Abstract
Objectives
The strain relaxation index (SRI), a novel diastolic functional parameter derived from tagged magnetic resonance imaging (MRI), is used to assess myocardial deformation during left ventricular relaxation. We investigated whether diastolic function indexed by SRI predicts heart failure (HF) and atrial fibrillation (AF) over an 8-year follow-up.
Methods
As a part of the multi-ethnic study of atherosclerosis, 1544 participants free of known cardiovascular disease (CVD) underwent tagged MRI in 2000–02. Harmonic phase analysis was used to compute circumferential strain. Standard parameters, early diastolic strain rate (EDSR) and the peak torsion recoil rate were calculated. An SRI was calculated as difference between post-systolic and systolic times of the strain peaks, divided by the EDSR peak. It was normalized by the total interval of relaxation. Over an 8-year follow-up period, we defined AF (n = 57) or HF (n = 36) as combined (n = 80) end-points. Cox regression assessed the ability of SRI to predict events adjusted for risk factors and markers of subclinical disease. Integrated discrimination index (IDI) and net reclassification index (NRI) of SRI, compared with conventional indices, were also assessed.
Results
The hazard ratio for SRI remained significant for the combined HF and AF end-points as well as for HF alone after adjustment. For the combined end-point, IDI was 1.5% (P < 0.05) and NRI was 11.4% (P < 0.05) for SRI. Finally, SRI was more robust than all other existing cardiovascular magnetic resonance diastolic functional parameters.
Conclusion
SRI predicts HF and AF over an 8-year follow-up period in a large population free of known CVD, independent of established risk factors and markers of subclinical CVD.
Keywords: Heart failure, Atrial, Fibrillation, Diastole, Magnetic resonance imaging
Introduction
Left ventricular diastolic dysfunction is a highly prevalent condition with strong associations with heart failure (HF) and atrial fibrillation (AF) established in previous cross-sectional studies. It has traditionally been thought that there is a similar pathophysiological mechanism underlying both diastolic HF and AF, secondary to abnormal diastolic function leading to elevated end-diastolic pressure; however, the specific role of abnormal myocardial diastolic deformation in this causation chain remains largely unclear1–6.
Myocardial circumferential strain and strain rate using cardiovascular magnetic resonance (CMR)-tagged images have been shown to accurately and reproducibly quantify deformation of the left ventricle (LV) through systole and diastole.7 Evaluation of diastolic function using CMR has, however, not been firmly established despite a number of prior efforts.8–11 Early diastolic strain rate (EDSR) and torsion recoil rate have been used as diastolic parameters, but have not yet been shown to predict cardiovascular events.8–10,12
In this prospective study, strain relaxation index (SRI), a measure of diastolic function based on strain from tagged magnetic resonance imaging (MRI), is introduced. The ability of SRI to predict incident HF, AF, and the combination of HF with AF in a large asymptomatic multi-ethnic population over an 8-year follow-up period is tested, and compared with the predictive abilities of EDSR and the torsion recoil rate. The improvement in discrimination and reclassification of events with the addition of the different diastolic functional parameters over and above conventional risk factors and markers of subclinical cardiovascular diseases (CVDs) is investigated.
Methods
Theoretical framework of strain relaxation index
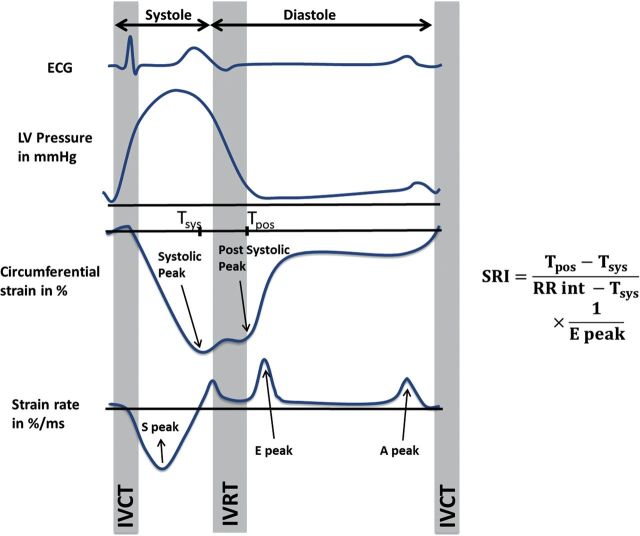
Figure 1 illustrates the deformation curves through the cardiac cycle. During the cardiac cycle, the circumferential strain reaches a minimum value (maximal shortening) at the peak systolic strain. In sequence, early left ventricular relaxation starts, followed shortly by the closure of the aortic valve (AVC). During the isovolumic relaxation time (IVRT), a positive peak can be observed in the circumferential strain rate curve following the AVC.13 The post-systolic strain peak, a minimum in the strain curve, can be observed at the end of the IVRT. After the opening of the mitral valve, a positive peak can be observed in the strain rate curve, the peak early diastolic strain rate.8
Figure 1.
This figure illustrating the calculation of the proposed SRI from the circumferential strain and strain rate curves. More negative strain values indicate greater circumferential shortening. SRI is calculated as the ratio of the duration of very early relaxation to that of the diastolic interval, divided by the early diastolic strain rate peak. The myocardial relaxation as imagined with a hypothetical pressure curve and electrocardiograph for reference. SRI: strain relaxation index; RR int: RR interval; S peak: peak systolic strain rate; IVCT: isovolumic contraction time; IVRT: isovolumic relaxation time; E peak: peak early diastolic strain rate; A peak: peak atrial-diastolic strain rate; Tsys: time of occurrence of peak systolic strain; Tpos: time of occurrence of post-systolic strain peak.
