Abstract
While the destructive actions of a cryoablative freeze cycle are long recognized, more recent evidence has revealed a complex set of molecular responses that provides a path for optimization. The importance of optimization relates to the observation that the cryosurgical treatment of tumors yields success only equivalent to alternative therapies. This is also true of all existing therapies of cancer that, while applied with curative intent; provide only disease suppression for periods ranging from months to years. Recent research has led to an important new understanding of the nature of cancer, which has implications for primary therapies, including cryosurgical treatment. We now recognize that a cancer is a highly organized tissue dependent on other supporting cells for its establishment, growth and invasion. Further, cancer stem cells are now recognized as an origin of disease and prove resistant to many treatment modalities. Growth is dependent on endothelial cells essential to blood vessel formation, fibroblasts production of growth factors, and protective functions of cells of the immune system. This review discusses the biology of cancer, which has profound implications for the diverse therapies of the disease, including cryosurgery. We also describe the cryosurgical treatment of diverse cancers, citing results, types of adjunctive therapy intended to improve clinical outcomes, and comment briefly on other energy-based ablative therapies. With an expanded view of tumor complexity, we identify those elements key to effective cryoablation and strategies designed to optimize cancer cell mortality with a consideration of the now recognized hallmarks of cancer.
Keywords: Cryoablation, cryosurgery, prostate cancer, cryosensitizers, cryotherapy
INTRODUCTION
Cryoablative therapies rely on controlled, local freezing caused by the removal of thermal energy (heat) from the tissues; hence, an energy-deprivation strategy. These procedures are grounded on well established cryobiological principles linked to the investigative work on the cryopreservation of cells and pathogenesis of frostbite. Cooper [25,26] first described a necrotic outcome, provided a tissue temperature of −20°C or colder, held for 1 minute or longer, was attained. This description in terms of nadir temperature and freeze duration provided the basis for cryoablative dosimetry and was a key to early clinical successes. Numerous other experimental and clinical reports have attempted to define the cryoablative dose in terms of temperature-time to assure complete tissue destruction. However, a precise definition of the “cryoablative dose” is difficult due to the diversity of opinions and practices as they related to procedural implementation, the existence of thermal gradients in frozen tissue (often related to cryoprobe performance characteristics), variations in regional blood flow, anatomical distinctions, the use of accessory warming device, cancers distinct phenotypic responses to a freeze-thaw stress, and the molecular signaling (survival and cascades death) of cells.
From a conceptual perspective, the idea of dosing originally relied on the presumption that only physical parameters related to the freeze-thaw cycle and tumor’s capillary support structure were determinate of cancer cell survival. In 1999 the first reports appeared implicating post-thaw cell stress responses as equally important to managing tumor ablation through gene regulated cell death pathways [55]. An evaluation of a variety of studies allows one to conclude that inhibition of survival stress signaling pathways with adjunctive agents can enhance the ablative effect of freezing. Further, studies have also shown that optimization of the physical factors associated with cryoprocedures can similarly affect treatment-dependent cell death [11]. Since a cancer cell population response to temperature excursions is typically “normal” or Gaussian[115], there is a population of cancer cells that may avoid freeze rupture and evoke cell survival mechanisms (pathways) to avoid apoptosis and secondary necrosis. This cancer cell survival response requires disruption for assured treatment efficacy. In practice, there is one caveat to the concept of predictable dose. Accurate tumor temperature measurement can be difficult due to thermocouple placement variation and even error in the thermocouple positioning especially when placed adjacent to blood vessels (heat sources). Hence, the temperature thresholds provided in this review are those “commonly accepted” within the field.
Numerous ablative therapies are currently in use for the treatment of cancers. While applied with curative intent, these therapies provide only modest success in disease suppression, not cure, as cancer cell mutation often enables resistance to therapy. This outcome is despite a half century of research revealing only modest improvements in durable response to diverse treatment strategies [28]. This disappointing absence of cure is related to the fact that “cancer” represents a group of more than 150 diseases linked to cellular genetic controls exhibiting defensive strategies with clear mutagenic responses.
A tumor is no longer considered a homogeneous mass of cells, growing without replicative control, causing the disruption of the architecture of primary and numerous secondary (metastatic) sites. Tumors are highly organized structures dependent on supporting cells for their establishment, growth and invasion. A hierarchy of intercellular commands provides for an “orderly” progression of the disease with accompanying defensive strategies that compromise both natural immunity and additive therapeutic interventions (i.e. radiation, chemotherapy, etc.) [27]. Cancer stem cells, now recognized as a potential origin of a tumor, lend unanticipated resiliency to the disease.
