Abstract
Background.
Age-related olfactory loss (presbyosmia) substantially decreases quality of life, presages neurodegenerative disease, impairs nutrition, and predicts mortality. We sought to determine how race is associated with olfactory loss in older American adults in order to inform both health care and policy.
Methods.
The National Social Life, Health and Aging Project interviewed a cross-sectional nationally representative probability sample of older adults in the United States. African Americans and Hispanics were oversampled, providing power to detect disparities for these subgroups. As part of an omnibus survey of demographic, social, psychological, and biological measures, National Social Life, Health and Aging Project assessed the ability to verbally identify odors by presenting five odor pens. Multivariate ordinal logistic regression quantified racial differences in odor identification, and then tested potential confounders.
Results.
African Americans and Hispanics had markedly worse olfactory function (controlling for gender and age) compared with whites (p < .001), twice the magnitude of gender differences, and comparable to aging 9 years. Cognition, household assets, and education accounted for the disparity found among Hispanics but not among African Americans. Moreover, other potential confounders, such as physical or mental health, including tobacco and alcohol use, did not account for the African American health disparity, which remained significant (p = .001) after including these factors.
Conclusions.
African Americans are more likely to suffer from presbyosmia, a health disparity not explained by gender, education, cognition, physical or mental health, and health behaviors. This novel health disparity may result from lifetime environmental exposures, diet, or genetic susceptibility. Dissecting the interactions among these putative mechanisms will provide insight into ameliorating this decline in critical human sensory function.
Key words: Aging, Olfaction, Race disparity, Gender.
Age-related olfactory loss (presbyosmia) predicts mortality (1) and is an important public health problem worldwide (2–4). In the United States, millions of older adults are affected and olfactory complaints overall lead to more than 200,000 physician visits annually. Because olfaction declines over time (4), the clinical impact will increase as the U.S. population ages. This sensory impairment of aging affects critical functions, such as nutrition (5), immunity (5), sensation of pleasure (6), detection of environmental hazards (7), mood, cognition, behavior, sexuality, and well-being, and therefore it poses a profound burden on older adults. Indeed, up to one third of older participants report dissatisfaction with their ability to smell (8), and approximately 50% are unable to detect the standard warning odor in natural gas (9). Importantly, decline in olfaction has been linked to several neurodegenerative conditions (10–12). Thus, olfactory sensory loss is related to factors that are critical to the physical well-being, social function, and quality of life of older adults. Despite its impact, human olfaction is relatively understudied, particularly in diverse populations.
The National Social Life, Health and Aging Project (NSHAP) is the first study of social relationships and health in a nationally representative probability sample of older adults. The NSHAP data set offers several advantages for studying presbyosmia including generalizability of findings across the U.S. population of older adults. The omnibus survey was designed to study interplay of sociological and medical issues in older persons (13–15), including health disparities (16). In order to characterize olfactory function during aging for the U.S. population, we designed a chemosensory module for inclusion in this home-based survey (17,18). NSHAP extends previous work in homogenous, nonnationally representative populations (3,4) by examining a national population sample that is racially diverse and by an exclusive focus on aging.
Identification of population subgroups at increased risk for poor health outcomes is now a paramount priority worldwide to improve medical care (19). Racial disparities in common and burdensome conditions that affect older adults, such as diabetes, heart disease, cancer, and other age-related sensory conditions (20), have become a focus of investigation recently in the United States and in Europe (21,22) with striking differences found across a variety of diseases. Despite its impact on human function, quality of life, and mortality, we have little information on potential disparities in olfactory function. Therefore, we tested the ability to identify odors in the NSHAP sample and utilized additional data on a variety of plausible social, psychological, and medical mechanisms that could underlie the effect.
Methods
Participants
In addition to being a nationally representative probability sample, NSHAP oversampled African Americans, Hispanics, men, and the oldest participants (those aged 75–84 at the time of screening) to increase power for analyzing race, gender, and age differences (23). In-home interviews were conducted by professional interviewers (National Opinion Research Center) with 3,005 community-dwelling older adults (1,455 men and 1,550 women) aged 57–85 years living throughout the United States between July 2005 and March 2006. The weighted participation rate was 75.5%, and item cooperation rates were high (24). The weighted distribution of demographic variables in the resulting sample closely matched those of the 2002 Current Population Survey.