The greater the difference between time to systolic and post-systolic strain peaks in the early stage of cardiac relaxation, the longer it takes to achieve the pressure drop required for diastolic filling. This is similar to the IVRT, which increases in the case of diastolic dysfunction.14–16 Moreover, the early diastolic strain rate (EDSR) decreases with diastolic dysfunction, indicating stiffer tissue.8 Therefore, the combination of early cardiac relaxation and tissue relaxation properties is proposed as an accurate indicator of diastolic LV function. SRI was calculated as follows:
The SRI was calculated as the difference between post-systolic (Tpos) and systolic (Tsys) times of the strain peaks divided by the early diastolic strain rate (EDSR) peak. The time difference was normalized by the difference between the cardiac inter-beat interval and the time-to-peak systolic strain, representing the total interval of relaxation. SRI is presented in ms/%.
Study population
The design and population characteristics of the multi-ethnic study of atherosclerosis (MESA) have been described previously.17,18 Briefly, MESA is a prospective, population-based observational cohort study of 6814 men and women representing four racial/ethnic groups (Caucasian, African-American, Hispanic, and Chinese-American), aged 45–84 years and free of clinical CVD at enrolment. As part of the baseline examination, between 2001 and 2002, a total of 5004 (73%) participants received comprehensive cardiac MRI studies at six field centres. The institutional review boards of all MESA field centres approved the study protocol, and all participants gave informed consent. Of the 5004 individuals who underwent cardiac MRI examination, 1617 with available clinical covariate data agreed to a slightly longer MRI examination to accommodate MRI tagging sequences. Of these participants, deformation data could not be analysed owing to data acquisition failure or insufficient quality for strain and strain rate determination in 73 participants. The remaining 1544 participants with complete circumferential strain, strain rate, and strain relaxation rate measurements were included in this analysis. Of these 1544 participants, 743 underwent a follow-up examination after an 8-year period, with MRI tagging as a part of the imaging protocol. Of these, 27 were excluded because of insufficient quality of determined strain or data acquisition failure. Tagged MR protocol and analysis methods remained the same in the baseline and follow-up visits.
Magnetic resonance imaging
Images were acquired in whole-body scanners using electrocardiogram triggered segmented k-space fast spoiled gradient-echo pulse sequences during breath holds. CMR myocardial horizontal and vertical tagging were performed on three LV short-axis slices (base, mid, and apex) by non-selective radiofrequency pulses separated by a spatial modulation of magnetization-encoding gradients. Parameters for imaging and analysis methods have been described previously.18
Short-axis-tagged slices were analysed by the harmonic phase method.19 Systolic and post-systolic circumferential strain peaks were assessed from the mid-wall mid-ventricular circumferential strain (Ecc) and strain rates through the cardiac cycle. These were then used to compute SRI and EDSR. Ecc values are conventionally negative to express circumferential shortening. Torsion curves were computed as previously described.20 The peak torsion recoil rate (deg/cm/ms) was calculated as the first minimum from the rate curve after peak torsion.
Follow-up and end-points
Events adjudicated as incident HF and AF as part of the MESA study were used as end-points. A telephone interviewer contacted each participant (or representative) every 6–9 months to inquire about all interim hospital admissions, cardiovascular outpatient diagnoses, and deaths. Two physicians reviewed all records for independent end-point classification and assignment of event dates.21
Criteria for HF as end-point included symptomatic HF diagnosed by a physician and patient receiving medical treatment for HF and (i) pulmonary oedema/congestion by chest X-ray, and/or (ii) dilated ventricle or poor LV function by echocardiography or ventriculography, or evidence of LV diastolic dysfunction. Participants who had a physician's diagnosis of HF were classified as having HF. Criteria for AF as end-point were if in-hospital AF was diagnosed according to ICD9 codes. The combined end-point was ascertained as the first-documented event of either HF or AF.
Conventional risk factor measures (age, race, gender; body mass index, smoking status, systolic blood pressure, use of hypertension medication, diabetes mellitus/impaired fasting glucose, low-density lipoprotein cholesterol, and total cholesterol),21 serum concentration of n-terminal pro-brain natriuretic peptide (NT-proBNP),22 and coronary calcium scores23,24 were obtained as explained previously.
Statistical analysis
Probability distributions of all continuous variables were graphically examined and tested by the goodness-of-fit tests for normality. Summary statistics were presented as mean/SD for continuous variables and as percentages for categorical variables. Natural logarithmic transformation was applied to SRI, EDSR, and NT-proBNP, since these variables have skewed distributions. The mean differences in diastolic function between the follow-up and baseline exams were assessed by the two-sided t-test based on participants who had both baseline and the follow-up MRI exams. AHA recommendations for evaluating novel cardiovascular risk factors25 were used for statistical analysis procedures.
Univariable Cox models were used to assess the prediction ability of diastolic function parameters separately on the time-to-event distribution of the combined end-point. Multivariable Cox models were used to assess the prediction ability of diastolic function parameters to the time-to-event distribution of the combined end-point with the addition of conventional risk factors. The hazard ratios (HRs) along with the corresponding 95% confidence intervals and P-values were used to make statistical inference on the covariate effects. The added value of diastolic function parameters to the existing model was calculated from the difference in the calculated Harrell's C-statistic and the significance of this difference.