Three sentinel changes in our understanding of cancer are in process. First, cancer stem cells (CSC) [17] are now accepted as key elements of tumorigenesis as well as a “cell-of-origin” (a mutated tissue stem cell) and can be highly resistant to radiation [45] and chemotherapy [121]. Second, tumor formation involves the recruitment of numerous non-cancer support cells that establish a microenvironment essential to tumor survival, growth and ultimate metastasis. These tumor-associated cells include endothelial cells essential to blood vessel formation, fibroblasts to serve various support functions, cells of the immune system which assume a protective role for the cancer cells by masking cancer immunogenicity from circulating immune cells (i.e. macrophages, etc.), nutritive serosal cells and mesenchymal cells [50]. Hence, the tumor microenvironment creates a protective neo-tissue environment that can serve to isolate the tumor from the various defense strategies of the body. Linked to each of the above is the growing body of evidence demonstrating that with successive therapeutic attempts, the cancer cells acquire progressively enhanced resistance to individual therapeutic modalities (i.e. chemotherapy, radiation, hormonal deprivation, etc.). For example, exposure to successive bouts of cytotoxic drugs results in the survival of approximately 20–30% of the population of the cancer cells as only those cells in dividing stages succumb to the toxic exposure. With follow up treatments each additional dose result in tumor-associated fibroblasts secreting a surface protective protein (Wnt 16B) which enhances cancer cell chemotherapeutic resistance [121]. In addition, other defensive strategies are brought into play such as the upregulation of membrane protein pumps that function to eliminate the chemotherapeutic agent. Radiation has also been shown to induce the same Wnt 16B response as well as yield the amplification of DNA repair/protective strategies and inhibition of apoptosis [45].
Cryoablation is unique as a treatment modality in that it is typically a monotherapy applied without follow up or successive treatments thereby denying cancer cells the opportunity to develop defensive mutations. Additionally, the freeze-thaw process results in a disruption of many of the principal characteristics, hallmarks, of cancer [51] (Table 1). These hallmarks are indicative of evolved capabilities of cancer to assure successful tumor growth in the face of diverse, well-established anti-tumor protective adaptations. Being an energy-deprivation therapy, cryoablation has allowed the opportunity to extend our understanding to include activation of freeze-induced molecular stress cascades and their manipulation. These strategies are providing a new therapeutic path, which holds promise for improved patient outcomes.
TABLE 1.
Hallmarks of Cancer- Classifications of Generally Recognized Survival Strategies used by Cancer
|
This review will evaluate and project principals to alter and improve today’s techniques for cryosurgery, recognize the inherent variability implicit in a cryoablative application and how that variability might prove beneficial, and provide insight into adjunctive strategies designed to increase the efficacy of cryosurgery.
CRYOABLATIVE INJURY MECHANISMS
Dosimetry
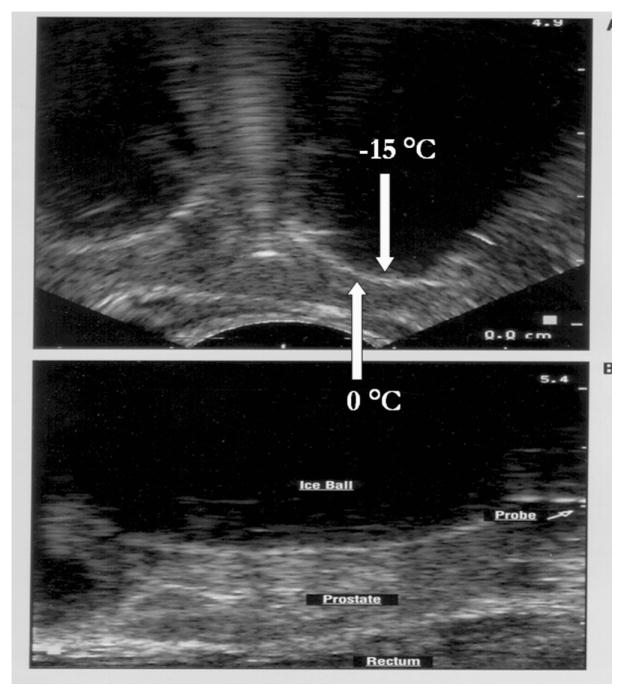
For nearly forty years since the 1960’s work of Cooper, significant and logical attempts have been expended in an effort to describe a cryosurgical “ablative dose.” The localized, sharply demarcated zone of the “ice ball” is a priori considered “lethal” especially when a second freeze-thaw cycle is included in the procedure (Figure 1). (NOTE: The temperatures indicated represent actual patient measurements.)
Figure 1. Transrectal Ultrasound Images of a Human Prostate Cryoablation Procedure.
Upper Image: Transverse view of prostate illustrating two “ice balls” advancing toward the rectum (bottom of image). The white hyperechoic rim (HER) represents the boundary between frozen (black shadowed area) and unfrozen tissue. Tissue temperature at the distal edge of the HER is nominally 0°C and approximately −15°C along the proximal edge of the HER. The position of the cryoprobe is obscured by the advancing ice. Lower Image: Sagital view the freezing process in the human prostate. Note the position of the cryoprobe shaft projecting from the frozen tissue mass.