NSHAP assessed a broad range of social, psychological, health, and demographic measures. Most relevant are (a) sociodemographic characteristics, (b) physical and psychological health, (c) sensory function including olfactory testing, and (d) health behaviors, detailed subsequently. The protocol was approved by the Institutional Review Boards of the University of Chicago and National Opinion Research Center; all respondents provided written, informed consent (25).
Olfactory Function Field Examination
Olfactory function was assessed using a validated odor identification task (26). The long NSHAP interview, conducted by field interviewers in the home, demanded the shortest version of the task that correlated highly with the long form, using five carefully selected odorants (17,26,27). Odors were presented one at a time, and respondents were asked to identify each from a set of four prompts in a forced choice protocol (26). Specifically, the interviewer held the pen for the participant to sniff and then presented a card with four picture and words, one matching the target odorant. The target odorants and corresponding response sets (correct odor in italics) were as follows: (a) chamomile, raspberry, rose, or cherry; (b) smoke, glue, leather, or grass; (c) orange, blueberry, strawberry, or onion; (d) bread, fish, cheese, or ham; and (e) chive, peppermint, pine, or onion. Odor pens were purchased from Burghart Messtechnik (Wedel, Germany) and stored and utilized according to the manufacturer’s instructions.
Race or Ethnicity
Race and ethnicity were coded using the standard National Institutes of Health classification based on two questions, “Do you consider yourself primarily white or Caucasian, black or African American, American Indian, Asian, or something else?” and “Do you consider yourself Hispanic or Latino?” African Americans who identified themselves as Hispanic were included in the African American category (n = 7) and American Indian, Asian, or others were combined into a single category. Twelve respondents provided insufficient race or ethnicity information and were excluded from the analysis.
Potential Confounding Variables
Cognitive function (specifically memory and mental arithmetic) was measured with a modified version of the Short Portable Mental Status Questionnaire (28). Education was defined by the highest degree or certification earned. Respondents also reported their net household assets (house, cars, or rental properties/businesses owned; financial assets including savings accounts, stocks, and pensions minus outstanding debt). Self-rated physical health was measured by a standard 5-point scale (excellent, very good, good, fair, or poor). Comorbid diseases were assessed with the Charlson Index modified for NSHAP (29). Frequencies of depressive symptoms, anxiety symptoms, and perceived stressors were measured with standard scales modified for survey use: 11-item Center for Epidemiologic Studies Depression (CES-D) scale, Hospital Anxiety Scale, and the Perceived Stress Scale (30). In all three measures, respondents chose the relevant symptom frequency: (a) rarely or none of the time, (b) some of the time, (c) occasionally, or (d) most of the time; the average score across all items was used in the analysis. Health behaviors affecting olfaction were current smoking, based on either salivary cotinine level (n = 2,219) or self-report (n = 709), and problem drinking, based on a combination of the CAGE alcoholism questionnaire (31) and frequency of having four or more drinks on one occasion in the last 3 months.
Statistical Analysis
The analysis treated the number of odors correctly identified (0–1 [combined], 2, 3, 4, or 5) as the dependent variable. Refusals to answer individual items were coded as incorrect, following the forced choice protocol. Multivariate ordinal logistic regression modeled the relationship between covariates of interest and olfactory identification. Odds ratios are reported per decade increase in age for ease of interpretation (also roughly equivalent to one SD). Multiple imputation was used when fitting Models 3 and 4 to address missing values for several of the covariates (Table 1; see Supplementary Methods and Supplementary Table 1 for complete details; [32]). Wald tests provided p values (all two-sided) and were inverted to obtain 95% confidence intervals (CI). Models were refit after excluding respondents with a prior head injury or nose surgery to confirm that this did not affect the results.
Table 1.