A secondary analysis, also using multivariable Cox models, was performed to test the ability of diastolic function parameters to predict the time-to-event probabilities for HF and/or AF independent of other established risk factors. Since some participants have missing covariates, this analysis was performed on a subset of the full cohort. Because of the design of the study, the missing covariates can be reasonably assumed to be missing at random. Models considered were those with the progressive addition of established risk factors to conventional risk factors—coronary calcium score24 (Model 1), LV mass index26 (Model 2), LV ejection fraction (Model 3), and NT-proBNP27 (Model 4). Calibration of the models was confirmed using the Gronnesby–Borgan tests to compare the expected and observed event rates across deciles for each model.
Integrated discrimination index (IDI) based on the Cox models was calculated to report the improvement in discrimination based on the survival probabilities with the addition of diastolic function parameters to the conventional risk factors.28 Net reclassification index (NRI) based on the Cox models was used to quantify the number of individuals correctly and incorrectly reclassified with the addition of the new biomarker into low-, intermediate-, and high-risk categories within 8 years.28 Risk categories of <5, 5–20, and >20% were used in the measurement of NRI for HF27,29 and the combined end-points. For AF29,30 as the end-point, categories were defined as <5, 5–15, and >15%.
Two-tailed P-values were <0.05 used for significance testing. All statistical analysis was done using the STATA v11.0 (StataCorp LP, College Station, TX, USA).
Results
Baseline characteristics of the participants are provided in Table 1. HF was present in 2.6% (n = 36) of the population, whereas AF was incident in 3.9% (n = 57) over the 8-year follow-up period. Fourteen participants had both AF and HF, and 12 with AF preceding HF. The incidence of HF and AF were associated with increased age, male gender, higher body mass index, higher systolic blood pressure, decreased early diastolic strain rate, and increased SRI.
Table 1.
Baseline characteristics
| Variable | Mean (SD) |
|||
|---|---|---|---|---|
| Overall (n = 1544) | HF (n = 36) | AF (n = 57) | Combined (n = 80) | |
| Age (years) | 65 ± 9.7 | 70.2 ± 8.2 | 70.7 ± 9.1 | 70.3 ± 8.7 |
| Body mass index (kg/m2) | 27.8 ± 4.7 | 28.9 ± 4.1 | 28.3 ± 4.1 | 28.6 ± 4.3 |
| Systolic blood pressure (mmHg) | 128 ± 20.7 | 134.8 ± 20.0 | 139.1 ± 22.5 | 136.9 ± 22.3 |
| HDL cholesterol (mg/dL) | 50.6 ± 14.5 | 49.1 ± 12.1 | 49 ± 14.1 | 48.8 ± 13.8 |
| Total cholesterol (mg/dL) | 194.2 ± 34.9 | 180.8 ± 26.9 | 183.9 ± 30.1 | 181.5 ± 28.8 |
| EDSR (%/ms) | 0.12 ± 0.06 | 0.10 ± 0.05 | 0.10 ± 0.05 | 0.10 ± 0.05 |
| log(SRI) (ms/%) | 0.78 ± 0.56 | 1.12 ± 0.45 | 1.01 ± 0.52 | 1.04 ± 0.51 |
| Torsion recoil rate (deg/cm/ms) | −19 ± 11 | −18 ± 13 | −21 ± 13 | −19 ± 13 |
| Variable | Proportion of participants (%) |
|||
| Overall (n = 1544) | HF (n = 36) | AF (n = 5) | Combined (n = 80) | |
| Men | 53 | 72.9 | 69.5 | 71.2 |
| Race | ||||
| Caucasian | 28.9 | 16.3 | 47.5 | 34.3 |
| Chinese-American | 14.6 | 10.8 | 11.9 | 10.9 |
| African-American | 27.8 | 27 | 16.9 | 21.9 |
| Hispanic | 28.7 | 45.9 | 23.7 | 32.9 |
| Smokers | ||||
| Former | 35.9 | 50 | 36.2 | 40.7 |
| Current | 11.3 | 11.1 | 12.1 | 11.1 |
| Diabetes/ impaired fasting glucose | 31.2 | 54 | 35.6 | 63 |
| Use of hypertension medication | 39.9 | 54.1 | 57.6 | 58.5 |
Shown are baseline characteristics of individuals who underwent tagged MRI at baseline and with information on conventional risk factors. For continuous variables, mean ± SD are given and for categorical variables, % are given.
SRI: strain relaxation index; EDSR: early diastolic strain rate; HF: heart failure; AF: atrial fibrillation; HDL: high-density lipoprotein.
In the longitudinal follow-up, logSRI increased significantly (P < 0.05) from 0.74 ± 0.58 at baseline to 1 ± 0.58 at follow-up in the population free of clinical events (n = 696). In the same period and using the same population, early diastolic strain rate (EDSR) decreased from 0.12 ± 0.06 to 0.10 ± 0.04 (P < 0.05). The torsion recoil rate increased from −19.3 ± 11.2 at baseline to −22.7 ± 9.4 at follow-up (P < 0.05). In those with HF (n = 7), an increase in logSRI (0.97 ± 0.42–1.65 ± 0.41, P < 0.05), a decrease in EDSR (0.09 ± 0.03–0.06 ± 0.03, P < 0.05), and no significant change in the torsion recoil rate (−8.2 ± 7.9 to −14.7 ± 4.5, P = NS) were seen from baseline to follow-up exams. In those with AF (n = 14), no significant changes were seen in logSRI (0.98 ± 0.93 to –0.76 ± 0.65, P = NS), EDSR (0.10 ± 0.06 to –0.11 ± 0.05, P = NS), and the torsion recoil rate (−9.6 ± 11.3 to −21.5 ± 10.3, P = NS) from baseline to follow-up exams.
