Abstract
Background
HMGB1, the most important member of the high mobility group box protein family, is a nuclear protein with different functions in the cell; it has a role in cancer progression, angiogenesis, invasion, and metastasis development. We studied the expression of HMGB1 and whether it is a prognostic factor in colorectal carcinoma.
Material/Methods
The study included 110 cases that were histopathologically diagnosed with colorectal carcinoma from the tissue samples acquired by surgical resection and biopsy in Antalya Education and Research Hospital between 2008 and 2012. HMGB1 expression was examined via immunohistochemical method.
Results
HMGB1 expression was evaluated as negative in 32 (44.4%) of the patients and as positive in 40 (55.6%) patients. There was no relation between the HMGB1 expression and sex, age, tumor invasion depth, and histological type. However, a significant relation was detected between the HMGB1 expression and lymph node status, metastasis status, and stage (p:<0.001, p:<0.001, p:<0.001, respectively). Similar results were obtained for the relations between the HMGB1 and histological grade, perineural invasion, lymphovascular invasion, and lymphocytic response (p<0.001, p<0.001, p<0.001, and p<0.001, respectively).
Conclusions
The results of our study demonstrate that HMGB1 overexpression has a significant role in tumor progression (especially migration of tumor cells) and tumor ability to metastasize in colorectal cancers; thus, it corroborates the idea that it might be an important prognostic factor.
MeSH Keywords: Colorectal Neoplasms - pathology, HMGB1 Protein - diagnostic use, Prognosis
Background
Colorectal carcinoma (CRC) ranks third among all cancer types in frequency and cancer-related deaths [1]. In Turkey, it is the second most common type among females and ranks fifth among males, according to 2006 data [2] and studies estimating the prognosis in CRC support this data. The 7th edition of AJCC (American Joint Committee on Cancer) added to the previously known high level preoperative CEA the following: satellite tumor deposits without residue lymph node characteristics and durability with the infiltrative limit of the carcinoma, tumor regression against neoadjuvant chemotherapy, circumferential surgical limit, microsatellite instability, perineural invasion (PNI), lymphovascular invasion (LVI), and KRAS mutation status [3].
The invasive carcinoma development process from normal colon epithelium is between 7 and 12 years [4]. During this time, many genetic and epigenetic factors play a role [5,6].
High mobility group box (HMGB) proteins are non-histone nuclear proteins with many different functions in the cell [7]. HMGB1, HMGB2, and HMGB3 are the members of the HMGB protein family. While the expressions of HMGB2 and HMGB3 are limited, HMGB1 expression is common and can be regulated with peripheral factors. Accumulating evidence indicates the role of HMGB1 in cancer progression, angiogenesis, invasion, and metastasis development. Existing studies suggest that HMGB1 may have an important role in cancer development [8–13].
In this study, we studied the expression of HMGB1 and whether it is a prognostic factor in colorectal carcinoma.
Material and Methods
Patient selection
Our study included 110 cases that were histopathologically diagnosed with colorectal carcinoma from the tissue samples acquired by surgical resection and biopsy in Antalya Education and Research Hospital between 2008 and 2012. We excluded 20 patients whose follow-ups and treatments were not carried out in our hospital. Samples of the remaining 90 patients were used for histopathological staging according to the 7th edition of American Joint Committee on Cancer (AJCC). HMGB1 expression was examined by immunohistochemical method. Due to technical reasons, we excluded 18 cases in which the immunohistochemical expression was not eligible for evaluation. As a result, overall, samples of 72 patients were examined. The information about demographic data such as age, sex, stage of disease, and treatments were obtained by searching patient files retrospectively.
Tissue preparation and immunohistochemical staining
Resection materials obtained after colorectal surgery were placed in 10% formaldehyde immediately after the process and fixed for 24 hours. After fixation, pathologically sampled tumor samples were buried into paraffin after routine tissue follow-up. Immunohistochemical staining was applied on cross-sections containing nominal tumor samples that were evaluated with hematoxylin and eosin staining. Cross-sections of 4 μm thickness prepared for immunohistochemical staining were deparaffinized in an oven at 60°C for 2 hours. Afterwards, they were kept in xylene for 30 minutes and 100% alcohol for 30 minutes, and washed with water. Laminas were kept in a solution buffered with 10% citrate in the microwave at maximum power (800 watts) for 15 minutes. Afterwards, the power was decreased by half for an additional 20 minutes in the microwave. Laminas brought out of the microwave were kept at room temperature for 20 minutes. Endogenous peroxidase activity was removed by being kept in 3% hydrogen peroxide for 10 minutes. Laminas washed with phosphate-buffered saline (PBS) were kept with protein blockage after having been treated with 3×5 PBS. After being kept in HMGB1 primary antibody (rabbit monoclonal, clone EPR3506, dilution 1:100, Abcam, Cambridge, MA, USA) for 60 minutes, they were washed in PBS for 5 minutes. Afterwards, they were treated with biotinylated secondary antibody (Vector Laboratories, Burlingham, CA) for 20 minutes and washed with PBS for 5 minutes. They were then kept with peroxidase conjugated antibody for 20 minutes. Afterwards, they were washed in PBS for 5 minutes. They were kept in chromogenic DAB for 5 minutes. Laminas were washed under tap water and then counterstained with hematoxylin. They were dehydrated, dried, and covered with Entellan.
