Leu16, Phe33, Glu65, Phe99, Leu132, Gly133 and Phe223 (Fig. 4 ▶ b). The pyridine N atom of 5PAL formed two hydrogen bonds with O∊1 and O∊2 of Glu65. The hydroxyl group at the C3 position formed a hydrogen bond with a catalytic water molecule that was the ligand of Zn1. The C3 hydroxyl group also formed a hydrogen bond with Tyr210 Oη. The carbonyl O atom formed a hydrogen bond to Gly133 N. The lactone ring formed a π-stacking with Phe223. The other 5PAL bound to a part of the protein surface formed by β-strands 1 and 2, α-helix 13 and the loop of 66–70 with a high B factor (42.55 Å2; Fig. 4 ▶ c). The pyridine N atom formed a hydrogen bond to Ser13 Oγ. The C3 hydroxyl group formed two hydrogen bonds to Glu70 O∊2 and Gln73 O∊1. The lactone O atom and carbonyl O atom formed hydrogen bonds to Lys258 Nζ. This binding of 5PAL on the surface may be an artifact of the crystal packing for two reasons. (i) 5PAL showed competitive but not allosteric inhibition, with only one binding site in the active region of 4-pyridoxolactonase (Jong & Snell, 1986 ▶; Funami et al., 2005 ▶). (ii) A part of this binding site, Arg32 in β-strand 2 and the 67–68 loop region, was fixed by the Gly166*–Asp168* region of an adjacent symmetrical molecule (not the crystallographic dimer), showing that crystal packing formed this binding site. The high B factor of 5PAL also supports this consideration.
Figure 4.
The binding modes of 5PAL. (a) Binding sites of 5PAL. 5PAL is shown as a pink stick model. 2F o − F c maps of 5PAL are also shown as grey meshes contoured at 1.0σ. (b) The binding mode of 5PAL at the active site shown in stereo. The catalytic water molecule, which was located 5.8 Å apart from the carbonyl C atom of 5PAL, is shown above the Zn1 and Zn2 molecules. (c) The binding mode of 5PAL at the protein surface.
The hydrolytic reaction in the class B β-lactamases is initiated when a hydroxide ion nucleophilically attacks the carbonyl C atom of a substrate (Palzkill, 2013 ▶; Bounaga et al., 1998 ▶). In the 4-pyridoxolactonase–5PAL complex, the carbonyl group of 5PAL pointed away from the candidate water molecule (5.8 Å between the carbonyl C atom and the water molecule), because the enzyme preferably recognized the C3 hydroxyl group of 5PAL over the carbonyl O atom. Thus, the lactone ring of 5PAL cannot be hydrolyzed but it can act as the competitive inhibitor through the binding at the active site.
The binding mode of 5PAL was compared with that of homoserine lactone (HSL) in the AiiA–HSL complex (PDB entry 2br6; Kim et al., 2005 ▶). The inhibitor HSL bound to AiiA through interactions with the two zinc ions (carbonyl O atom⋯Zn1 and lactone O atom⋯Zn2) as shown in Fig. 5 ▶(a). When the substrate 4PAL is placed in the same position as HSL, the methyl group at the C2 position makes a collision with the side chains of Glu65 and Phe99. This indicates that 4PAL interacts with the zinc ions in a different manner from the case of the AiiA–HSL complex. It also suggests that 4-pyridoxolactonase has a different catalytic mechanism from that of AiiA.
Figure 5.
The comparison of the structures between the 4-pyridoxolactonase–5PAL and AiiA–HSL complexes, and the simulated binding modes of 4PAL, the dianion intermediate and 4PA in 4-pyridoxolactonase. (a) The comparison of the 4-pyridoxolactonase–5PAL (green) and AiiA–HSL (PDB entry 2br6; white) complexes shown in stereo. Interactions are shown as thick and thin broken lines, respectively. (b) The simulated binding mode of the 4PAL complex. The distance between the carbonyl C atom and the catalytic water (3.1 Å) is shown as a red broken line. (c) The simulated binding mode of the dianion intermediate (DAI). (d) The simulated binding mode of 4PA. (e) Superimposition of all structures. All models are superimposed on the 5PAL complex coloured in green.