Prediction of a combined end-point of HF and AF as well as HF and AF
Using Kaplan–Meier survival curves, SRI showed robust prediction of HF, AF, and the combined end-points across tertiles (Figure 2). The HRs for SRI and EDSR alone were both significant for the combined end-point in the univariate analysis and after adjustment for conventional risk factors, with values favouring SRI. C-statistics showed better performance for SRI when compared with EDSR; with significant improvement in HF and a trend towards statistical significance for the combined end-point (Table 2). The torsion recoil rate did not predict HF, AF, or the combined end-point.
Figure 2.
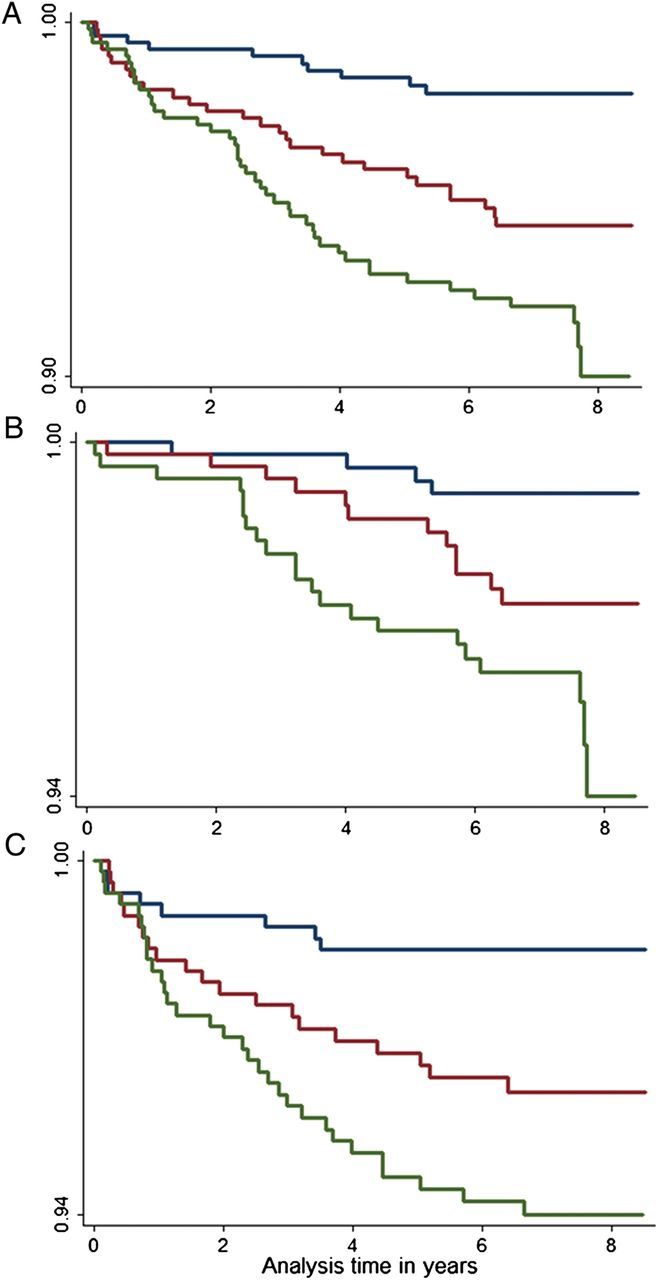
This figure show the Kaplan–Meier survival curves for combined (A), HF (B), and AF (C), end-points across tertiles of log(SRI). Individuals were free of AF or HF at baseline. log(SRI) expressed as median (minimum, maximum) across three tertiles were Q1: 0.119 (−0.2.239, 0.544), Q2: 0.805(0.544, 1.034), and Q3: 1.378(1.034, 3.471). P < 0.001 for all trends.
Table 2.
Prediction and discrimination assessment on the combined end-point of HF and/or AF for CMR-derived diastolic parameters (n = 1544)
| HR (95% CI) |
Discrimination |
||||
|---|---|---|---|---|---|
| Univariate | Multivariate | AUC | Difference | P | |
| Combined (n = 80) | |||||
| log(EDSR) | 0.32 (0.19–0.53) | 0.51 (0.30–0.84) | 0.763 | 0.005 | 0.556 |
| log(SRI) | 2.54 (1.76–3.66) | 1.88 (1.29–2.74) | 0.774 | 0.016 | 0.099 |
| Torsion recoil rate | 1.01 (0.99–1.03) | – | – | – | – |
| HF (n = 36) | |||||
| log(EDSR) | 0.26 (0.12–0.55) | 0.45 (0.21–0.97) | 0.786 | 0.013 | 0.342 |
| log(SRI) | 3.22 (1.91–5.43) | 2.25 (1.30–3.89) | 0.803 | 0.030 | 0.039 |
| Torsion recoil rate | 1.02 (0.98–1.05) | – | – | – | – |
| AF (n = 57) | |||||
| log(EDSR) | 0.39 (0.22–0.69) | 0.57 (0.32–1.02) | 0.774 | 0.003 | 0.612 |
| log(SRI) | 2.35 (1.52–3.62) | 1.77 (1.13–2.76) | 0.783 | 0.009 | 0.421 |
| Torsion recoil rate | 0.99 (0.97–1.02) | – | – | – | – |
End-point is the participants who had atrial fibrillation and heart failure, whichever happened first. In multivariate analysis, adjustments were made for age, race, gender; body mass index, smoking status, systolic blood pressure, use of hypertension medication, diabetes mellitus/impaired fasting glucose, low-density lipoprotein (LDL) cholesterol, total cholesterol, and log(SRI).