Evaluation of immunohistochemically stained sections
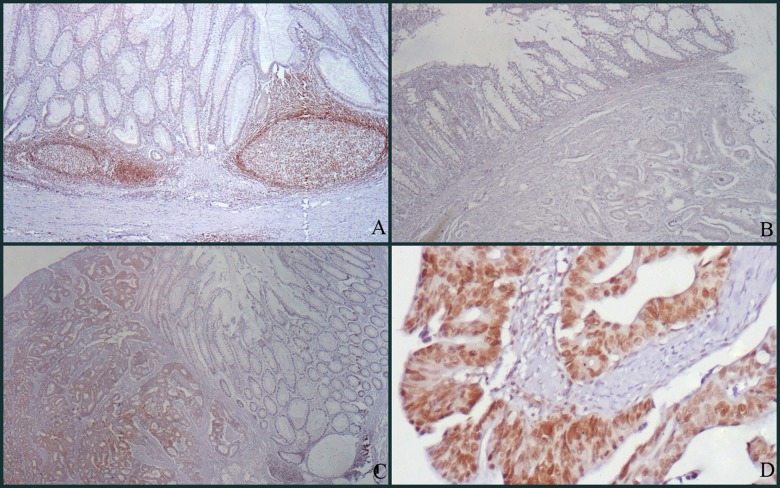
Expression rates for the positive tumor cells in the specimens were evaluated by 2 pathologists who were unaware of the patients’ clinical features (DS, AS). Although there was no HMBG1 expression on non-neoplastic colorectal surface epithelium and gland epithelium, there was a strong nuclear staining in lymphoid follicles in the stroma (Figure 1A). This nuclear staining observed in lymphocytes was used as the positive internal control in the evaluation of cases. Vascular structures, fibroblasts, smooth-muscle cells, vessel endothelium, vessel wall, neural structures, and adipocytes within the cross-section showed no staining. Absence of expression in these structures was used as the negative internal control in immunohistochemical evaluation. In carcinoma cases with HMGB1 expression, staining was nuclear and accompanied by weak cytoplasmic staining. This cytoplasmic staining was ignored and nuclear staining was evaluated. HMGB1 staining was assessed by a relatively simple, reproducible scoring method. The staining intensity was scored as 0 (negative), 1 (weak), 2 (medium), and 3 (strong). Extent of staining was scored as 0 (0%), 1 (1–25%), 2 (26–50%), 3 (51–75%), and 4 (76–100%), according to the percentages of the positive nuclear staining areas of tumor cells in relation to the whole carcinoma area. The sum of the intensity and extent score was used as the final staining score (0 to 7). This relatively simple, reproducible scoring method gives highly concordant results between independent evaluators and has been used in previous studies [11,14,15]. Tumors with a final staining score of 3 or higher were considered to be positive. HMGB1 staining examples are shown in Figure 1B, 1C, and 1D at various scores.
Figure 1.
(A) There is no staining in non-neoplastic surface epithelium, whereas strong nuclear staining is observed in lymphoid follicles (HMBG1 ×100). (B) Staining score 0 (negative): There is no staining in non-neoplastic surface epithelium and tumor cells (HMBG1 ×100). (C) Staining score 5 (positive): No staining is observed in non-neoplastic surface epithelium, but adenocarcinoma is stained diffusely and is moderately positive (HMBG1 ×100). (D) Staining score 6 (positive): There is diffuse and strong nuclear staining in adenocarcinoma and accompanying weakly positive cytoplasmic staining is observed (HMBG1 ×200).
Statistical analysis
Statistical analyses were carried out using SPSS software for Windows 15.0. Suitability of variables to normal dispersion was observed by using visual (histograms and probability graphics) and analytical methods (Kolmogorov-Smirnov and Shapiro-Wilk tests). In Kolmogorov-Smirnov test, p values above 0.05 are considered as normal dispersion. Differences between groups were observed by using chi-squared and the Mann-Whitney U test. Kaplan-Meier survival analysis was performed for the relation of each immunohistochemical positive and negative result with survival. Statistical differences had been confirmed by log-rank testing. P values under 0.05 were considered significant.