Distributions of Variables Used in Analysis
| Variables | Weighted % | N | ||
|---|---|---|---|---|
| Odors correctly identified | 2,928 | |||
| 0 | 1.1 | |||
| 1 | 2.4 | |||
| 2 | 5.0 | |||
| 3 | 13.9 | |||
| 4 | 29.3 | |||
| 5 | 48.5 | |||
| Sex (% men) | 48.9 | 2,928 | ||
| Race or ethnicity | 2,928 | |||
| White | 80.8 | |||
| African American | 9.9 | |||
| Hispanic (non-African American) | 6.9 | |||
| Others | 2.5 | |||
| Education | 2,928 | |||
| <High school | 18.4 | |||
| High school graduate or equivalent | 26.7 | |||
| Some college | 30.2 | |||
| Bachelors or higher | 24.8 | |||
| Self-rated physical health | 2,917 | |||
| Poor | 6.8 | |||
| Fair | 18.0 | |||
| Good | 29.4 | |||
| Very good | 32.7 | |||
| Excellent | 13.2 | |||
| Current smoker | 19.0 | 2,928 | ||
| Problem drinking | 25.8 | 2,928 | ||
| Weighted Mean | Range | SD | N | |
| Age | 68.0 | 57–85 | 7.8 | 2,928 |
| Cognition (SPMSQ*) | 9.3 | 0–10 | 1.2 | 2,928 |
| Household assets (log10) | 5.2 | 0–7.3 | 1.2 | 1,817 |
| Comorbidity index | 1.8 | 0–10.5 | 1.8 | 2,928 |
| Depressive symptoms | 1.5 | 1–3.9 | 0.5 | 2,923 |
| Anxiety symptoms | 1.5 | 1–4 | 0.5 | 2,724 |
| Perceived stressors | 1.4 | 1–4 | 0.6 | 2,726 |
*SPMSQ = Short Portable Mental Status Questionnaire.
To verify the adequacy of the ordinal logistic models, two-parameter item response models were also fit that incorporated the covariates through a structural component (26). In addition, ordinal logistic regression models were fit after excluding one identification item at a time to ensure that a single odor was not unduly affecting the results (Supplementary Table 2).
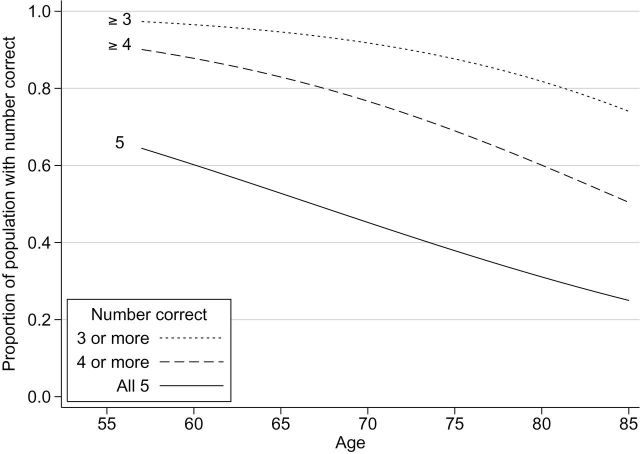
Olfactory performance is presented graphically by plotting the predicted probabilities of getting all five, four or more, or three or more odors correct based on logistic regression models corresponding to each performance criterion.
Analyses were weighted using the weights distributed with the data set to account for differential probabilities of selection and differential nonresponse; design-based standard errors were calculated using the linearization method (33) together with the strata and primary sampling unit indicators provided with the data set. Statistical analyses were conducted with Stata Version 12.1 (Stata Corp LP).
Results
Of the 3,005 NSHAP respondents, 98% completed the olfactory module. Only 49% of the population responded correctly to all five odorants, 78% got four or more correct, 92% met the less stringent performance criterion of three or more correct, and 97% got two or more correct. We used each of these increasingly liberal criteria to model olfactory performance. The distributions of each of the variables used in the analysis are provided in Table 1.
Olfactory performance declined steadily across age groups. The likelihood of getting all five items correct declined from 0.64 at age 57 to only 0.25 by age 85 (Figure 1). Similar declines with age (ie, similar slopes on the logit scale) were observed for the less stringent performance criteria. Controlling for differences in age, men scored lower than women; specifically, their odds of meeting a given performance criterion were 29% less than women’s odds (OR = 0.71, 95% CI = 0.62–0.80). This difference in performance between men and women is equivalent to the effect of a 5 year increase in age. Moreover, the magnitude of this gender difference was the same across ages (p = .50 for the interaction between gender and age).
