Abstract
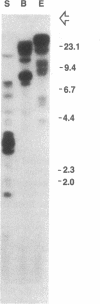
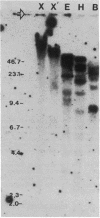
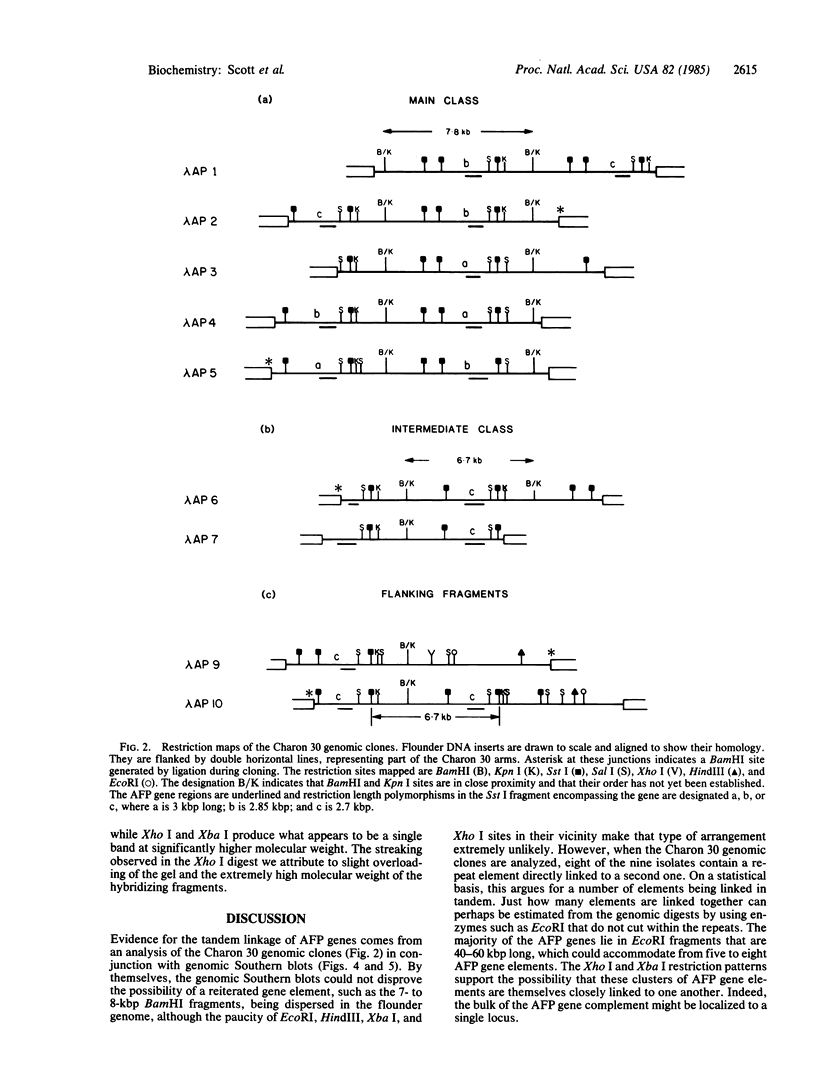
We have used genomic Southern blots and restriction maps of genomic clones to examine the organization of the antifreeze protein multigene family in the winter flounder. The majority of the approximately equal to 40 antifreeze protein (AFP) genes in this fish are present in 7- to 8-kilobase-pair (kbp) elements of DNA, which are iterated as tandem direct repeats. Each repeat contains a single antifreeze protein gene that is 1 kbp long, and all of these genes have the same transcriptional orientation. Although the repeated elements are highly homologous, they do show some restriction site and restriction length polymorphisms. When flounder genomic DNA is digested with restriction endonucleases that do not cut within the repeats, most of the antifreeze protein genes reside in fragments that are at least 40 kbp long, representing clusters of five or more repeats in tandem. After genomic DNA is digested with Xba I or Xho I, these genes are present in fragments of exceptionally high molecular weight, suggesting that the clusters themselves are grouped together in the genome. The AFP gene locus may have evolved by gene amplification as recently as 10(6) years ago in response to the onset of the Cenozoic ice age.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callan H. G. The organization of genetic units in chromosomes. J Cell Sci. 1967 Mar;2(1):1–7. doi: 10.1242/jcs.2.1.1. [DOI] [PubMed] [Google Scholar]
- Davies P. L., Hough C., Scott G. K., Ng N., White B. N., Hew C. L. Antifreeze protein genes of the winter flounder. J Biol Chem. 1984 Jul 25;259(14):9241–9247. [PubMed] [Google Scholar]
- Davies P. L., Roach A. H., Hew C. L. DNA sequence coding for an antifreeze protein precursor from winter flounder. Proc Natl Acad Sci U S A. 1982 Jan;79(2):335–339. doi: 10.1073/pnas.79.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries A. L. Antifreeze peptides and glycopeptides in cold-water fishes. Annu Rev Physiol. 1983;45:245–260. doi: 10.1146/annurev.ph.45.030183.001333. [DOI] [PubMed] [Google Scholar]
- Devries A. L., Lin Y. Structure of a peptide antifreeze and mechanism of adsorption to ice. Biochim Biophys Acta. 1977 Dec 20;495(2):388–392. doi: 10.1016/0005-2795(77)90395-6. [DOI] [PubMed] [Google Scholar]
- Duman J. G., de Vries A. L. Isolation, characterization, and physical properties of protein antifreezes from the winter flounder, Pseudopleuronectes americanus. Comp Biochem Physiol B. 1976;54(3):375–380. doi: 10.1016/0305-0491(76)90260-1. [DOI] [PubMed] [Google Scholar]
- Ericson D. B., Wollin G. Pleistocene climates and chronology in deep-sea sediments. Science. 1968 Dec 13;162(3859):1227–1234. doi: 10.1126/science.162.3859.1227. [DOI] [PubMed] [Google Scholar]
- Fletcher G. L. Circannual cycles of blood plasma freezing point and Na+ and Ci- concentrations in Newfoundland winter flounder (Pseudopleuronectes americanus): correlation with water temperature and photoperiod. Can J Zool. 1977 May;55(5):789–795. doi: 10.1139/z77-103. [DOI] [PubMed] [Google Scholar]
- Fourney R. M., Fletcher G. L., Hew C. L. Accumulation of winter flounder antifreeze messenger RNA after hypophysectomy. Gen Comp Endocrinol. 1984 Jun;54(3):392–401. doi: 10.1016/0016-6480(84)90153-9. [DOI] [PubMed] [Google Scholar]