Results
A total of 72 CRC patients – 32 (44.4%) females and 40 (55.6%) males – were included in the study. Mean age of patients was 62.2±13.7 years (range 32–83 years). Conventional adenocarcinoma was determined in 59 (81.9%) of the patients, mucinous adenocarcinoma was determined in 10 (15.3%), and signet ring cell carcinoma was determined in 3 (4.2%) patients. T2 disease was detected in 3 patients (4.2%), T3 disease was found in 50 patients (69.4%), and T4 disease was found in 19 patients (26.4%). Regional lymph node metastasis was positive in 44 (61.2%) patients and negative in 28 patients (38.9%). Distant metastasis was found in 16 (22.2%) patients and there were no distant metastases in 56 (77.8%) patients. The most frequent metastasis site was the liver, with a determination rate of 13.9% (10 patients).
When patients had been evaluated according to their stages, we found that 1 patient (1.4%) was in stage 1, 25 patients (34.7%) were in stage 2, 31 patients (43.1%) were in stage 3, and 15 patients (20.8%) were in Stage 4. When patient samples were evaluated in terms of histological grades, 47 patients (65.3%) had grade 1 tumor and 25 patients (34.7%) had grade 2 tumor. Lymphocytic response (LR) was determined in 24 (33.3%) patients, perineural invasion (PNI) was determined in 55 (76.4), and lymphovascular invasion (LVI) was determined in 56 (77.8%).
HMGB1 expression was evaluated as negative in 32 (44.4%) patients and positive in 40 (55.6%) patients. There were no relations between HMGB1 expression and sex, age, tumor invasion depth, or histological type (p: 0.289, p: 0.475, p: 0.185, and p: 0.709; respectively). There was a significant relation between HMGB1 expression with lymph node status, metastasis status, and stage (p:<0.001, p:<0.001, p:<0.001; respectively). Also, a significant relation was determined between HMGB1 expression and histological grade, PNI, LVI, and LR (p:<0.001, p:<0.001, p:<0.001, p:<0.001, respectively) (Table 1).
Table 1.
Relation between demographic and tumor characteristics of patients based on HMGB1 expression.
| HMGB1 negative N (%) |
HMGB1 positive N (%) |
P Value | |
|---|---|---|---|
| Gender | 0.289 | ||
| Female | 12 (37.5) | 20 (50) | |
| Male | 20 (62.5) | 20 (50) | |
|
| |||
| Age | 63±14.5 | 61.7±13.1 | 0.475 |
|
| |||
| T stage | 0.185 | ||
| T2 | 3 (9.4) | 0 | |
| T3 | 22 (68.8) | 28 (70) | |
| T4 | 7 (21.8) | 12 (30) | |
|
| |||
| N status | <0.001 | ||
| Node negative | 23 (71.9) | 5 (12.5) | |
| Node positive | 9 (28.1) | 35 (87.5) | |
|
| |||
| M status | <0.001 | ||
| Metastasis negative | 31 (96.9) | 25 (62.5) | |
| Metastasis positive | 1 (3.1) | 15 (37.5) | |
|
| |||
| Stage | <0.001 | ||
| Stage 1 | 1 (3.1) | 0 | |
| Stage 2 | 23 (71.9) | 2 (5) | |
| Stage 3 | 7 (21.9) | 24 (60) | |
| Stage 4 | 1 (3.1) | 14 (35) | |
|
| |||
| Histological subtypes | 0.709 | ||
| Adenocarcinoma | 26 (81.3) | 33 (82.5) | |
| Signet ring cell carcinoma | 5 (15.6) | 5 (12.5) | |
| Mucinous adenocarcinoma | 1 (3.1) | 2 (5) | |
|
| |||
| Grade | <0.001 | ||
| Grade 1 | 29 (90.6) | 18 (45) | |
| Grade 2 | 3 (9.4) | 22 (55) | |
|
| |||
| Perineural invasion | <0.001 | ||
| Negative | 15 (46.9) | 2 (5) | |
| Positive | 17 (53.1) | 38 (95) | |
|
| |||
| Lymphovascular invasion | <0.001 | ||
| Negative | 15 (46.9) | 1 (2.5) | |
| Positive | 17 (53.1) | 39 (97.5) | |
|
| |||
| Lymphocytic response | <0.001 | ||
| Negative | 13 (40.6) | 35 (87.5) | |
| Positive | 19 (59.4) | 5 (12.5) | |
The average follow-up duration was 36.9 months. Due to inadequate follow-up time, we were unable to gain information about the median survival period in survival analysis. Average survival of patients was 66.8±4.5 months (95% confidence interval 58–75.6 months). When survival periods were evaluated in terms of cancer stages, we found a statistically significant difference (p: 0.032). Median survival in stage 4 patients was 29.3±0.14 (95% confidence interval 29.07–29.6).