Figure 1.
Olfactory function of older adults in the United States, adjusted for sex. Proportion of the study cohort answering the specified number (three or more, four or more, or five) of olfactory test questions correct on the y-axis. Age in years on the x-axis.
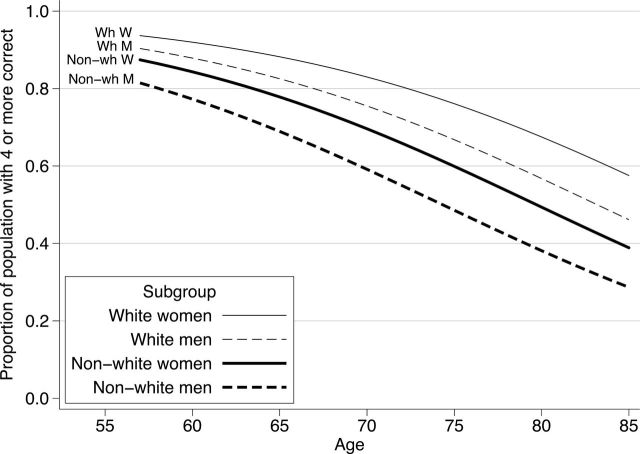
Consistent with other reported health disparities, non-White respondents had poorer olfactory performance than whites (Figure 2). Controlling for age and gender, non-Whites had 47% lower odds of meeting a given performance criterion than whites (OR = 0.53, 95% CI = 0.42–0.65)—a difference equal to a 9 year increase in age or almost twice the estimated gender difference (Table 2, Model 1). There was no evidence that the magnitude of this disparity differed with age (p = .24 for the interaction between race or ethnicity and age) or that it was greater in men or women (p = .75 for the interaction between race or ethnicity and gender).
Figure 2.
Olfactory function of older adults in the United States, stratified by race or ethnicity and sex. Proportion of the study cohort answering four or more olfactory test questions correct on the y-axis. Age in years on the x-axis.
Table 2.
Results From Ordinal Logistic Regression Models Fit to the Number of Odors Identified Correctly (five odors total, N = 2,928)
| Covariates | Odds Ratio (95% Confidence Interval), p Value | |||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |
| Age (decades) | 0.48 (0.42, 0.54), <.001 | 0.48, (0.42, 0.54), <.001 | 0.52 (0.46, 0.60), <.001 | 0.52 (0.46, 0.59), <.001 |
| Sex (men vs women) | 0.70 (0.62, 0.80), <.001 | 0.70 (0.62, 0.79), <.001 | 0.65 (0.56, 0.75), <.001 | 0.68 (0.58, 0.79), <.001 |
| Race or ethnicity (vs white) | ||||
| All non-White | 0.53 (0.42, 0.65), <.001 | |||
| African American | 0.46 (0.33, 0.63), <.001 | 0.61 (0.46, 0.81), .001 | 0.61 (0.46, 0.82), .001 | |
| Hispanic, non-African American | 0.63 (0.45, 0.89), .009 | 0.91 (0.65, 1.29), .59 | 0.94 (0.65, 1.35), .73 | |
| Others | 0.55 (0.29, 1.02), .06 | 0.62 (0.36, 1.08), .09 | 0.66 (0.40, 1.09), .10 | |
| Cognition (SPMSQ*) | 1.32 (1.21, 1.43), <.001 | 1.30 (1.19, 1.41), <.001 | ||
| Education† | 1.16 (1.07, 1.27), .001 | 1.14 (1.04, 1.24), .006 | ||
| Household assets (log10) | 1.07 (0.95, 1.19), .27 | 1.02 (0.91, 1.15), .68 | ||
| Self-rated physical health‡ | 1.13 (1.02, 1.25), .02 | |||
| Comorbidity index | 1.04 (0.98, 1.10), .16 | |||
| Depressive symptoms | 0.78 (0.61, 1.01), .06 | |||
| Anxiety symptoms | 1.28 (1.02, 1.61), .04 | |||
| Perceived stressors | 0.94 (0.76, 1.16), .54 | |||
| Current smoker | 0.94 (0.75, 1.19), .61 | |||
| Problem drinking | 0.88 (0.71, 1.09), .24 | |||
*SPMSQ = Short Portable Mental Status Questionnaire.