3.6. Docking simulation
Determination of the structures of 4-pyridoxolactonase–4PAL (substrate) and 4-pyridoxolactonase–4PA (product) complexes was not successful. Therefore, docking simulations of the structures were performed. A docking simulation of the structure of the 4-pyridoxolactonase–dianion intermediate (a reaction intermediate) complex was also performed. The best binding models were obtained based on the binding mode of 5PAL with binding energies of −5.15 kcal mol−1 for 4PAL, −15.29 kcal mol−1 for the dianion intermediate and −8.06 kcal mol−1 for 4PA. 4-Pyridoxolactonase showed the highest affinity towards the dianion intermediate.
4PAL bound to the similar position of 5PAL, and the carbonyl O atom pointed towards the catalytic water (Fig. 5 ▶ b). The pyridine N atom of 4PAL formed a hydrogen bond to Glu65 O∊1 (2.8 Å). The C3 hydroxyl group formed two hydrogen bonds to the catalytic water (2.7 Å) and Tyr210 Oη (2.7 Å). In contrast to the binding of 5PAL, the carbonyl O atom of 4PAL interacted with Tyr210 Oη (3.0 Å), Zn1 (2.7 Å) and the catalytic water (2.5 Å). The ring structure of the substrate formed a π-stacking with Phe223. The distance between the carbonyl C atom and the catalytic water (3.1 Å) was reasonable for nucleophilic attack of the water. Although an interaction between a substrate and Zn2 which stabilizes the substrate binding and the subsequent intermediate formation has been observed in other subclass B3 enzymes (Palzkill, 2013 ▶; Momb et al., 2008 ▶), no interaction was observed between the substrate and Zn2 in 4-pyridoxolactonase. The simulated dianionic intermediate moved towards the zinc ions owing to its negative charges (Fig. 5 ▶ c). The C3 hydroxyl group formed a hydrogen bond to Tyr210 Oη (2.6 Å) and became the ligand of Zn2 (1.8 Å). One of the deduced anionic O atoms interacted with Asp100 Oδ1 (2.6 Å) and became the ligand of Zn1 (2.0 Å). The other anionic oxygen derived from the carbonyl O atom of the substrate formed a hydrogen bond to Tyr210 Oη (2.8 Å) and became the ligand of Zn1 (1.7 Å). The lactone O atom weakly interacted with Zn1 (2.9 Å). In the simulated 4PA complex (Fig. 5 ▶ d), the C3 hydroxyl group formed a hydrogen bond to Tyr210 Oη (2.8 Å) and became the ligand of Zn2 (1.8 Å). The C4 carboxyl group also became the ligand of Zn1 (1.7 Å) and interacted with Asp100 Oδ1 (2.7 Å). The carboxyl group occupied almost the same position as the catalytic water in the 4PAL complex (Fig. 5 ▶ e).
3.7. Catalytic mechanism
4-Pyridoxolactonase coordinated two zinc ions in the manner of the subclass B3 enzymes of the class B β-lactamase (Fig. 3 ▶ a). Therefore, it was assumed that the enzyme binds the substrate and catalyzes the reaction as in the subclass B3 enzymes. However, the simulated binding mode of the substrate was different from that of the subclass B3 enzymes: no interaction was observed between the lactone O atom of the substrate and Zn2. In this mode of binding, the anionic oxygen intermediate cannot be stabilized as discussed previously (Momb et al., 2008 ▶). The simulated binding modes of the substrate, intermediate and product give one possible catalytic mechanism (Fig. 6 ▶). The catalysis follows a subclass B2-like mechanism in which a dianion intermediate is formed as follows (Bounaga et al., 1998 ▶). (i) 4PAL binds to the active site. The carbonyl O atom of 4PAL interacts with Zn1 and forms a hydrogen bond to Tyr210 Oη. These interactions polarize the carbonyl group. Glu65 and Phe223 stabilize the proper substrate binding for initiation of the reaction. (ii) A catalytic water molecule between two zinc ions attacks the polarized carbonyl C atom to form a tetrahedral intermediate. Zn2 and Asp100 enhance the nucleophilicity of the water molecule. The O− (from the carbonyl O atom) of the tetrahedral intermediate coordinates to Zn1 by moving to the zinc ions. In accompaniment with this movement, the C3 hydroxyl group and the new hydroxyl group coordinate to Zn2 and Zn1, respectively. This movement also removes the interaction between the intermediate and Glu65 which neutralized the charge of the pyridine N atom. (iii) The dianion tetrahedral intermediate is then formed by transfer of a proton. The coordination of the C3 hydroxyl group with Zn2 weakens the interaction between Asp100 and Zn2, and makes the transfer of a proton between Asp100 and the new hydroxyl group easy, as discussed previously (Momb et al., 2008 ▶). (iv) Finally, the product is formed and becomes the ligand of the zinc ions. One cycle of the catalysis is completed by dissociation of the product. A new water molecule occupies the vacated place for the next catalytic cycle. The simulated binding energy of 4PA (−8.06 kcal mol−1) is larger than that of 4PAL (−5.15 kcal mol−1). This suggests that some conformational changes may occur during the formation and release of the product.