- Hew C. L., Fletcher G. L., Ananthanarayanan V. S. Antifreeze proteins from the shorthorn sculpin, Myoxocephalus scorpius: isolation and characterization. Can J Biochem. 1980 May;58(5):377–383. doi: 10.1139/o80-049. [DOI] [PubMed] [Google Scholar]
- Hew C. L., Yip C. The synthesis of freezing-point-depressing protein of the winter flounder Pseudopleuronectus americanus in Xenopus laevis oocytes. Biochem Biophys Res Commun. 1976 Aug 9;71(3):845–850. doi: 10.1016/0006-291x(76)90908-6. [DOI] [PubMed] [Google Scholar]
- Jackson J. A., Fink G. R. Gene conversion between duplicated genetic elements in yeast. Nature. 1981 Jul 23;292(5821):306–311. doi: 10.1038/292306a0. [DOI] [PubMed] [Google Scholar]
- Klein H. L., Petes T. D. Intrachromosomal gene conversion in yeast. Nature. 1981 Jan 15;289(5794):144–148. doi: 10.1038/289144a0. [DOI] [PubMed] [Google Scholar]
- Long E. O., Dawid I. B. Repeated genes in eukaryotes. Annu Rev Biochem. 1980;49:727–764. doi: 10.1146/annurev.bi.49.070180.003455. [DOI] [PubMed] [Google Scholar]
- Petes T. D. Unequal meiotic recombination within tandem arrays of yeast ribosomal DNA genes. Cell. 1980 Mar;19(3):765–774. doi: 10.1016/s0092-8674(80)80052-3. [DOI] [PubMed] [Google Scholar]
- Pickett M. H., Hew C. L., Davies P. L. Seasonal variation in the level of antifreeze protein mRNA from the winter flounder. Biochim Biophys Acta. 1983 Jan 20;739(1):97–104. doi: 10.1016/0167-4781(83)90049-0. [DOI] [PubMed] [Google Scholar]
- Pickett M., Scott G., Davies P., Wang N., Joshi S., Hew C. Sequence of an antifreeze protein precursor. Eur J Biochem. 1984 Aug 15;143(1):35–38. doi: 10.1111/j.1432-1033.1984.tb08335.x. [DOI] [PubMed] [Google Scholar]
- Roberts J. M., Axel R. Gene amplification and gene correction in somatic cells. Cell. 1982 May;29(1):109–119. doi: 10.1016/0092-8674(82)90095-2. [DOI] [PubMed] [Google Scholar]
- Schaffner W., Gross K., Telford J., Birnstiel M. Molecular analysis of the histone gene cluster of psammechinus miliaris: II. The arrangement of the five histone-coding and spacer sequences. Cell. 1976 Aug;8(4):471–478. doi: 10.1016/0092-8674(76)90214-2. [DOI] [PubMed] [Google Scholar]
- Slaughter D., Fletcher G. L., Ananthanarayanan V. S., Hew C. L. Antifreeze proteins from the sea raven, Hemitripterus americanus. Further evidence for diversity among fish polypeptide antifreezes. J Biol Chem. 1981 Feb 25;256(4):2022–2026. [PubMed] [Google Scholar]
- Slaughter D., Hew C. L. Radioimmunoassay for the antifreeze polypeptides of the winter flounder: seasonal profile and immunological cross-reactivity with other fish antifreezes. Can J Biochem. 1982 Aug;60(8):824–829. doi: 10.1139/o82-103. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Szostak J. W., Wu R. Unequal crossing over in the ribosomal DNA of Saccharomyces cerevisiae. Nature. 1980 Apr 3;284(5755):426–430. doi: 10.1038/284426a0. [DOI] [PubMed] [Google Scholar]
- Tartof K. D. Unequal mitotic sister chromatid exchange and disproportionate replication as mechanisms regulating ribosomal RNA gene redundancy. Cold Spring Harb Symp Quant Biol. 1974;38:491–500. doi: 10.1101/sqb.1974.038.01.053. [DOI] [PubMed] [Google Scholar]
- Thomashow L. S., Milhausen M., Rutter W. J., Agabian N. Tubulin genes are tandemly linked and clustered in the genome of trypanosoma brucei. Cell. 1983 Jan;32(1):35–43. doi: 10.1016/0092-8674(83)90494-4. [DOI] [PubMed] [Google Scholar]