†Treated as a continuous measure using integer scores for educational level.
‡Treated as a continuous measure using integer scores for health level (higher scores, better health).
NSHAP’s non-White respondents are a diverse group representative of the U.S. population, composed of African Americans, Hispanics, and other less prevalent groups. Both Hispanics and African Americans had worse olfactory identification abilities. There was no significant difference detected among the three non-White groups (p = .44). Thus, controlling for age and gender, each of the three non-White groups scored lower than whites to approximately the same degree (Table 2, Model 2).
This novel finding of racial differences in the ability to identify odors could result from other differences between the groups that also affect olfaction. To test this, we first introduced covariates indicating cognitive decline and education because of their known association with olfactory identification and net household assets as a measure of socioeconomic status. Only declining cognition and less education were associated with poorer olfactory performance (Table 2, Model 3). Each additional error in the SPMSQ (ie, a one-point decrease) was associated with a 24% reduction in olfactory performance (OR = 1.32, 95% CI = 1.21–1.43). More education was associated with better performance, with an increase in one educational level associated with a 16% increase achieving performance criteria (OR = 1.16, 95% CI = 1.07–1.27). In contrast, there was no evidence of an association between household assets and odor identification score (p = .27).
Controlling for these cognitive and socioeconomic variables reduced the difference between Hispanics and whites, which was no longer statistically significant. The difference between African Americans and whites—while also reduced slightly—remained both substantial and statistically significant (OR = 0.61, 95% CI = 0.46–0.81). Thus, differences in cognition and education account for much of the difference in olfactory identification ability between Hispanics and whites but not in African Americans.
Because disparities in the health of African Americans place them at greater risk for a variety of diseases, we added covariates capturing physical health, mental health, as well as smoking and problem drinking, two health behaviors known to have deleterious effects on olfaction (Table 2, Model 4). There was little evidence of an association between the number of comorbid diseases and olfactory performance (p = .16), although self-rated physical health was associated with better performance (OR = 1.13, 95% CI = 1.02–1.25). There was weak evidence that depressive symptoms were associated with poor olfactory identification (p = .06). In contrast, an increase in anxiety symptoms within the normal range was associated with better performance (p = .04). Finally, although both current smoking and problem drinking were associated with a slight reduction in olfactory performance, neither was significant (p = .61 and .24, respectively).
African Americans still suffered reduced olfactory performance, despite controlling for known health disparities in physical disease, mental health, and health behaviors, along with cognitive and socioeconomic variables. These other health disparities did not change the magnitude of the difference (Table 2, Model 3 vs 4).
These conclusions did not change when persons with a history of nasal surgery (n = 208) or head trauma (n = 127) were excluded. Results were also robust to modeling the data in other ways such as item response models. We further sought to determine if lack of familiarity with one particular odor across groups accounted for the effects seen, but this was not the case (Supplementary Table 2). Finally, these results were similar when analyzed without imputed data (Supplementary Table 1).
Discussion
About 22% of older American adults were unable to identify four or more of the five odors presented, a prevalence similar to the 24.5% prevalence of olfactory dysfunction found in a population from Beaver Dam, WI, where olfaction was measured using the San Diego Odor Identification Test (34). In both studies, four picture and word prompts were provided in a combined test of odor detection and identification.
Importantly, we show for the first time that the well-established superior performance of women versus men in olfactory function is maintained across the entire age range from 57 to 85 years old, equivalent to aging 5 years. This sex difference is not due to imbalances in socioeconomic status, physical and mental health, or common health behaviors. Because this sex difference is already evident in children (35), it could arise from differences in olfactory sensitivity or verbal fluency. Nonetheless, the similar prevalence of olfactory dysfunction between the sexes as it declines across age groups suggests that there is no sex difference in the mechanism of olfactory aging.