Figure 6.
A proposed catalytic mechanism of 4-pyridoxolactonase. Coordination bonds are shown as black lines. π-Stacking is shown as an ellipse (black broken line). Hydrogen bonds and interactions with zinc ions are shown as black broken lines.
In this study, we elucidated the crystal structure of 4-pyridoxolactonase and proposed the catalytic mechanism based on the docking simulation. The mechanism follows that found in subclass B2 enzymes and differs from that proposed in the AHL lactonases AiiA (Momb et al., 2008 ▶) and AiiB (Liu et al., 2007 ▶), which catalyze the reaction following the mechanism of the subclass B3 enzymes. Although Asp100 in 4-pyridoxolactonase was conserved as Asp108 in AiiA (Fig. 5 ▶ a) and Asp115 in AiiB, respectively, Glu65 and Phe223 in 4-pyridoxolactonase were replaced by Ile73 and Gly207 in AiiA (Fig. 5 ▶ a) and Ala80 and Val230 in AiiB, respectively. There is no alternative functional group in AHL, such as the C3 hydroxyl group in 4PAL. These differences probably resulted in the difference of the catalytic mechanisms. 4-Pyridoxolactonase shows activity towards AHL, which is the substrate of AiiA and AiiB (Funami et al., 2005 ▶). The structure of the 4-pyridoxolactonase/AHL complex and the reaction mechanism of AHL catalyzed by 4-pyridoxolactonase will provide detailed information regarding the catalytic mechanism of class B β-lactamases. We are continuing our study of this enzyme using X-ray crystallography, site-directed mutagenesis and kinetic analysis.
Supplementary Material
PDB reference: 4-pyridoxolactonase, 3aj3
PDB reference: 3aj0
PDB reference: complex with 5PAL, 4keq
References
- Afonine, P. V., Grosse-Kunstleve, R. W., Echols, N., Headd, J. J., Moriarty, N. W., Mustyakimov, M., Terwilliger, T. C., Urzhumtsev, A., Zwart, P. H. & Adams, P. D. (2012). Acta Cryst. D68, 352–367. [DOI] [PMC free article] [PubMed]
- Auld, D. S. (2001). Biometals, 14, 271–313. [DOI] [PubMed]
- Bounaga, S., Laws, A. P., Galleni, M. & Page, M. I. (1998). Biochem. J. 331, 703–711. [DOI] [PMC free article] [PubMed]
- Burg, R. W. & Snell, E. E. (1969). J. Biol. Chem. 244, 2585–2589. [PubMed]
- Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. (2010). Acta Cryst. D66, 486–501. [DOI] [PMC free article] [PubMed]
- Funami, J., Yoshikane, Y., Kobayashi, H., Yokochi, N., Yuan, B., Iwasaki, K., Ohnishi, K. & Yagi, T. (2005). Biochim. Biophys. Acta, 1753, 234–239. [DOI] [PubMed]
- Garau, G., García-Sáez, I., Bebrone, C., Anne, C., Mercuri, P., Galleni, M., Frère, J.-M. & Dideberg, O. (2004). Antimicrob. Agents Chemother. 48, 2347–2349. [DOI] [PMC free article] [PubMed]
- Holm, L. & Rosenström, P. (2010). Nucleic Acids Res. 38, 545–549. [DOI] [PMC free article] [PubMed]
- Jones, S. & Thornton, J. M. (1996). Proc. Natl Acad. Sci. USA, 93, 13–20.