SRI: strain relaxation index; EDSR: early diastolic strain rate; Combined, AF, or HF; HF: heart failure; AF: atrial fibrillation; AUC: area under the curve.
In a subset of the cohort (n = 1255; patients characteristics in Tables 3 and 4), SRI had consistent performance as an independent predictor of HF or AF after progressive adjustment to computed tomography-derived calcium score, LV mass index, LV ejection fraction, and serum NT-proBNP in addition to the conventional risk factors. In comparison, EDSR had significant predictive power independent of only calcium score, but not with the addition of LV mass index.
Table 3.
Baseline characteristics for secondary analysis in the subpopulation
| Variable | Mean (SD) |
|||
|---|---|---|---|---|
| Overall (n = 1255) | HF (n = 28) | AF (n = 49) | Combined (n = 65) | |
| Age (year) | 65.3 ± 9.6 | 71.1 ± 7.5 | 70.9 ± 9.4 | 70.7 ± 8.6 |
| Body mass index (kg/m2) | 27.6 ± 4.7 | 29.0 ± 4.2 | 28.1 ± 4.2 | 28.4 ± 4.3 |
| Systolic blood pressure (mmHg) | 128.2 ± 20.7 | 134.9 ± 19.8 | 140.4 ± 21.5 | 138.2 ± 21.6 |
| HDL cholesterol (mg/dL) | 50.6 ± 14.6 | 49.7 ± 12.7 | 49.8 ± 14.6 | 49.7 ± 14.3 |
| Total cholesterol (mg/dL) | 194 ± 34.9 | 181 ± 27.6 | 186.6 ± 30 | 183.9 ± 29.2 |
| log(BNP) (pg/mL) | 4 ± 1.19 | 5.51 ± 1.39 | 5.13 ± 1.29 | 5.14 ± 1.34 |
| LV mass index (g/m1.7) | 61.3 ± 14.2 | 76.4 ± 21.4 | 69.5 ± 19.5 | 70.2 ± 18.6 |
| LVEF (%) | 69 ± 7.6 | 63.7 ± 11.6 | 67.9 ± 10.9 | 67.3 ± 10.6 |
| EDSR (%/ms) | 0.12 ± 0.06 | 0.10 ± 0.04 | 0.10 ± 0.04 | 0.10 ± 0.04 |
| log(SRI) (ms/%) | 0.76 ± 0.60 | 1.07 ± 0.42 | 1.01 ± 0.52 | 1.00 ± 0.49 |
| Variable | Proportion of participants (%) |
|||
| Overall (n = 1255) | HF (n = 28) | AF (n = 49) | Combined (n = 65) | |
| Men | 54.5 | 71.4 | 71.4 | 70.7 |
| Race | ||||
| Caucasian | 29.9 | 17.9 | 51.1 | 38.5 |
| Chinese-American | 16.2 | 10.7 | 10.2 | 10.8 |
| African-American | 23.9 | 21.4 | 14.2 | 16.9 |
| Hispanic | 30 | 50 | 24.5 | 33.8 |
| Smokers | ||||
| Former | 36.5 | 51.9 | 37.5 | 42.2 |
| Current | 10.8 | 11.1 | 12.5 | 10.9 |
| Diabetes/impaired fasting glucose | 31.9 | 60.7 | 36.7 | 46.2 |
| Use of hypertension medication | 39.5 | 50 | 55.1 | 55.4 |
| Calcium score categories | ||||
| 0 | 44.2 | 17.8 | 16.4 | 20.1 |
| 1–100 | 27.6 | 25 | 28.6 | 27.6 |
| 101–300 | 14.1 | 14.3 | 16.3 | 15.4 |
| >300 | 14.1 | 42.9 | 48.7 | 36.9 |
Shown are baseline characteristics of individuals who underwent tagged MRI at baseline and with information on conventional risk factors. For continuous variables, mean ± SD are given and for categorical variables, % are given.
SRI: strain relaxation index; EDSR: early diastolic strain rate; BNP: brain natriuretic peptide.
Table 4.
Prediction and discrimination assessment on the combined end-point of HF and/or AF for CMR-derived diastolic parameters (n = 1255, 65 events)
| log(EDSR) |
log(SRI) |
|||||
|---|---|---|---|---|---|---|
| HR (95% CI) | AUC | Difference | HR (95% CI) | AUC | Difference | |
| Model 1 | 0.53 (0.30–0.92) | 0.784 | 0.007 | 1.81 (1.17–2.79) | 0.791 | 0.014 |
| Model 2 | 0.59 (0.34–1.05) | – | – | 1.72 (1.11–2.67) | 0.808 | 0.010 |
| Model 3 | 0.60 (0.34–1.06) | – | – | 1.72 (1.11–2.66) | 0.808 | 0.010 |
| Model 4 | 0.61 (0.34–1.09) | – | – | 1.77 (1.13–2.76) | 0.827 | 0.006 |
End-point is the participants who had atrial fibrillation or heart failure combined. In multivariate analysis, adjustments to different variables were made for each model. Model 1: age, race, gender; body mass index, smoking status, systolic blood pressure, diastolic blood pressure, use of hypertension medication, diabetes mellitus/impaired fasting glucose, LDL cholesterol, total cholesterol, categories of coronary calcium, and log(SRI); Model 2: Model 1 + LV mass index; Model 3: Model 2 + LV ejection fraction; Model 4: Model 3 + log(BNP).