Controlling for age and sex, there were striking racial health disparities in the ability to identify odors that were almost twice the magnitude of the sex difference and equivalent to aging 9 years. We acknowledge the complexity of race as a construct in social, health, and genetic studies. Here, participants classified themselves by racial group using the standard National Institutes of Health items measuring both race and ethnicity. Among Hispanics, the health disparity was explained primarily by education attained and cognitive abilities at the time of testing, both of which affect cognitive processing and verbal fluency, similar to other Hispanic health disparities (36). These results are consistent with the fact that less education is associated with worse health and, among whites, in our sample, with poorer olfactory function (Supplementary Table 3).
In contrast, substantial olfactory disparities persist between older African Americans and whites after controlling for cognitive function and education and assets. A number of physical and mental health variables, known to affect olfaction, might also explain this health disparity. Indeed, we found that differences in physical health, depressive symptoms, and anxiety symptoms were associated with olfactory function confirming what others have reported. However, the race disparity for African Americans remained identical after controlling for these additional factors. Likewise, health behaviors, such as smoking and problem drinking, did not explain the health disparity. Accurate assessment of self-reported financial information is a sensitive topic in survey research, but our core results are not heavily dependent on the inclusion of assets, and thus this issue does not invalidate our main findings.
We hypothesize that this disparity could arise from other factors not measured in this study, a limitation of this secondary data analysis. Exposure to olfactory toxins in the urban (pollutants), work (chemicals), or home (mold) environments all degrade olfactory abilities and exposure to such toxins is higher among African Americans (37,38). The connection between olfaction and established cultural differences across the life course should also be explored, such as differential early life development, childhood and adult nutrition, exercise, work environments, or participation in war. It is well established that minorities face greater adversity in life and are subject to increased social vulnerability leading to negative health effects (39). Interestingly, some data suggest that neurodegenerative diseases are more prevalent and morbid among African Americans, which supports our findings given the relationship between olfactory decline and these conditions (40). Genetic differences due to distinct ancestry exist in different populations. However, to date, no study has identified specific genetic variation that underlies susceptibility to or resilience to olfactory loss. In sum, race likely serves as a proxy for differential environmental exposures and life experiences, which may interact with biological differences in the olfactory system, should they exist.
These cross-sectional results highlight the need to perform longitudinal studies to estimate within-person decline in olfactory function with age and its association with important social and health factors. This will be especially important for determining how much, if any, of the population difference described here is due to accelerated olfactory aging. Moreover, the pattern “writ large” in the African American population likely generalizes to any set of people experiencing similar risk factors for olfactory decline.
These results have several important implications. Our data show that older African Americans and Hispanics in the United States are more likely to suffer from olfactory dysfunction, with potentially grave consequences for their health and social life. Minority populations in other developed nations may face similar sensory disparities. Given the critical role that olfaction plays in safety, nutrition and diet, sensation of pleasure, quality of life, and even mortality, this represents a major burden to underserved populations. Clinicians should recognize that race, along with the conventionally recognized factors of gender and age, is an important risk factor for olfactory function in older age. Identifying the underlying determinants of these differences will allow us to design strategies—at the molecular, organismal, behavioral, environmental, or societal level—that can prevent or mitigate the great burden that they cause.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
The National Health, Social Life and Aging Project (NSHAP) is supported by the National Institutes of Health, including the National Institute on Aging, the Office of Women’s Health Research, the Office of AIDS Research, and the Office of Behavioral and Social Sciences Research (R01AG021487). Support was also provided by the National Institute on Aging (AG029795, K23 AG036762, and T32000243), the McHugh Otolaryngology Research Fund, the American Geriatrics Society, and the Institute of Translational Medicine at The University of Chicago (KL2RR025000 and UL1RR024999). No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
The authors declare no conflicts of interest. Jamie M. Phillips and James Lane provided logistical support. We thank Robert M. Naclerio, MD, Fuad M. Baroody, MD, (both, the University of Chicago), and Raymond J. Cho, MD, PhD (University of California–San Francisco) for useful editorial comments provided generously. Johann Lundstrom, PhD (Monell Chemical Senses Center), designed the olfactory testing in conjunction with M.K.M. and Thomas Hummel, MD (University of Dresden Medical School), developed the test protocol in National Social Life, Health and Aging Project (NSHAP). Stacy Tessler Lindau, MD, MAPP (The University of Chicago), made significant contribution to the design of the biomeasure component of NSHAP wave 1. We gratefully acknowledge the participation of the NSHAP respondents.