If this concept was accurate, the “ablative dose” would simply equal the volume of frozen tissue located concentric to a given isotherm. Cooper (1964) [25] defined the “lethal dose” as −20°C for 1 minute. Later Neel (1971) [88] re-defined the lethal dose as −60°C where as Staren (1997) [119] identified −70°C as the target temperature (refer to Clinical Application section). These reports, however, did not adequately address the issue of time-at-temperature. More recently, −40°C has emerged as the target temperature based on a variety of in vitro and animal studies as well as the physics of pure water which supports the suggestion that small volumes (“cell sized”) of liquid water do not have the ability to undercool (remain a liquid) much below −40°C. Accordingly, this suggests that all freezable liquid water in a cell would be expected to crystallize near −40°C resulting in the formation of lethal intracellular ice. Taken together, our knowledge of the physics of water, our nascent understanding of the biology of cells at low temperatures and the conclusions drawn from pre-clinical experimentation, it is often taught that −40°C for “a few minutes” represents a targeted “lethal dose” [38]. While the precision of targeted tissue freezing has limitations, secondary destructive effects occur following thawing. Upon thawing, a necrotic cascade is launched culminating in a pathologically defined state, “coagulative necrosis” [41]. This state exists primarily due to the damaged endothelial cell lining of the microvasculature supplying the targeted tissue and the resulting edema and inflammation. Accordingly, the freeze/thaw process has additive, deleterious consequences due to the “solution effects” attendant to the freezing process (i.e. freeze concentration of solutes) and the critical role played by post-thaw vascular stasis.
Dosimetry implies a discrete time-energy relationship. The challenge of precise “dosing” within a cryoablative procedure is found in (a) the discontinuity in temperature (the thermal gradient measured across the “ice ball”), (b) the time of exposure within a given isothermal boundary, (c) the frequency of exposure (variations in the freeze-thaw cycle), and (d) the differential rates of cooling and thawing across the geometry of the “ice ball.” As a cryoprobe (the heat sink) or cluster of probes is activated, heat is extracted from the targeted tissue at a rapid rate adjacent to the probe and at reduced rates distant from the probe surface. Accordingly, any given cell within the targeted zone will experience distinct cooling and thawing rates, varying exposure temperatures and shortening times of exposure to a given temperature with distance from the probe. Confounding the dosing challenge is the inherent biological variability found within the tissue target. Cellular responses to a low temperature insult can vary with cell type (neoplastic vs. normal) and with reproductive stage of a given cell type (cell cycle). Further, the vascular supply (proximity to large vessels) can vary within patients as can the vasoconstrictive response of the arterioles supplying blood to the tissue. As chilling progresses through a tissue mass, reflex vasodilatory responses may cause zones of elevated temperature possibly resulting in undertreated foci. This effect is most pronounced at the “ice ball” periphery and with widespread (spaced) probe placement. These response differentials are poorly understood and provide a challenge to the concept of uniform cryotherapeutic dosimetry.
Further, the full extent of the deleterious effects may not manifest for hours, days and even weeks post-thaw. Molecular-based cellular events (i.e., apoptosis) have recently been implicated as contributing to the cascade of cell death related events [11,55]. These events may augment therapeutic effectiveness of a cryosurgical procedure especially at and near the freeze zone periphery.
Good cryosurgical technique designed to yield complete destruction of the cancerous tissues must take advantage of the full spectrum of cell death dependent inductive factors. Therefore, knowledge of the interplay between these factors and their additive effects is necessary to support progressive improvement in surgical outcomes. Ideally, an optimized cryosurgical procedure would be one in which the thermal variables are sufficiently controlled so as to provide uniformity across the targeted tissue. Unfortunately, a prescription cannot be written in terms of minutes at nadir temperature. In practice, the “dose” is the application of a freeze/thaw cycle that results in the attainment of the therapeutic goal. That is, all of the target tissue must be adequately frozen. This is determined by observation (for skin lesions), thermometry, imaging, or electrophysiological measurement (in cardiac applications) and combinations of such information. If a malignant tumor is the target, for security and safety, dosing requires a second freeze. Hence, we are unable to provide an exact quantitative statement of “dose” as is often attempted in heat-based therapies where a thermal dose is commonly (and controversially) defined as the Cumulative Equivalent Minutes at 43°C [112]. Factors contributing to an effective cryoablative dose include a complex of physical (ice – intra- versus extracellular) and biological (i.e., chill sensitivity at sub-freezing temperatures, the hypothermic continuum[7], and the molecular responses attendant to hypothermia) parameters [104].
Mechanisms of Injury
The foundations of an effective dosing stratagem are grounded in a clear understanding of the mechanisms of cellular injury associated with a freeze-thaw excursion and therefore the targeted tissues response.
The Tissue Response to Freezing
The multiple factors contributing to the freezing insult (i.e. cooling rate, nadir temperature, duration and rate of thaw) determine the response of the targeted tissue, which may range between inflammation to cellular destruction. Whether the extent of freezing is contained in the periphery of the lesion or widespread, an inflammatory response occurs. With severe freezing, further and complete destruction of cells will result as a consequence of physical disruption caused by intracellular ice. Diverse cell types often demonstrate sensitivity differences to cold injury. The exploitation of these differences for therapeutic purposes, whether for ablation or preservation of cells and tissues, depends on these dichotomous responses [9,13,60,76,91,118,120,128]. Prostate cancer cells have demonstrated distinct freeze susceptibilities correlated to the androgen receptor expression [62].