SRI: strain relaxation index; EDSR: early diastolic strain rate; AUC: area under the curve.
Discrimination and reclassification
For the combined end-point, there was a significant improvement in discrimination of risks for events and non-events as assessed by IDI with the addition of SRI to the conventional risk factor model of 1.5% (P = 0.001). The IDI for HF and AF as end-points were 1.1% (P = 0.13) and 1.0% (P = 0.017), respectively. The values for the combined end-point, HF, and AF using EDSR were 1.3% (P = 0.006), 0.9% (P = 0.19), and 0.7% (P = 0.05) respectively.
Risk category reclassification (NRI) was higher for the prediction of combined end-points when compared with only the conventional risk factors. NRI for the combined end-point using SRI and EDSR were 11.4% (P = 0.007) and 9.5% (P = 0.044), respectively. The improvement in the net reclassification was both a result of upward reclassification of events to higher risk categories and a downward reclassification of non-events. The NRI for HF and AF individually were not significant.
Discussion
In a large population free of known CVD at baseline, diastolic function from circumferential strain curves showed a powerful independent ability for the prediction of HF and AF over an 8-year follow-up period. The addition of diastolic function to conventional risk factors significantly improved discrimination and reclassification for the combined HF and AF end-point. SRI, a robust and sensitive SRI to assess diastolic dysfunction by tagged CMR images, showed improved prediction, discrimination, and reclassification abilities in comparison with previously proposed CMR diastolic function parameters, such as EDSR and torsion recoil rate.
HF and AF are linked to a similar pathologic pathway, as both can be mediated by diastolic dysfunction secondary to similar cardiovascular risk factors. These risk factors have been associated with myocardial intracellular and extracellular, as well as electrophysiological changes that combine to create LV dysfunction, leading to both HF and AF.1,31 In our study, both HF and AF had a similar association with CMR diastolic parameters, again suggesting that these two conditions likely share significant similar causal pathways.
Diastolic dysfunction is related to both diagnostic and prognostic aspects of HF and AF. In fact, diastolic function may be the earliest parameter to become altered in progressive LV dysfunction.32 In this regard, clinical events of HF and AF have shown important relations to diastolic dysfunction as assessed by echocardiography, although such relationships have not been uniformly consistent in previous studies. Echocardiography has been used to predict HF and AF using parameters based on the diastolic phase (e.g. IVRT, deceleration time) and/or accounting for both early and late diastolic filling periods (E/A ratio).3–6 CMR has proven to be the most accurate method to assess cardiac structure and systolic function. However, the assessment of diastolic dysfunction by CMR has not been established. Despite previous efforts, no CMR-derived diastolic parameter so far showed robust prediction ability for clinical events. In this study, we demonstrate that SRI is a robust predictor of clinical events known to be associated with impaired diastolic function, namely HF and AF.
The extent of post-systolic shortening, the local minimum found on the strain curve prior to the time of peak early diastolic strain rate, has been used to study the influence of ischaemia in segmental myocardial dysfunction.33,34 However, in this study, we demonstrate that it is also a component of normal myocardial mechanical physiology.35–38 The interval between the occurrence of the post-systolic peak and the peak systolic strain (analogous to the IVRT) is a measure of cardiac relaxation and is influenced by increased arterial impedance, intracellular calcium overload, and diastolic filling pressures.15 The early-diastolic strain rate (EDSR), on the other hand, is mainly a measure of ventricular filling reflecting chamber stiffness due to fibrosis, myocyte loss, and changes in LV geometry. SRI, on the other hand, accounts for both the active (time difference between peaks) and passive processes (EDSR) of relaxation, possibly underlying its increased predictive power relative to other CMR-derived indices of diastolic impaired performance. Indeed, in this study, we show that SRI, as it combines diverse factors related to the very early relaxation period, has a better predictive ability when compared with the EDSR or torsion recoil rate. Multivariate analysis revealed that SRI was an independent predictor of HF or AF after adjustment for conventional risk factors, calcium score categories, CMR-derived LV mass index, LV ejection fraction, and NT-proBNP. Moreover, in comparison with baseline values, we demonstrate a temporal (after an 8-year follow-up) increase in SRI and a decrease in EDSR values, which are consistent with the diastolic functional decline associated with aging.
AF and HF with a preserved ejection fraction have emerged as cardiovascular epidemics. LV diastolic dysfunction measurement and grading by non-invasive means could be a crucial component as a complement to diagnosis in the clinical setting. The diagnostic relevance of imaging-based diastolic function indices and their application across different modalities is crucial. We have shown a diastolic function parameter that, in addition to providing robust prediction information and improved discrimination and reclassification, has the potential to be applied across different modalities. In addition to tagged MRI as shown here, other strain estimation methods, such as speckle tracking echocardiography, can potentially be used to derive SRI.