References
- 1. Gopinath B, Sue CM, Kifley A, Mitchell P. The association between olfactory impairment and total mortality in older adults. J Gerontol A Biol Sci Med Sci. 2012;67:204–209 [DOI] [PubMed] [Google Scholar]
- 2. Wysocki CJ, Gilbert AN. National Geographic Smell Survey. Effects of age are heterogenous. Ann N Y Acad Sci. 1989;561:12–28 [DOI] [PubMed] [Google Scholar]
- 3. Brämerson A, Johansson L, Ek L, Nordin S, Bende M. Prevalence of olfactory dysfunction: the skövde population-based study. Laryngoscope. 2004;114:733–737 [DOI] [PubMed] [Google Scholar]
- 4. Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. J Am Med Assoc. 2002;288:2307–2312 [DOI] [PubMed] [Google Scholar]
- 5. Schiffman SS, Graham BG. Taste and smell perception affect appetite and immunity in the elderly. Eur J Clin Nutr. 2000;54:S54–S63 [DOI] [PubMed] [Google Scholar]
- 6. Wolfe JM, Kluender KR, Levi DM, et al. Sensation & Perception. 2nd ed. Sunderland, MA: Sinauer Associates; 2008 [Google Scholar]
- 7. Santos DV, Reiter ER, DiNardo LJ, Costanzo RM. Hazardous events associated with impaired olfactory function. Arch Otolaryngol Head Neck Surg. 2004;130:317–319 [DOI] [PubMed] [Google Scholar]
- 8. Wysocki CJ, Pelchat ML. The effects of aging on the human sense of smell and its relationship to food choice. Crit Rev Food Sci Nutr. 1993;33:63–82 [DOI] [PubMed] [Google Scholar]
- 9. Cain WS, Stevens JC. Uniformity of olfactory loss in aging. Ann N Y Acad Sci. 1989;561:29–38 [DOI] [PubMed] [Google Scholar]
- 10. Kovács T. Mechanisms of olfactory dysfunction in aging and neurodegenerative disorders. Ageing Res Rev. 2004;3:215–232 [DOI] [PubMed] [Google Scholar]
- 11. Schubert CR, Carmichael LL, Murphy C, Klein BE, Klein R, Cruickshanks KJ. Olfaction and the 5-year incidence of cognitive impairment in an epidemiological study of older adults. J Am Geriatr Soc. 2008;56:1517–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wilson RS, Schneider JA, Arnold SE, Tang Y, Boyle PA, Bennett DA. Olfactory identification and incidence of mild cognitive impairment in older age. Arch Gen Psychiatry. 2007;64:802–808 [DOI] [PubMed] [Google Scholar]
- 13. Cornwell EY, Waite LJ. Social disconnectedness, perceived isolation, and health among older adults. J Health Soc Behav. 2009;50:31–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Drum ML, Shiovitz-Ezra S, Gaumer E, Lindau ST. Assessment of smoking behaviors and alcohol use in the national social life, health, and aging project. J Gerontol B Psychol Sci Soc Sci. 2009;64:i119–i130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357:762–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qato DM, Lindau ST, Conti RM, Schumm LP, Alexander GC. Racial and ethnic disparities in cardiovascular medication use among older adults in the United States. Pharmacoepidemiol Drug Saf. 2010;19:834–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boesveldt S, Lindau ST, McClintock MK, Hummel T, Lundstrom JN, Lindstrom JN. Gustatory and olfactory dysfunction in older adults: a national probability study. Rhinology. 2011;49:324–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schumm LP, McClintock M, Williams S, et al. Assessment of sensory function in the National Social Life, Health, and Aging Project. J Gerontol B Psychol Sci Soc Sci. 2009;64:i76–i85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bulatao RA, Anderson NB. Understanding Racial and Ethnic Differences in Health in Late Life: A Research Agenda. Washington, DC: National Academies Press; 2004 [PubMed] [Google Scholar]
- 20. Lin FR, Thorpe R, Gordon-Salant S, Ferrucci L. Hearing loss prevalence and risk factors among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2011;66:582–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Vogli R, Gimeno D, Kivimaki M. Socioeconomic inequalities in health in 22 European countries. N Engl J Med. 2008;359:1290; author reply 1290–1290 [DOI] [PubMed] [Google Scholar]