- Jong, Y.-J. & Snell, E. E. (1986). J. Biol. Chem. 261, 15112–15114. [PubMed]
- Kim, M. H., Choi, W.-C., Kang, H. O., Lee, J. S., Kang, B. S., Kim, K.-J., Derewenda, Z. S., Oh, T.-K., Lee, C. H. & Lee, J.-K. (2005). Proc. Natl Acad. Sci. USA, 102, 17606–17611. [DOI] [PMC free article] [PubMed]
- Krissinel, E. & Henrick, K. (2007). J. Mol. Biol. 372, 774–797. [DOI] [PubMed]
- Laskowski, R. A., MacArthur, M. W., Moss, D. S. & Thornton, J. M. (1993). J. Appl. Cryst. 26, 283–291.
- Liu, D., Momb, J., Thomas, P. W., Moulin, A., Petsko, G. A., Fast, W. & Ringe, D. (2008). Biochemistry, 47, 7706–7714. [DOI] [PMC free article] [PubMed]
- Liu, D., Thomas, P. W., Momb, J., Hoang, Q. Q., Petsko, G. A., Ringe, D. & Fast, W. (2007). Biochemistry, 46, 11789–11799. [DOI] [PubMed]
- Machida, S., Yu, Y., Singh, S. P., Kim, J.-D., Hayashi, K. & Kawata, Y. (1998). FEMS Microbiol. Lett. 159, 41–46. [DOI] [PubMed]
- Matsuda, S., Yokochi, N., Yoshikane, Y., Kobayashi, J., Huy, C. N., Baba, S., Kuramitsu, S., Mikami, B. & Yagi, T. (2009). Acta Cryst. F65, 886–889. [DOI] [PMC free article] [PubMed]
- Momb, J., Wang, C., Liu, D., Thomas, P. W., Petsko, G. A., Guo, H., Ringe, D. & Fast, W. (2008). Biochemistry, 47, 7715–7725. [DOI] [PMC free article] [PubMed]
- Morris, G. M., Huey, R., Lindstrom, W., Sanner, M. F., Belew, R. K., Goodsell, D. S. & Olson, A. J. (2009). J. Comput. Chem. 30, 2785–2791. [DOI] [PMC free article] [PubMed]
- Murshudov, G. N., Skubák, P., Lebedev, A. A., Pannu, N. S., Steiner, R. A., Nicholls, R. A., Winn, M. D., Long, F. & Vagin, A. A. (2011). Acta Cryst. D67, 355–367. [DOI] [PMC free article] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol. 276, 307–326. [DOI] [PubMed]
- Palzkill, T. (2013). Ann. N. Y. Acad. Sci. 1277, 91–104. [DOI] [PMC free article] [PubMed]
- Schüttelkopf, A. W. & van Aalten, D. M. F. (2004). Acta Cryst. D60, 1355–1363. [DOI] [PubMed]
- Sheldrick, G. M. (2010). Acta Cryst. D66, 479–485. [DOI] [PMC free article] [PubMed]
- Terwilliger, T. C. (2000). Acta Cryst. D56, 965–972. [DOI] [PMC free article] [PubMed]
- Terwilliger, T. C. (2003). Acta Cryst. D59, 38–44. [DOI] [PMC free article] [PubMed]
- Vagin, A. & Teplyakov, A. (2010). Acta Cryst. D66, 22–25. [DOI] [PubMed]
- Vaguine, A. A., Richelle, J. & Wodak, S. J. (1999). Acta Cryst. D55, 191–205. [DOI] [PubMed]
- Wang, Z., Fast, W., Valentine, A. M. & Benkovic, S. J. (1999). Curr. Opin. Chem. Biol. 3, 614–622. [DOI] [PubMed]
- Winn, M. D. et al. (2011). Acta Cryst. D67, 235–242.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: 4-pyridoxolactonase, 3aj3
PDB reference: 3aj0
PDB reference: complex with 5PAL, 4keq