The limitations of the study include the small number of events as the MESA cohort includes only those without any CVD at baseline. A comparison of prediction powers of diastolic function from MRI with that from echocardiography could not be performed because echocardiographic data was not obtained at baseline. Tag fading can be a problem in measuring motion from harmonic phase in tagged MRI.11 This is particularly true at mid-to-late diastole. In this study, the parameters measured were at very early and early diastole, when the effects of tag fading are minimal, if any. Of the 73 studies that were excluded, 32 (<2%) were excluded because of problems from tag fading affecting the acquisition.
In conclusion, we show that diastolic function assessed by SRI derived from tagged CMR studies provides robust predictive information for the future development of HF and AF over an 8-year follow-up period in a multi-ethnic asymptomatic population without CVD at baseline. SRI accounts for both myocardial relaxation and tissue compliance, and predicts HF and AF independent of established risk factors and conventional markers of subclinical CVD, such as coronary calcium score and left ventricular hypertrophy.
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org/ (UID: NCT00005487).
Conflict of interest: none declared.
References
- 1.Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure. Circulation. 2009;119:2516–25. doi: 10.1161/CIRCULATIONAHA.108.821306. [DOI] [PubMed] [Google Scholar]
- 2.Leite-Moreira AF. Current perspectives in diastolic dysfunction and diastolic heart failure. Heart. 2006;92:712–8. doi: 10.1136/hrt.2005.062950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsang TSM, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Risks for atrial fibrillation and congestive heart failure in patients ≥65 years of age with abnormal left ventricular diastolic relaxation. Am J Cardiol. 2004;93:54–8. doi: 10.1016/j.amjcard.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Tsang TSM, Gersh BJ, Appleton CP, Tajik AJ, Barnes ME, Bailey KR, et al. Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J Am Coll Cardiol. 2002;40:1636–44. doi: 10.1016/s0735-1097(02)02373-2. [DOI] [PubMed] [Google Scholar]
- 5.Aurigemma GP, Gottdiener JS, Shemanski L, Gardin J, Kitzman D. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: The Cardiovascular Health Study. J Am Coll Cardiol. 2001;37:1042–8. doi: 10.1016/s0735-1097(01)01110-x. [DOI] [PubMed] [Google Scholar]
- 6.Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC, et al. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–63. doi: 10.1001/jama.2011.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castillo E, Osman NF, Rosen BD, El-Shehaby I, Pan L, Jerosch-Herold M, et al. Quantitative assessment of regional myocardial function with MR-tagging in a multi-center study: interobserver and intraobserver agreement of fast strain analysis with Harmonic Phase (HARP) MRI. J Cardiovasc Magn Res. 2005;7:783–91. doi: 10.1080/10976640500295417. [DOI] [PubMed] [Google Scholar]
- 8.Ennis DB, Epstein FH, Kellman P, Fananapazir L, McVeigh ER, Arai AE. Assessment of regional systolic and diastolic dysfunction in familial hypertrophic cardiomyopathy using MR tagging. Magn Reson Med. 2003;50:638–42. doi: 10.1002/mrm.10543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edvardsen T, Rosen BD, Pan L, Jerosch-Herold M, Lai S, Hundley WG, et al. Regional diastolic dysfunction in individuals with left ventricular hypertrophy measured by tagged magnetic resonance imaging—the Multi-Ethnic Study of Atherosclerosis (MESA) Am Heart J. 2006;151:109–14. doi: 10.1016/j.ahj.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Dong SJ, Hees PS, Siu CO, Weiss JL, Shapiro EP. MRI assessment of LV relaxation by untwisting rate: a new isovolumic phase measure of τ. Am J Physiol Heart Circul Physiol. 2001;281:H2002–9. doi: 10.1152/ajpheart.2001.281.5.H2002. [DOI] [PubMed] [Google Scholar]
- 11.Garot J. The study of diastole by tagged MRI: are we nearly there yet? Eur Heart J. 2004;25:1376–7. doi: 10.1016/j.ehj.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 12.Thompson RB, Paterson I, Chow K, Cheng-Baron J, Scott JM, Esch BT, et al. Characterization of the relationship between systolic shear strain and early diastolic shear strain rates: insights into torsional recoil. Am J Physiol Heart Circul Physiol. 2010;299:H898–907. doi: 10.1152/ajpheart.00353.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Khoury DS, Thohan V, Torre-Amione G, Nagueh SF. Global diastolic strain rate for the assessment of left ventricular relaxation and filling pressures. Circulation. 2007;115:1376–83. doi: 10.1161/CIRCULATIONAHA.106.662882. [DOI] [PubMed] [Google Scholar]
- 14.Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: part I. Circulation. 2002;105:1387–93. doi: 10.1161/hc1102.105289. [DOI] [PubMed] [Google Scholar]
- 15.Rudko R, Przewlocki T, Pasowicz M, Biernacka B, Kablak-Ziembicka A, Tracz W. IVRT′/IVRT index is a useful tool for detection of elevated left ventricular filling pressure in patients with preserved ejection fraction. Echocardiography. 2008;25:473–81. doi: 10.1111/j.1540-8175.2008.00644.x. [DOI] [PubMed] [Google Scholar]
