Abstract
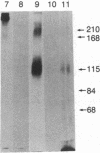
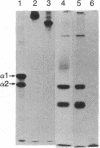
Type IX collagen was isolated as a native protein from chicken embryo sternal cartilages and purified to homogeneity. Chondroitin and/or dermatan sulfate were bound covalently to one of the three polypeptide chains present in this protein containing collagenous and noncollagenous domains. Type IX collagen could be metabolically labeled with both radioactive sulfate and glycine. The protein containing either of these labels was sensitive to digestion by bacterial collagenase as well as chondroitinase ABC. Besides the glycosaminoglycans, type IX collagen contains asparagine-linked carbohydrate chains because the protein could be labeled with radioactive mannose and no glycosaminoglycans other than those mentioned above were present. The melting curve indicated that, in contrast to interstitial collagens, this molecule contains at least two disulfide-bonded collagenous domains with distinct thermal stabilities.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayad S., Abedin M. Z., Grundy S. M., Weiss J. B. Isolation and characterisation of an unusual collagen from hyaline cartilage and intervertebral disc. FEBS Lett. 1981 Jan 26;123(2):195–199. doi: 10.1016/0014-5793(81)80286-4. [DOI] [PubMed] [Google Scholar]
- Benya P. D., Shaffer J. D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982 Aug;30(1):215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Sage H. Structurally distinct collagen types. Annu Rev Biochem. 1980;49:957–1003. doi: 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- Bruckner P., Eikenberry E. F. Procollagen is more stable in cellulo than in vitro. Eur J Biochem. 1984 Apr 16;140(2):397–399. doi: 10.1111/j.1432-1033.1984.tb08115.x. [DOI] [PubMed] [Google Scholar]
- Bruckner P., Mayne R., Tuderman L. p-HMW-collagen, a minor collagen obtained from chick embryo cartilage without proteolytic treatment of the tissue. Eur J Biochem. 1983 Nov 2;136(2):333–339. doi: 10.1111/j.1432-1033.1983.tb07746.x. [DOI] [PubMed] [Google Scholar]
- Bruckner P., Prockop D. J. Proteolytic enzymes as probes for the triple-helical conformation of procollagen. Anal Biochem. 1981 Jan 15;110(2):360–368. doi: 10.1016/0003-2697(81)90204-9. [DOI] [PubMed] [Google Scholar]
- Burgeson R. E., Hollister D. W. Collagen heterogeneity in human cartilage: identification of several new collagen chains. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1124–1131. doi: 10.1016/s0006-291x(79)80024-8. [DOI] [PubMed] [Google Scholar]
- Duance V. C., Shimokomaki M., Bailey A. J. Immunofluorescence localization of type-M collagen in articular cartilage. Biosci Rep. 1982 Apr;2(4):223–227. doi: 10.1007/BF01136720. [DOI] [PubMed] [Google Scholar]
- Fessler J. H., Fessler L. I. Biosynthesis of procollagen. Annu Rev Biochem. 1978;47:129–162. doi: 10.1146/annurev.bi.47.070178.001021. [DOI] [PubMed] [Google Scholar]
- Furthmayr H., Timpl R. Characterization of collagen peptides by sodium dodecylsulfate-polyacrylamide electrophoresis. Anal Biochem. 1971 Jun;41(2):510–516. doi: 10.1016/0003-2697(71)90173-4. [DOI] [PubMed] [Google Scholar]
- Gibson G. J., Kielty C. M., Garner C., Schor S. L., Grant M. E. Identification and partial characterization of three low-molecular-weight collagenous polypeptides synthesized by chondrocytes cultured within collagen gels in the absence and in the presence of fibronectin. Biochem J. 1983 May 1;211(2):417–426. doi: 10.1042/bj2110417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G. J., Schor S. L., Grant M. E. Effects of matrix macromolecules on chondrocyte gene expression: synthesis of a low molecular weight collagen species by cells cultured within collagen gels. J Cell Biol. 1982 Jun;93(3):767–774. doi: 10.1083/jcb.93.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann D. J., Magloire H., Ricard-Blum S., Joffre A., Couble M. L., Ville G., Herbage D. Light and electron immunoperoxidase localization of minor disulfide-bonded collagens in fetal calf epiphyseal cartilage. Coll Relat Res. 1983 Jul;3(4):349–357. doi: 10.1016/s0174-173x(83)80016-8. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Heinegård D. Aggregation of cartilage proteoglycans. I. The role of hyaluronic acid. J Biol Chem. 1974 Jul 10;249(13):4232–4241. [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lee S. L., Piez K. A. Type II collagen from lathyritic rat chondrosarcoma: preparation and in vitro fibril formation. Coll Relat Res. 1983 Mar;3(2):89–103. doi: 10.1016/s0174-173x(83)80036-3. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y., Olsen B. R. Synthesis and characterization of cDNA encoding a cartilage-specific short collagen. Proc Natl Acad Sci U S A. 1984 May;81(10):3014–3018. doi: 10.1073/pnas.81.10.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noro A., Kimata K., Oike Y., Shinomura T., Maeda N., Yano S., Takahashi N., Suzuki S. Isolation and characterization of a third proteoglycan (PG-Lt) from chick embryo cartilage which contains disulfide-bonded collagenous polypeptide. J Biol Chem. 1983 Aug 10;258(15):9323–9331. [PubMed] [Google Scholar]
- Oike Y., Kimata K., Shinomura T., Nakazawa K., Suzuki S. Structural analysis of chick-embryo cartilage proteoglycan by selective degradation with chondroitin lyases (chondroitinases) and endo-beta-D-galactosidase (keratanase). Biochem J. 1980 Oct 1;191(1):193–207. doi: 10.1042/bj1910193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I., Tuderman L., Guzman N. A. The biosynthesis of collagen and its disorders (first of two parts). N Engl J Med. 1979 Jul 5;301(1):13–23. doi: 10.1056/NEJM197907053010104. [DOI] [PubMed] [Google Scholar]
- Reese C. A., Mayne R. Minor collagens of chicken hyaline cartilage. Biochemistry. 1981 Sep 15;20(19):5443–5448. doi: 10.1021/bi00522a014. [DOI] [PubMed] [Google Scholar]
- Reese C. A., Wiedemann H., Kühn K., Mayne R. Characterization of a highly soluble collagenous molecule isolated from chicken hyaline cartilage. Biochemistry. 1982 Mar 2;21(5):826–830. doi: 10.1021/bi00534a002. [DOI] [PubMed] [Google Scholar]
- Ricard-Blum S., Hartmann D. J., Herbage D., Payen-Meyran C., Ville G. Biochemical properties and immunolocalization of minor collagens in foetal calf cartilage. FEBS Lett. 1982 Sep 20;146(2):343–347. doi: 10.1016/0014-5793(82)80949-6. [DOI] [PubMed] [Google Scholar]
- Schmid T. M., Conrad H. E. A unique low molecular weight collagen secreted by cultured chick embryo chondrocytes. J Biol Chem. 1982 Oct 25;257(20):12444–12450. [PubMed] [Google Scholar]
- Schmid T. M., Linsenmayer T. F. A short chain (pro)collagen from aged endochondral chondrocytes. Biochemical characterization. J Biol Chem. 1983 Aug 10;258(15):9504–9509. [PubMed] [Google Scholar]
- Shimokomaki M., Duance V. C., Bailey A. J. Identification of a new disulphide bonded collagen from cartilage. FEBS Lett. 1980 Nov 17;121(1):51–54. doi: 10.1016/0014-5793(80)81265-8. [DOI] [PubMed] [Google Scholar]
- Shimokomaki M., Duance V. C., Bailey A. J. Identification of two further collagenous fractions from articular cartilage. Biosci Rep. 1981 Jul;1(7):561–570. doi: 10.1007/BF01116305. [DOI] [PubMed] [Google Scholar]
- Shinomura T., Kimata K., Oike Y., Noro A., Hirose N., Tanabe K., Suzuki S. The occurrence of three different proteoglycan species in chick embryo cartilage. Isolation and characterization of a second proteoglycan (PG-Lb) and its precursor form. J Biol Chem. 1983 Aug 10;258(15):9314–9322. [PubMed] [Google Scholar]
- van der Rest M., Mayne R., Ninomiya Y., Seidah N. G., Chretien M., Olsen B. R. The structure of type IX collagen. J Biol Chem. 1985 Jan 10;260(1):220–225. [PubMed] [Google Scholar]
- von der Mark K., van Menxel M., Wiedemann H. Isolation and characterization of a precursor form of M collagen from embryonic chicken cartilage. Eur J Biochem. 1984 Feb 1;138(3):629–633. doi: 10.1111/j.1432-1033.1984.tb07961.x. [DOI] [PubMed] [Google Scholar]
- von der Mark K., van Menxel M., Wiedemann H. Isolation and characterization of new collagens from chick cartilage. Eur J Biochem. 1982 May;124(1):57–62. doi: 10.1111/j.1432-1033.1982.tb05905.x. [DOI] [PubMed] [Google Scholar]