Histopathology of the cryogenic lesion reveals a central coagulation necrosis surrounded by a relatively thin peripheral zone (freeze margin). The margin is a region in which cell destruction may initially be incomplete. Tissue beyond this margin represents an unfrozen hypothermic zone. Post-thaw histology reveals a hyperemic border surrounding the previously frozen volume along with a congested central zone. Within a few days, the central zone becomes edematous and necrotic. The freeze margin or border is critical to therapeutic outcome. Tissue temperatures in the margin range from 0° to −20°C during the freezing steps yielding temperatures at which cancer cell survival is possible. Within this marginal area, apoptosis and secondary necrosis provide important mechanisms of continued cell death. Following thawing, tissue repair is initiated by an immediate infiltration of inflammatory cells (lymphocytes and macrophages) in response to release of cyto- and chemokines from damaged cells. In the weeks and even months that follow, the necrotic tissue is slowly removed by phagocytotic activity of the inflammatory cells and replaced by a fibrous, collagenous scar. A distinct asset to tissue repair and healing of cryoablation is the preservation of the collagen matrix architecture. In contrast, heating does not produce a clear demarcation of the peripheral treatment boundary as the boundary expands over the week post-treatment. The interior of the heated target zone is characterized by charring, boiling and even popping. With cold denaturation, a complete loss of the tissue architecture is noted which impacts wound repair and tissue remodeling.
The Mechanisms of Cryogenic Injury
The mechanisms of cell death initiated by cryoablation represent a cascade, which includes direct injury to the cells caused by ice crystal formation, failure of the microcirculation following thawing, and the induction of apoptosis and necrosis. Extracellular ice crystal formation removes water from the cells, which in turn produces deleterious metabolic disturbances related to the freeze concentration of solutes, a process referred to as the “solution effects”. Ice crystals cause mechanical damage due to shearing forces affecting cell membrane integrity especially in highly organized tissues [38]. Intracellular ice crystal formation occurs secondarily in the freeze zone and is lethal. The loss of blood supply due to vascular stasis in the volume of previously frozen tissue occurs soon after thawing increasing the probability that the cells will not survive. While the relative contribution of these two mechanisms of injury can be debated, they are clearly synergistic to cryoinjury [8,10,38,39,54,55].
Both intrinsic (mitochondrial-related) and extrinsic (membrane-related) apoptosis have been shown to affect cell death in a cryogenic lesion [22,23,52,59,105,139]. Disruption of the normal function of mitochondria through the influence of the Bcl-2 family of proteins is critical to the intrinsic apoptotic cell death pathways. Bax, a pro-apoptotic protein found in the cytoplasm, increases immediately after thawing. Analysis of Bcl-2, a pro-survival protein, and Bax reveal that immediately post-thaw, there is a mitochondrial-based signal promoting cell death. Bcl-2 overexpression is typical in many kinds of cancer, including prostate, and can protect the cancer cells from various therapeutic strategies [63,85,100]. Overexpression of Bcl-2 in vitro in a prostate cancer cell model has been reported to not affect the efficacy of cryoablation alone, but did provide protection to these cells when exposed in combination with chemotherapeutic agents [23]. An extension of the apoptotic cell death cascade occurs with activation of the extrinsic pathway at lower temperatures [105]. In this regard, it has been recently reported that exposure to ultracold freezing temperatures (below −30°C) results in the rapid initiation and progression of the extrinsic membrane-mediated apoptotic pathway [105]. This recent discovery has now added a molecular component to cancer cell death in the core of a cryogenic lesion where historically intracellular ice formation was believed to be the predominant mechanism of cell death.
Technique – The Freeze-Thaw Cycle
Ideal cryosurgical technique requires that the tissue is frozen rapidly, thawed slowly and completely, and then is exposed to a second freeze cycle. Proper technique accomplishes the goal of achieving a lethal temperature in the target tissues while assuring a safe margin around the tumor. Every phase of the freeze-thaw cycle, including the cooling rate, nadir tissue temperature, duration of freezing, and thawing rate, may be injurious. Rapid cooling increases the probability of lethal intracellular ice crystal formation and should be induced as fast as possible [38,56] recognizing; however, that maximum cooling rates are a function of cryogen type and cryoprobe design. Repetition of the freeze-thaw cycle subjects the tissues to a repeat and amplification of the injurious events. This double freeze is often considered to be important to ensure proper destruction of malignant tumors.
One factor, nadir temperature, in cryosurgical technique is critical, as cell death is progressive as tissues experience deeper freezing. Cancer cell viability sharply decreases with declining temperature, and it is evident that the majority of cells die as temperatures approach −40°C. However, some cancer cells have been shown to survive at lower temperatures further necessitating a double freeze-thaw cycle to −40°C [38,63].
Thawing rate also affects cancer cell survival. Slow thawing affords a longer interval of exposure to subfreezing temperatures after nadir temperature induced damage. Rapid thawing rates are less damaging and are to be avoided. However, in clinical practice thawing of the periphery of the freeze zone is passive (slow) as it relies on body heat as the source of heat energy for the thaw. Cryosurgical devices with thaw mechanisms can only provide heat to the freeze zone adjacent to the cryoprobe, which serves to loosen cryoprobes for removal and repositioning. Rapid thawing adjacent to a cryoprobe may not impact cell survival in this region.