When survival was examined with single-variable analysis, there were no relations between sex, primary tumor location, tumor invasion depth, nodal status, histological grade, PNI, LVI, and LR (p: 0.879, p: 0.335, p: 0.594, p: 0.473, p: 0.453, p: 0.245, p: 0.934, and p: 0.873, respectively). We found a significant relation between histological sub-type and survival (p: 0.028). Mean survival in patients with conventional adenocarcinoma histology was 67.6±4.9 months (95% confidence interval 57.8–77.4) and mean survival in patients with signet ring cell histology was 17.6±6.7 months (95% confidence interval 4.4–30.8). No statistically significant relation was detected between HMGB1 expression and survival (p: 0.920).
Discussion
Evidence supporting the role of HMGB1 in cancer progression, angiogenesis, invasion, and metastasis development has been steadily accumulating [16]. Existing studies suggest that HMGB1 may have an important role in tumor progression beyond cancer development. The relation of HMGB1 overexpression with lymph node metastasis presence and advanced stage in hepatocellular carcinoma, head-neck, and esophagus squamous cell carcinoma, cervix uteri, and ovary carcinoma was demonstrated [17–21]. In our study, there was a significant relation determined between HMGB1 lymph node status, metastasis status, stage, and histological grade, PNI, LVI, and LR.
HMGB1 was initially defined as chromatin-related protein with high acidic and basic amino acid content [22]. HMGB1 is a nuclear protein that acts as a chromatin binding factor. HMGB1 exists in the nuclei of both cancerous and normal cells. HMGB1 modifies the interaction of DNA with transcription factors like p53 steroid hormone receptors by non-specifically binding to a smaller groove of DNA, and this plays a role in DNA repair, transcription, differentiation, extracellular signalization, and somatic recombination [23].
HMGB1 has affinity for different structures of DNA. These include supercoiled, single-stranded, B- and Z-DNA, DNA mini-circles, 4-way junctions, looped structures, hemi-catenated DNA, and triplex DNA [23]. Native HMGB1 released from tumor cells inhibits DNA replication. The effect of native HMGB1 decreases after acetylation. Besides recombinant HMGB1 phosphorylated by in vitro protein, kinase C cannot inhibit replication [12].
HMGB1 also enhances activity of some transcription factors related with cancer development. These include p53, p73, retinoblastoma protein, transcription factors such as Rel/NF-κB family, and estrogen receptor, which is a nuclear hormone receptor [13,24–26].
HMGB1 is released from cells that went through necrosis or were exposed to chemotherapy. It was shown in cell culture studies that HMGB1 may show DNA damage caused by chemotherapy [27]. HMGB1 has paradoxical effects in carcinogenesis. HMGB1 stimulates tumor neo-angiogenesis and enhances protective anti-tumoral T-cell response [28]. HMGB1 released from dead tumor cells stimulates mature dendritic cells and completes the tumor antigen presentation process by interacting with TLR-4 so it enhances the immune response against the tumor [29].
The role of the immune system in carcinogenesis is complex and includes high-level interaction between genetically modified cells and adaptive and natural immune cells. HMGB1 alerts the natural immune system about stress and excessive or irregular cell death. HMGB1 is transported outside of the cell in its role as a danger signal or inflammatory mediator. This can occur in 2 ways: active transport from live inflammatory cells, or passive release from necrotic or stressed cells. Anticancer treatments cause cell death and passive release of HMGB1. Also, activated leucocytes secrete HMGB1 in the tumor microenvironment [30].
HMGB1 is able to inhibit apoptosis by different pathways. HMGB1 overexpression suppresses caspase-3 and caspase-9 activity; thus, it inhibits significant steps in apoptosis. HMGB1 overexpression was shown to regulate c-IAP2, which is an antiapoptotic protein. In colorectal cancer, cytochrome apoptosis inhibitor protein 2 (c-IAP2) levels are related to HMGB1 expression [31]. Cell line studies indicate that HMGB1 inhibits the expression of Bak, which is a member of the proapoptotic Bcl-2 family [32].
Rapid tumor growth causes a decrease in intensity of microvessels, chronic hypoxia, and formation of necrotic foci. Antigenic factors are released from hypoxic and necrotic areas and inflammatory cells such as macrophages immigrate to necrotic foci. Macrophages are enabled to release angiogenic cytokines and growth factors. HMGB1 activation results in NF(Kappa)B activation and this enables release of leucocyte adhesion molecules and pro-inflammatory cytokines, and so enhances inflammation and angiogenesis [33].