- 22. Jha AK, Fisher ES, Li Z, Orav EJ, Epstein AM. Racial trends in the use of major procedures among the elderly. N Engl J Med. 2005;353:683–691 [DOI] [PubMed] [Google Scholar]
- 23. Qato DM, Lindau ST, Conti RM, Schumm LP, Alexander GC. Racial and ethnic disparities in cardiovascular medication use among older adults in the United States. Pharmacoepidemiol Drug Saf. 2010;19:834–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. O’Muircheartaigh C, Eckman S, Smith S. Statistical design and estimation for the national social life, health, and aging project. J Gerontol B Psychol Sci Soc Sci. 2009;64:i12–i19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smith S, Jaszczak A, Graber J, et al. Instrument development, study design implementation, and survey conduct for the national social life, health, and aging project. J Gerontol B Psychol Sci Soc Sci. 2009;64:i20–i29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schumm LP, McClintock M, Williams S, et al. Assessment of sensory function in the National Social Life, Health, and Aging Project. J Gerontol B Psychol Sci Soc Sci. 2009;64:i76–i85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mueller C, Renner B. A new procedure for the short screening of olfactory function using five items from the “Sniffin’ Sticks” identification test kit. Am J Rhinol. 2006;20:113–116 [PubMed] [Google Scholar]
- 28. Pfeiffer E. A Short Portable Mental Status Questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–441 [DOI] [PubMed] [Google Scholar]
- 29. Katz JN, Chang CL, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84 [DOI] [PubMed] [Google Scholar]
- 30. Shiovitz-Ezra S, Leitsch S, Graber J, Karraker A. Quality of life and psychological health indicators in the national social life, health, and aging project. J Gerontol B Psychol Sci Soc Sci. 2009;64:i30–i37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ewing JA. Detecting alcoholism. The CAGE questionnaire. J Am Med Assoc. 1984;252:1905–1907 [DOI] [PubMed] [Google Scholar]
- 32. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New york: John Wiley & sons; 1987 [Google Scholar]
- 33. Binder DA. On the variances of asymptotically normal estimators from complex surveys. Int Stat Rev. 1983;51:279–292 [Google Scholar]
- 34. Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. J Am Med Assoc. 2002;288:2307–2312 [DOI] [PubMed] [Google Scholar]
- 35. Richman RA, Wallace K, Sheehe PR. Assessment of an abbreviated odorant identification task for children: a rapid screening device for schools and clinics. Acta Paediatr. 1995;84:434–437 [DOI] [PubMed] [Google Scholar]
- 36. Albrecht SS, McVeigh KH. Investigation of the disparity between New York City and national prevalence of nonspecific psychological distress among Hispanics. Prev Chronic Dis. 2012;9:E52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Calderón-Garcidueñas L, Franco-Lira M, Henríquez-Roldán C, et al. Urban air pollution: influences on olfactory function and pathology in exposed children and young adults. Exp Toxicol Pathol. 2010;62:91–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mohai P, Lantz PM, Morenoff J, House JS, Mero RP. Racial and socioeconomic disparities in residential proximity to polluting industrial facilities: evidence from the Americans’ Changing Lives Study. Am J Public Health. 2009;99:S649–S656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shuey KM, Willson AE. Cumulative disadvantage and black-white disparities in life-course health trajectories. Res Aging. 2008;30:200–225 [Google Scholar]
- 40. Chin AL, Negash S, Hamilton R. Diversity and disparity in dementia: the impact of ethnoracial differences in Alzheimer disease. Alzheimer Dis Assoc Disord. 2011;25:187–195 [DOI] [PMC free article] [PubMed] [Google Scholar]