On the matter of freeze duration, physician instinct (anecdotal evidence) guides toward longer durations. Few basic research studies have been conducted to provide lab-based evidence pertinent to duration except that a significant increase in cell death is noted when freeze duration was increased from five minutes to ten minutes [63]. In this study, it was further noted that exposure intervals of less than 1 minute and a given temperature often yielded significantly reduced cell death compared to freeze durations of greater than one minute.
CLINICAL APPLICATIONS
Skin cancer
Small skin cancers are easily and commonly managed by cryosurgery, using a spray of liquid nitrogen from a handheld device, which also may be fitted with a metal probe for application to the cancer. Treatment may be combined with preliminary curettage. The cure rate is very high, about 99% [65,97]. Topical Imiquimod has been used as an adjuvant to cryosurgery with good results [42]. Moh’s micrographic surgery is a common preferred competitor with a similar high success rate. Good comparative controlled studies of these diverse treatments are scarce. Surgical excision is the standard of care, but diverse other methods of treatment have been used [117,125].
Lung cancer
Patients who have lung cancer which is not suitable for resection because of advanced disease or poor general condition have been treated by cryosurgery, which has been performed at thoracotomy or by percutaneous routes. The extensive experience of Niu and associates with 625 patients is noteworthy [90]. The adverse effects after treatment included hemoptysis, pneumothorax, and pleural effusion, which were generally not a significant problem. The 3-year survival rate was 32%. Cryosurgery has also been used to treat the lung cancer and its metastases to sites such as the liver, adrenal and bone with a 1-year survival of about 53% [6].
Breast cancer
Interest is evident in the treatment of small early-stage breast cancers by image-guided percutaneous ablative techniques [141]. In clinical trials with these techniques, cryosurgery has been rated as safe and effective [70,98]. Clearly randomized trials are needed to support the wider acceptance of ablative approaches offering local control with minimal side effects.
Esophagus
In the treatment of esophageal cancer, surgical excision is commonly required. However cryosurgical techniques using a spray of cryogen, commonly liquid nitrogen via endoscopy, have found use for dysplastic mucosal disease and early-stage cancer [49]. Benefits are achieved in about 70% of patients. In addition to cryosurgery, alternate options in endoscopic ablative techniques include mucosal resection, photodynamic therapy and radiofrequency [21].
Hepatic cancers
Hepatic cancers have been treated by cryosurgery for many years [37]. Recently they have been treated by ultrasound- guided percutaneous techniques, which have been considered safe and effective [20,68]. In comparison with other techniques of ablation, the complication rate with cryosurgery was considered high [78]. The selection of patients may have been a factor in this result. Cryosurgery has found use in the 15–20% of patients with liver metastases from colon cancer. The survival rates are comparable to radiofrequency resection, which also is commonly used [89,96].
Kidney tumors
Kidney tumors commonly require surgical excision, the standard of care, but small tumors in selected patients have been treated by image-guided cryoablation, often by percutaneous techniques. Though surgical excision is the standard of care for the small tumors (<4 cm), image-guided, cryosurgical ablation via percutaneous or laparoscopic techniques have yielded promising results and are judged to be safe effective treatments [33,123]. Location of the tumor on the posterior surface is favorable to the use of percutaneous cryoablation [72]. Recent reports compare cryoablation with radiofrequency ablation revealing that the oncological treatment results and the complication rate are similar in the two techniques, however further comparative studies are needed [4,34,35].
Prostate cancer
Radical prostatectomy has long been the standard of care, but this treatment has significant morbidity. Cryosurgical treatment has had extensive trial and has been recognized as an acceptable alternative to radical excision [5,24]. However, cryosurgery too has morbidity related to freezing extraprostatic tissues during ablation of the prostate. This has led to recent high interest in focal treatment by cryosurgery, a technique of unilateral nerve-sparing ablation introduced by Onik (2004) [93]. Currently a variety of ablative techniques are used in focal treatment, including MRI- guided laser ablation and MRI-guided focused ultrasound which are judged as promising options in therapy [16], but a wide range of choice in treatment is evident in literature [1,18]. Only a small percentage of prostate cancers, however, are sufficiently localized to be considered for focal therapy. As such, this method is viewed as a treatment choice between radical treatment methods, such as prostatectomy and radiotherapy, or watchful waiting. A central challenge to focal treatment effectiveness relates to the ability to anatomically localize portions of the gland containing cancer versus those that are cancer-free. Clinical trials dedicated to evaluating focal therapy remain needed but will be complex and difficult.
Bone tumors
If benign or with low metastatic potential, bone tumors can be treated by tissue-conserving techniques, commonly by intralesional excision by curettage followed by cryosurgery [36]. After curettage, cryosurgery devitalizes an additional margin of tissue around the lesion, which helps insure the intent to cure. The treated bone is weakened by resorption and prone to fracture, so support during healing is needed and provided by cementation, bone grafts, or internal fixation. Cumulative data shows more than 90% recurrence- free outcomes with a 5-year average follow up. In comparison with other treatment options, curettage with adjunctive cryosurgery and related supportive measures yields the most effective treatment. Aggressive cancers usually require excision and adjunctive chemotherapy or irradiation. Cryosurgical techniques can provide palliative benefits in cancers metastatic to bone.