In addition to NF-KB, HMGB1 can also stimulate angiogenesis by activating factors such as vascular endothelial growth factor (VEGF) [34]. Wang et al., in their study of the relation between HMGB1 expression and angiogenesis in samples from patients with bladder cancer, reported that HMGB1 is associated with CD34 and VEGF, which are angiogenesis indicators [35]. HMGB1 is associated with pathological stage of a tumor. Real-time PCR showed an increase in HMGB1 mRNA expression as tumor stage rises.
Because of these reasons, HMGB1 and its receptor, RAGE, have become important in target treatment. Blockage of RAGE, which mediates extracellular effects of HMGB1, may inhibit growth or progression of tumors. Various strategies have been evaluated for blocking the HMGB1 signal, such as management of the extracellular ligand-binding section of sRAGE, blockage of Fab fragments derived from anti-RAGE, and/or anti-HMGB1 IgG [36].
In the liver tumor model, ethyl pyruvate (EP) inhibits tumor growth in a dose-dependent manner. Even delayed treatment with EP significantly suppresses tumor growth. Increased serum IL6 and HMGB1 levels after tumor injection were significantly reduced in animals treated with EP [37]. EP A549 stimulates necrosis-apoptosis exchange in adenocarcinoma cells and inhibits release of HMGB1 [38].
Tumor cells incubated with oxaliplatin preserve HMGB1 in cytotoxic concentrations, even longer than other agents used in potent cytolytic cells [39]. Extracellular effects of HMGB1 are prevented by being kept inside the cell.
As an antioxidant, Quercetin (3,3′,4′,5,7-Pentahydroxyflavone dihydrate) has anti-inflammatory effects. It reduces oxidative damage by regulating NO, IL6, and TNF-α release [40]. Quercetin treatment significantly decreases circulatory levels of HMGB1 in animals with settled endotoxemia. In macrophage cultures, Quercetin inhibits both HMGB1 release and the activation of MAPK and Nf-kB of 2 signal pathways critical in cytokine release induced by HMGB1 [41]. Cell cycle regulation, interaction with type 2 estrogen binding areas, and some effects including tyrosine kinase inhibition of Quercetin makes it a potential anti-cancer agent [42].
Cytarabine resistance was shown to be 8–50 times decreased when short interfering RNA (siRNA) and HMGB1 expression were inhibited in human carcinoma cells [26]. These cells were shown to have lost their invasion and metastasis ability by HMGB1 gene expression being inhibited by siRNA in a gastric cancer cell culture [43].
Moriwaka et al. determined that the HMGB1 concentration in primary tumor tissue in Dukes C patients among CRC patients was higher when compared to Dukes B patients. They demonstrated that macrophage number decreased in both metastatic and non-metastatic lymph nodes among Dukes C patients. They reported that HMGB1 secreted by the primary tumor decreased the macrophage number in localized lymph nodes and weakened the anti-metastatic defense [44]. Similar results were obtained by Kuniyasu et al. [45]. These 2 studies prove that HMGB1 has, at least, facilitating effects on lymph node metastasis and increasing effects on tumor growth in CRC. Higher ratios of HMGB1 overexpression was determined in advanced stage patients and patients with positive lymph node in our study, and our results support these publications.
In stage 3B colon cancer patients, Peng et al. examined the relation between CD45RO, which demonstrates the tumor infiltrating lymphocyte (TIL) intensity, and HMGB1 expression, using immunohistochemical methods. In that study, TIL intensity and HMBG1 expression was found to be inversely proportional. Researchers claimed that HMGB1 distorted the local immune response and played a role in carcinogenesis [46]. Distinctively, we assessed LR against tumor in our study. We found approximately 4.7 times less LR in patients with HMGB1 overexpression. These findings support the hypothesis that HMGB1 overexpression distorts the host immune response.
In addition to HMBG1 overexpression suppressing the LR, determination of increased lymph node metastasis in patients can again be explained by the anti-cancer immunity suppression. It was demonstrated that antigen-presenting CD205-positive intra-tumoral dendritic cell number decreased in patients with colorectal cancer with HMGB1 overexpression and lymph node metastasis [47].
HMGB1 and its receptor, RAGE, are indicators of tumor progression in CRC. In an immunohistochemical study, HMGB1 overexpression was determined as 55.7% in CRC cases. In our cases, almost the same ratio (55.6%) of HMGB1 overexpression was determined. In that study, HMGB1 overexpression was found to be related to tumor invasion, lymph node status, distant metastasis, and stage of the disease, similar to our study. It was found also to be related to overall survival and was demonstrated to be an independent predictor of worse prognosis, based on multivariate analyses [11]. Fahmueller et al. similarly demonstrated that HMBG1 expression was related to worse prognosis in CRC patients [48]. Due to an insufficient follow-up period, our study was insufficient to determine survival.