- 16.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure—abnormalities in active relaxation and passive stiffness of the left ventricle. New Engl J Med. 2004;350:1953–9. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 17.Bild DE, Bluemke DA, Burke GL, Detrano R, Roux AVD, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 18.Fernandes VRS, Cheng S, Cheng YJ, Rosen B, Agarwal S, McClelland RL, et al. Racial and ethnic differences in subclinical myocardial function: the Multi-Ethnic Study of Atherosclerosis. Heart. 2011;97:405. doi: 10.1136/hrt.2010.209452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osman NF, McVeigh ER, Prince JL. Imaging heart motion using harmonic phase MRI. IEEE Trans Med Imaging. 2000;19:186–202. doi: 10.1109/42.845177. [DOI] [PubMed] [Google Scholar]
- 20.Yoneyama K, Gjesdal O, Choi EY, Wu CO, Hundley WG, Gomes AS, et al. Age, sex, and hypertension-related remodeling influences left ventricular torsion assessed by tagged cardiac magnetic resonance in asymptomatic individuals: the multi-ethnic study of atherosclerosis. Circulation. 2012;126:2481–90. doi: 10.1161/CIRCULATIONAHA.112.093146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bluemke DA, Kronmal RA, Lima JAC, Liu K, Olson J, Burke GL, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–55. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi E-Y, Bahrami H, Wu CO, Greenland P, Cushman M, Daniels LB, et al. N-terminal Pro-B-Type natriuretic peptide, left ventricular mass, and incident heart failure: the Multi-Ethnic Study of Atherosclerosis. Circ Heart Fail. 2012;5:727–34. doi: 10.1161/CIRCHEARTFAILURE.112.968701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) Study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 24.Lakoski SG, Greenland P, Wong ND, Schreiner PJ, Herrington DM, Kronmal RA, et al. Coronary artery calcium scores and risk for cardiovascular events in women classified as ‘low risk’ based on Framingham risk score: the multi-ethnic study of atherosclerosis (MESA) Arch Intern Med. 2007;167:2437. doi: 10.1001/archinte.167.22.2437. [DOI] [PubMed] [Google Scholar]
- 25.Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MSV, et al. Criteria for evaluation of novel markers of cardiovascular risk. Circulation. 2009;119:2408–16. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chirinos JA, Segers P, De Buyzere ML, Kronmal RA, Raja MW, De Bacquer D, et al. Left ventricular mass. Hypertension. 2010;56:91–8. doi: 10.1161/HYPERTENSIONAHA.110.150250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melander O, Newton-Cheh C, Almgren P, Hedblad B, Berglund G, Engström G, et al. Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA. 2009;302:49–57. doi: 10.1001/jama.2009.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pencina MJ, D'Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith JG, Newton-Cheh C, Almgren P, Struck J, Morgenthaler NG, Bergmann A, et al. Assessment of conventional cardiovascular risk factors and multiple biomarkers for the prediction of incident heart failure and atrial fibrillation. J Am Coll Cardiol. 2010;56:1712–9. doi: 10.1016/j.jacc.2010.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D'Agostino Sr RB, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. The Lancet. 2009;373:739–45. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality. Circulation. 2003;107:2920–5. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 32.Brutsaert D, Sys S. Relaxation and diastole of the heart. Physiol Rev. 1989;69:1228–315. doi: 10.1152/physrev.1989.69.4.1228. [DOI] [PubMed] [Google Scholar]
- 33.Skulstad H, Edvardsen T, Urheim S, Rabben SI, Stugaard M, Lyseggen E, et al. Postsystolic shortening in ischemic myocardium. Circulation. 2002;106:718–24. doi: 10.1161/01.cir.0000024102.55150.b6. [DOI] [PubMed] [Google Scholar]
- 34.Urheim S, Edvardsen T, Steine K, Skulstad H, Lyseggen E, Rodevand O, et al. Postsystolic shortening of ischemic myocardium: a mechanism of abnormal intraventricular filling. Am J Physiol Heart Circul Physiol. 2003;284:H2343–50. doi: 10.1152/ajpheart.00320.2002. [DOI] [PubMed] [Google Scholar]
- 35.Sengupta PP, Krishnamoorthy VK, Korinek J, Narula J, Vannan MA, Lester SJ, et al. Left ventricular form and function revisited: applied translational science to cardiovascular ultrasound imaging. J Am Soc Echocardiogr. 2007;20:539. doi: 10.1016/j.echo.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voigt JU, Lindenmeier G, Exner B, Regenfus M, Werner D, Reulbach U, et al. Incidence and characteristics of segmental postsystolic longitudinal shortening in normal, acutely ischemic, and scarred myocardium. J Am Soc Echocardiogr. 2003;16:415–23. doi: 10.1016/s0894-7317(03)00111-1. [DOI] [PubMed] [Google Scholar]
- 37.Zwanenburg J, Götte M, Kuijer J, Heethaar R, Van Rossum A, Marcus J. Timing of cardiac contraction in humans mapped by high-temporal-resolution MRI tagging: early onset and late peak of shortening in lateral wall. Am J Physiol Heart Circul Physiol. 2004;286:H1872–80. doi: 10.1152/ajpheart.01047.2003. [DOI] [PubMed] [Google Scholar]
- 38.Sengupta PP, Khandheria BK, Korinek J, Wang J, Jahangir A, Seward JB, et al. Apex-to-base dispersion in regional timing of left ventricular shortening and lengthening. J Am Coll Cardiol. 2006;47:163–72. doi: 10.1016/j.jacc.2005.08.073. Research Support, N.I.H., Extramural. [DOI] [PubMed] [Google Scholar]