Other Sites
Cryosurgical techniques have had limited use or clinical trials for cancers in diverse other sites which have not resulted in wide or general use. In the oral cavity, cryosurgery is used for a variety of benign diseases, but excision or irradiation is the preferred treatment. In other sites, including cancers of the eye, brain, larynx, bronchus, uterus, urinary bladder and pancreas, cryosurgery has had limited clinical use [40]. Excision, irradiation or other ablative techniques are preferred treatments in most of these sites.
ADJUNCTIVE THERAPY
The need to enhance the efficacy of cryosurgery in the treatment of cancer has led to the use of adjunctive therapeutic agents that have been diverse in nature [47]. In general, the goal of adjunctive therapy is to enhance the destructive activity of freezing at the border of the frozen zone where the sub- temperature is elevated between −40°C and −0.5°C. The intended effect is to cause an enhanced cell death response to cold stress via activation of apoptosis. Many challenges remain in the selection, dose, and use of the appropriate agents. Adjunctive chemotherapy with selected anti-neoplastic drugs, such as 5-Flouracil or Imiquimod, enhances cell injury via cytotoxicity when used for skin cancers. Transarterial chemoembolization, as an adjunct to cryoablation, has proven useful in the management of large metastatic liver cancers [83]. In general, however, chemotherapy as an adjunct to cryosurgery has limited usefulness because of the resistance of cancer cells. Experimental work in vivo using intratumor injection chemotherapy has shown enhanced benefit [67]. Further, extensive studies using in vitro prostate cancer models support the notion that when using chemotherapy as an adjunct to cryoablation enhanced cancer cell death at elevated subfreezing temperatures can be attained even when exposure is to subclinical (low dose) chemotherapeutic agents such as 5-FU, Taxotare, and Cisplatin [22,23,63].
Immunomodulating agents are used to stimulate an immunologic response. In an excellent review, Sabel has concluded that cryoablation alone has not produced a consistent immunologic response, whether in the direction of stimulation or suppression and that the clinical reports of the adjunctive use on immunostimulants lack certainty of benefit [107]. Experimental work in vivo on the induction of tumor immunity as an adjunct to cryoablation continues because the prospects of benefit [76]. While the current body of direct evidence based studies do not support a cryoimmune response, anecdotal clinical experience and observations supports the involvement, impact, and potential of immunomodulation.
Irradiation, in its diverse techniques, has been used often as an adjunct to cryosurgery. Irradiation from an external source is common, but many other techniques of irradiation are alternate approaches to therapy. Brachytherapy is a common choice; however, the use of irradiation via these implanted radioactive seeds as an adjunct to cryosurgery is less common. Although uncommon, this technique has been successfully used in the treatment of locally advanced cancer of the pancreas [137].
Other agents have been used as an adjunct therapy in experimental trials in vivo. Tumor necrosis factor alpha (TNF-a), used in a prostate cancer model in nude mice enhanced tumor cell injury by promotion of inflammation and neutrophil infiltration and by endothelial cell apoptosis [58]. Antifreeze proteins have been shown to affect the rate and morphology of ice crystal formation and increase cell destruction in a prostate cancer grown in mice and rats [84,99]. Vitamin D3 has been shown in cell culture and animal studies to be a highly effective cryosensitizing agent [12]. While diverse in nature and specific mechanisms (pathways) of action, a common theme of enhanced therapeutic outcome has emerged through the use of adjunctive pretreatment of cancer prior to cryoablation. Through various in vitro, in vivo, and clinical reports, improved cancer destruction at mild sub-freezing temperatures following brief low dose exposure to general or specific apoptotic inducing agents has been shown. In some cases, such as the use of vitamin D3 as a sensitizing agent, complete prostate cancer cell death has been demonstrated following freezing to temperatures as warm as −10°C in both in vitro and in vivo models [12,61,111].
COMPETITIVE ABLATIVE THERAPIES
Cure is possible by extirpation or ablation when the tumor is localized. Yet for disseminated disease, we presently lack curative strategies. Precise staging tools are essential to determine if the tumor is localized or not. In this section, we examine the strengths and weaknesses of each type of ablative therapy in comparison with each other and with surgical excision. The diverse techniques have been broadly divided into thermal and non-thermal methods. The mechanisms of cryoablative damage are more completely described when compared to heat-based procedures [11,12,61,111].
Thermal Ablation
Radiofrequency ablation
Radiofrequency ablation energy destroys tissue by heating to temperatures above 50°C [48,79]. Treatment by RF energy requires thin needle electrodes placed in the tissue in or around the tumor, usually by image guidance. Electric current in the radiofrequency range (4 to 500 Hz) causes resistive heating and destroys the tissue. Current exits the body via grounding electrode pads on the skin.