Morikawa et al. demonstrated that HMGB1 induced apoptosis with JNK activation in Kupffer cells, which are the elements of the reticulo-endothelial system in the liver, and monocyte-dendritic cells [44]. We determined a statistically significant relation between HMGB1 overexpression and liver metastasis. Theoretically, we suggest that liver metastasis ratio can be minimized with the blockage of HMGB1 and/or its receptor RAGE. Demonstration of HMGB1 overexpression and its receptor, RAGE, in many tumors gives hope that HMGB1 ligand or its receptor has potential for the treatment of these tumors [49].
Conclusions
Our results prove that HMGB1 overexpression is significant in tumor progression, especially migration of tumor cells, as well as gaining the ability to metastasize. Our findings lead us to think that HMBG1 is a significant prognostic factor in CRC. We believe that while developing treatment strategies for CRC, HMGB1 could be an important treatment target.
Footnotes
Conflict of interest
We have no financial interest or conflict of interest in association with this work.
Source of support: Departmental sources
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Eser S, Yakut C, Özdemir R, et al. Cancer incidence rates in Turkey in 2006: a detailed registry based estimation. Asian Pac J Cancer Prev. 2010;11:1731–39. [PubMed] [Google Scholar]
- 3.Edge SB, Byrd DR, Compton CC, et al. Editors: AJCC (American Joint Committee on Cancer) Cancer Staging Manual, 7thed. New York: Springer; 2010. [Google Scholar]
- 4.Kim GP, Colangelo LH, Wieand HS, et al. Prognostic and predictive roles of high-degree microsatellite instability in colon cancer: a National Cancer Institute – National Surgical Adjuvant Breast and Bowel Project collaborative study. J Clin Oncol. 2007;25:767–72. doi: 10.1200/JCO.2006.05.8172. [DOI] [PubMed] [Google Scholar]
- 5.Han DP, Zhu QL, Cui JT, et al. Polo-like kinase 1 is overexpressed in colorectal cancer and participates in the migration and invasion of colorectal cancer cells. Med Sci Monit. 2012;18(6):BR237–46. doi: 10.12659/MSM.882900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao L, Bai L, Nan Qz. Activation of Rho GTPase Cdc42 promotes adhesion and invasion in colorectal cancer cells. Med Sci Monit Basic Res. 2013;19:201–7. doi: 10.12659/MSMBR.883983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, McCauley MJ, Maher LJ, III, et al. Mechanism of DNA flexibility enhancement by HMGB proteins. Nucleic Acids Res. 2009;37:1107–14. doi: 10.1093/nar/gkn1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kostova N, Zlateva S, Ugrinova I, Pasheva E. The expression of HMGB1 protein and its receptor RAGE in human malignant tumors. Mol Cell Biochem. 2010;337:251–58. doi: 10.1007/s11010-009-0305-0. [DOI] [PubMed] [Google Scholar]
- 9.Sharma A, Ray R, Rajeswari MR. Overexpression of high mobility group (HMG) B1 and B2 proteins directly correlates with the progression of squamous cell carcinoma in skin. Cancer Invest. 2008;26:843–51. doi: 10.1080/07357900801954210. [DOI] [PubMed] [Google Scholar]
- 10.Gnanasekar M, Thirugnanam S, Ramaswamy K. Short hairpin RNA (shRNA) constructs targeting high mobility group box-1 (HMGB1) expression leads to inhibition of prostate cancer cell survival and apoptosis. Int J Oncol. 2009;34:425–31. [PubMed] [Google Scholar]
- 11.Yao X, Zhao G, Yang H, et al. Overexpression of high-mobility group box 1 correlates with tumor progression and poor prognosis in human colorectal carcinoma. J Cancer Res Clin Oncol. 2010;136:677–84. doi: 10.1007/s00432-009-0706-1. [DOI] [PubMed] [Google Scholar]
- 12.Rhodes DR, Yu J, Shanker K. Large-scale meta-analysis of cancer microarray data identifies common transcriptional profiles of neoplastic transformation and progression. Proc Natl Acad Sci USA. 2004;101:9309–14. doi: 10.1073/pnas.0401994101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Court EL, Ann Smith M, Avent ND, et al. DNA microarray screening of differential gene expression in bone marrow samples from AML, non-AML patients and AML cell lines. Leuk Res. 2004;28:743–53. doi: 10.1016/j.leukres.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Soumaoro LT, Uetake H, Higuchi T, et al. Cyclooxygenase-2 expression: a significant prognostic indicator for patients with colorectal cancer. Clin Cancer Res. 2004;10:8465–71. doi: 10.1158/1078-0432.CCR-04-0653. [DOI] [PubMed] [Google Scholar]
- 15.Masunaga R, Kohno H, Dhar DK, et al. Cyclooxygenase-2 expression correlates with tumor neovascularization and prognosis in human colorectal carcinoma patients. Clin Cancer Res. 2000;6:4064–68. [PubMed] [Google Scholar]
- 16.Shen X, Hong L, Sun H, et al. The expression of high-mobility group protein box 1 correlates with the progression of non-small cell lung cancer. Oncol Rep. 2009;22:535–39. doi: 10.3892/or_00000468. [DOI] [PubMed] [Google Scholar]
- 17.Liu F, Zhang Y, Peng Z, et al. High expression of high mobility group box 1 (hmgb1) predicts poor prognosis for hepatocellular carcinoma after curative hepatectomy. J Transl Med. 2012;10:135. doi: 10.1186/1479-5876-10-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chuangui C, Peng T, Zhentao Y. The expression of high mobility group box 1 is associated with lymph node metastasis and poor prognosis in esophageal squamous cell carcinoma. Pathol Oncol Res. 2012;18:1021–27. doi: 10.1007/s12253-012-9539-3. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Xi B, Zhao Y, et al. High-mobility group protein B1 (HMGB1) is a novel biomarker for human ovarian cancer. Gynecol Oncol. 2012;126:109–17. doi: 10.1016/j.ygyno.2012.03.051. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Xie C, Zhang X, et al. Elevated expression of HMGB1 in squamous-cell carcinoma of the head and neck and its clinical significance. Eur J Cancer. 2010;46:3007–15. doi: 10.1016/j.ejca.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 21.Hao Q, Du XQ, Fu X, Tian J. Expression and clinical significance of HMGB1 and RAGE in cervical squamous cell carcinoma. Zhonghua Zhong Liu Za Zhi. 2008;30:292–95. [PubMed] [Google Scholar]
- 22.Alexandrova EA, Beltchev BG. Acetylated HMG1 protein interacts specifically with homologous DNA polymerase alpha in vitro. Biochem Biophys Res Commun. 1988;154:918–27. doi: 10.1016/0006-291x(88)90227-6. [DOI] [PubMed] [Google Scholar]
- 23.Kang HJ, Lee H, Choi HJ, et al. Non-histone nuclear factor HMGB1 is phosphorylated and secreted in colon cancers. Lab Invest. 2009;89:948–59. doi: 10.1038/labinvest.2009.47. [DOI] [PubMed] [Google Scholar]
- 24.Jantzen HM, Admon A, Bell SP, Tjian R. Nucleolar transcription factor hUBF contains a DNA-binding motif with homology to HMG proteins. Nature. 1990;344:830–36. doi: 10.1038/344830a0. [DOI] [PubMed] [Google Scholar]
- 25.Topalova D, Ugrinova I, Pashev IG, Pasheva EA. HMGB1 protein inhibits DNA replication in vitro: a role of the acetylation and the acidic tail. Int J Biochem Cell Biol. 2008;40:1536–42. doi: 10.1016/j.biocel.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 26.Livesey KM, Tang D, Zeh HJ, Lotze MT. Not just nuclear proteins: ‘novel’ autophagy cancer treatment targets – p53 and HMGB1. Curr Opin Investig Drugs. 2008;9:1259–63. [PubMed] [Google Scholar]
- 27.Jiao Y, Wang HC, Fan SJ. Growth suppression and radiosensitivity increase by HMGB1 in breast cancer. Acta Pharmacol Sin. 2007;28:1957–67. doi: 10.1111/j.1745-7254.2007.00669.x. [DOI] [PubMed] [Google Scholar]
- 28.Krynetskaia NF, Phadke MS, Jadhav SH, Krynetskiy EY. Chromatin-associated proteins HMGB1/2 and PDIA3 trigger cellular response to chemotherapy-induced DNA damage. Mol Cancer Ther. 2009;8:864–72. doi: 10.1158/1535-7163.MCT-08-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito N, DeMarco RA, Mailliard RB, et al. Cytolytic cells induce HMGB1 release from melanoma cell lines. J Leukoc Biol. 2007;81:75–83. doi: 10.1189/jlb.0306169. [DOI] [PubMed] [Google Scholar]
- 30.Campana L, Bosurgi L, Rovere-Querini P. HMGB1: a two-headed signal regulating tumor progression and immunity. Curr Opin Immunol. 2008;20:518–23. doi: 10.1016/j.coi.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 31.Völp K, Brezniceanu ML, Bösser S, et al. Increased expression of high mobility group box 1 (HMGB1) is associated with an elevated level of the antiapoptotic c-IAP2 protein in human colon carcinomas. Gut. 2006;55:234–42. doi: 10.1136/gut.2004.062729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brezniceanu ML, Völp K, Bösser S, et al. HMGB1 inhibits cell death in yeast and mammalian cells and is abundantly expressed in human breast carcinoma. FASEB J. 2003;17:1295–97. doi: 10.1096/fj.02-0621fje. [DOI] [PubMed] [Google Scholar]