RF ablation has been used for tumors of the liver, lung, kidney, breast, and bone [53,114]. The method is most practical for tumors 3 cm or smaller. For hepatocellular tumors a successful ablation may be expected in 70 – 90% [44,71,138]. Results of RF ablation are similar to those of percutaneous alcohol injection [3,46]. Success rate decreases with increasing tumor size and the presence of cirrhosis [110]. The role of RF ablation in breast cancer remains to be determined. In kidney tumors, RFA has yielded long term outcomes comparable to excision [92]. In painful bone metastases, RF and cryosurgery offer similar benefits, but few controlled studies have been conduced [87]. The heat being transferred into the tissue may dissipate into adjacent tissues and produce undesirable local effects. Infections of the necrotic tissue can also be a problem.
Reports on recurrence rates after RF in hepatocellular carcinoma vary but approach 50% [127]. However, Rhim et al. (2008) report a 3000 patient experience with complete ablation in 96.7% and a five-year survival rate of 58% [104]. Post-procedure pain is extremely low during the first 24 hours <4 on a 10-point numerical rating scale and similar to those of irreversible electroporation (IRE) [86].
Microwave ablation
Microwave ablation creates heat by excitation of water molecules, increasing kinetic energy and elevating the tissue temperature. Most microwave ablation devices operate at 2.45 GHz, delivering 60W, and can produce tissue temperatures as high as 150°C. Microwave devices deliver energy via antennas, which are of various designs and offer several types of tissue-heating patterns. Newer devices operate at 915Hz, use water cooled antennas and can deliver 80W [122].
Microwave ablation has been used for tumors of the liver, lung, breast, and bone [114]. When used for cancer of the liver, the results are similar to those achieved by RF [116]. When used in lung cancers, microwave ablation achieved about a 50 to 60% five-year survival [132,136]. Higher microwave power can cause injury to other tissues especially the skin. Other reported injuries include liver abscess, perforation of the colon, tumor seeding, pleural effusion, hemorrhage, fever, and pain [69].
High-intensity focused ultrasound (HIFU)
HIFU uses ultrasound waves focused transcutaneously or transrectally, heating the tissue and producing necrosis. Treatment is guided by ultrasound or MRI. When used for breast tumors, a transducer is placed on the skin over the tumor. The high-frequency pressure waves lies in the range of 0.5 to 4.0 MHz. A temperature of 56°C or more must be produced and held for at least a second [114].
The results using HIFU are dependent upon patient selection, ablation margin, treatment time and related factors [113]. When used for liver tumors, accurate imaging is crucial [81]. When used for prostate cancer the technique usually requires a foregoing transurethral resection of the prostate. Results are highly dependent on patient selection, the definitions used for biochemical recurrence and the number of biopsies taken at follow-up visits [135]. Blana et al. (2008) provided outcome data for patients with localized prostate with mean follow-up of 4.8 years [14]. Disease-free survival rate was 66%; urinary incontinence rate 8% and 44.7% of the 76 potent patients became impotent post-treatment. In another study, the overall biochemical recurrence was 59.5% in an average of 13.8 months. Recently, a 51% biochemical failure and 77% positive biopsies were reported thirteen months following HIFU [129]. Concerns over HIFU has been that the length of the dose does not match the prostate’s geometry; treatment effect “between the columns” of heat and that real-time observation is not possible. Villers reported that 48 patients with a pre-treatment PSA of 6.0 had PSA of 2.6 and 3.6 at six and twelve-months, respectively [131]. Treated lobes were biopsy negative in 92% vs. 84% in contralateral lobes.
Laser therapy
Interstitial laser therapy functions by a refraction of laser light on the tumor. This action absorbs the protons and produces heat, which causes coagulation necrosis [106]. The technique requires image-guided percutaneous insertion of fibers into the center of the tumor. Tissue temperature is measured at the periphery of the tumor. Laser energy is delivered until the temperature reaches 60°C. Two types of laser therapies are available: laser-induced interstitial thermotherapy (LITT) and photodynamic therapy (PDT). LITT is also referred to as interstitial laser photocoagulation and has similar mechanisms of action as hyperthermia. With PDT, a photosensitizing agent is injected at intervals of varying length to assure distribution of the agent followed by laser activation. Three types of lasers are used: carbon dioxide lasers, argon lasers, and neodymium:yttrium-aluminum-garnet (Nd:YAG) lasers. The first two types of lasers are mainly used in skin cancer. Nd:YAG is generally used for tumors in internal organs (i.e. esophagus and colon).
Interstitial laser therapy has been successful in 67% of patients with early breast cancers [31]. It is best suited to invasive ductal cancers 2 cm or less in diameter [130] and in fibroadenomas [133]. The technique also has been used for liver tumors, breast cancers metastatic in the liver [74] and for palliation of advanced breast cancers [2]. Laser therapy has been used for primary and salvage treatment of supraglottic carcinoma [57]. MRI-guided laser ablation was judged to be a promising option for focal treatment of prostatic cancer [1,16,29].