- 33.Schlueter C, Weber H, Meyer B, et al. Angiogenetic signaling through hypoxia: HMGB1: an angiogenetic switch molecule. Am J Pathol. 2005;166:1259–63. doi: 10.1016/S0002-9440(10)62344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sitohy B, Nagy J, Dvorak H. Anti-VEGF/VEGFR therapy for cancer: reassessing the target. Cancer Res. 2012;72:1909–14. doi: 10.1158/0008-5472.CAN-11-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang W, Jiang H, Zhu H, et al. Overexpression of high mobility group box 1 and 2 is associated with the progression and angiogenesis of human bladder carcinoma. Oncol Lett. 2013;5:884–88. doi: 10.3892/ol.2012.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ellerman JE, Brown CK, de Vera M, et al. Masquerader: high mobility group box-1 and cancer. Clin Cancer Res. 2007;13:2836–48. doi: 10.1158/1078-0432.CCR-06-1953. [DOI] [PubMed] [Google Scholar]
- 37.Liang X, Chavez AR, Schapiro NE, et al. Ethyl pyruvate administration inhibits hepatic tumor growth. J Leukoc Biol. 2009;86:599–607. doi: 10.1189/jlb.0908578. [DOI] [PubMed] [Google Scholar]
- 38.Lim SC, Choi JE, Kim CH, et al. Ethyl pyruvate induces necrosis-to-apoptosis switch and inhibits high mobility group box protein 1 release in A549 lung adenocarcinoma cells. Int J Mol Med. 2007;20:187–92. [PubMed] [Google Scholar]
- 39.da Dong XE, Ito N, Lotze MT, et al. High mobility group box I (HMGB1) release from tumor cells after treatment: implications for development of targeted chemoimmunotherapy. J Immunother. 2007;30:596–606. doi: 10.1097/CJI.0b013e31804efc76. [DOI] [PubMed] [Google Scholar]
- 40.Dias AS, Porawski M, Alonso M, et al. Quercetin decreases oxidative stress, NF-kappaB activation, and iNOS overexpression in liver of streptozotocin-induced diabetic rats. J Nutr. 2005;135:2299–304. doi: 10.1093/jn/135.10.2299. [DOI] [PubMed] [Google Scholar]
- 41.Tang D, Kang R, Xiao W, et al. Quercetin Prevents Lipopolysaccharide-induced HMGB1 Release and Proinflammatory Function. Am J Respir Cell Mol Biol. 2009;41:651–60. doi: 10.1165/rcmb.2008-0119OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamson DW, Brignall MS. Antioxidants and cancer, part 3: quercetin. Altern Med Rev. 2000;5:196–208. [PubMed] [Google Scholar]
- 43.Li ZJ, Song B, Liu J, et al. Inhibitory effect of silencing of HMGB1 gene expression on the invasive and metastatic abilities of MGC-803 gastric cancer cells. Zhonghua Zhong Liu Za Zhi. 2013;35:244–48. doi: 10.3760/cma.j.issn.0253-3766.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 44.Moriwaka Y, Luo Y, Ohmori H, et al. HMGB1 attenuates anti-metastatic defense of the lymph nodes in colorectal cancer. Pathobiology. 2010;77:17–23. doi: 10.1159/000272950. [DOI] [PubMed] [Google Scholar]
- 45.Kuniyasu H, Yano S, Sasaki T, et al. Colon cancer cell-derived high mobility group 1/amphoterin induces growth inhibition and apoptosis in macrophages. Am J Pathol. 2005;166:751–60. doi: 10.1016/S0002-9440(10)62296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng RQ, Wu XJ, Ding Y, et al. Co-expression of nuclear and cytoplasmic HMGB1 is inversely associated with infiltration of CD45RO+ T cells and prognosis in patients with stage IIIB colon cancer. BMC Cancer. 2010;16:496. doi: 10.1186/1471-2407-10-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kusume A, Sasahira T, Luo Y, et al. Suppression of dendritic cells by HMGB1 is associated with lymph node metastasis of human colon cancer. Pathobiology. 2009;76:155–62. doi: 10.1159/000218331. [DOI] [PubMed] [Google Scholar]
- 48.Fahmueller YN, Nagel D, Hoffmann RT, et al. Immunogenic cell death biomarkers HMGB1, RAGE, and DNAse indicate response to radioembolization therapy and prognosis in colorectal cancer patients. Int J Cancer. 2013;15:2349–58. doi: 10.1002/ijc.27894. [DOI] [PubMed] [Google Scholar]
- 49.Tang D, Kang R, Zeh HJ, III, Lotze MT. High-mobility group box 1 and cancer. Biochim Biophys Acta. 2010;1799:131–40. doi: 10.1016/j.bbagrm.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]