Cryosurgery
This ablative therapy uses freezing to destroy tumors and requires the use of cryosurgical apparatus cooled by a variety of cryogens. Modern technology commonly uses argon as the cryogen, used in apparatus that cools by the Joule-Thomson principle, and features the use of 10 – 17 ga. cryoprobes, placed percutaneously into the tumor. Spraying the cryogen on the tissue is commonly done in the treatment of small skin tumors with a hand-held device. Automated equipment is used for application of one or more probes to the tumor and for initiating the flow of the cryogenic agent. As previously discussed, the tissue response to freezing ranges from inflammation to necrosis. Basic cryosurgical technique requires that the tumor be frozen rapidly, thawed slowly and then exposed to a repeated freeze/thaw cycle. A tissue temperature of −40°C should be achieved in the repeated freeze/thaw cycle. The use of imaging techniques is critical to the procedure in providing an image of the advancing ice front. However, sound waves do not penetrate the ice front thereby causing acoustic shadowing distal to the ice front (Figure 2). In the periphery where some cell survival may be expected because of the elevated freeze temperature, apoptosis (and possibly autophagy) contributes to cell death [11].
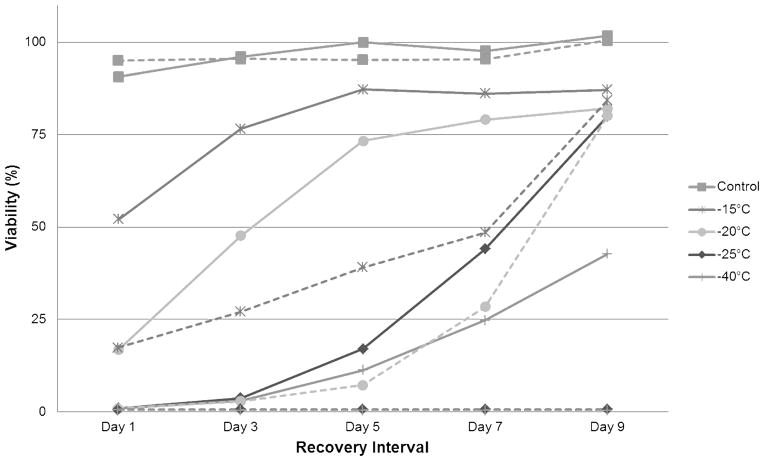
Figure 2. Freeze Response Profiles of an Androgen Sensitive and Insensitive Human Prostate Cancer Phenotype.
Androgen sensitive LNCaP cells (dotted lines) are a p53 positive and androgen receptor positive prostate cancer phenotype whereas PC-3 cells (solid lines) are p53 negative and androgen receptor negative. The androgen insensitive PC-3 cells have been shown to be more tolerant to freezing injury as illustrated by their recovery following exposure to −25°C and −40°C in comparison to the LNCaP cells which are completely destroyed at those temperatures.
In the treatment of cancer of the prostate, the long term disease-free survival for low to moderate risk disease states was about 77% [24]. Cryoablation has been compared favorably with external radiotherapy and brachytherapy [5,73]. Recently substantial interest is evident for focal or partial prostatic cryotherapy. The stimulus for this is the high incidence of erectile dysfunction after whole gland treatment whether radical prostatectomy or total gland cryosurgery. Cancer of the liver has had extensive usage of cryosurgery. The best results are achieved with small single tumors no larger than 2 −3 cm. Short term results are competitive with RFA [75]. Cryosurgery of kidney tumors yields a survival rate of 98%. RFA is associated with a trend toward a higher recurrence rate [48]. Cryoablation can eliminate breast cancers (1.5 cm. or smaller) if no DCIS exists [108] and may also be used for palliative benefits.
NON-THERMAL ABLATION
Alcohol injection
This method of ablation consists of the percutaneous injection of pure alcohol into the target tissue under image-guidance. The alcohol draws water from the cell, producing dehydration and cell destruction. Multiple injections may be needed, depending upon the size of the tumor [64]. This method has been used for tumors of the liver, kidney, pancreas, thyroid and others. In liver tumors, 2 cm or smaller, the 5 year survival rate was comparable to RFA [101]. Alcohol injection has been used as an adjunct to RFA and in combination with transarterial chemoembolization and with microwave ablation in the treatment of liver tumors [19,95,103,124,134]. A meta-analysis concluded that RF ablation was superior to percutaneous alcohol injection [43]. Randomized studies comparing this technique with surgical resection have been inconclusive [110].
Electroporation
Electroporation uses a high energy to produce short high-voltage (1500–3000 V) pulses of DC electric current (25–45A) to enhance the permeability of cell membranes to chemotherapeutic agents [126]. The pulses are delivered to the tissue through needle electrodes of variable length. Pulses of about 100 microseconds create an electrical field that opens the cell membranes by creating nano-sized pores. The membrane can be permanently permeabilized and eventually rupture, killing the cell. The pores can also be short-lived, in which case the membrane recovers. This is known as reversible electroporation. The goal is to permit the entry of drugs or chemical agents. When the molecule delivered to the cell is a chemotherapeutic drug, the process is called electrochemotherapy (ECT).
The chemotherapeutic agents most commonly used are bleomycin and cisplatin. This method may be used to treat cancers of the skin, head and neck, liver, kidney and prostate gland [66,77,94]. ECT increases the cytotoxicity of cisplatin without increasing the dosage. At the end of a trial on metastatic breast cancer all patients showed an objective response [102]. Side effects included muscle spasms for the duration of the treatment caused by the electric pulses. Redness and swelling subsided within 4 weeks and there was minimal scarring. Clinical experience is small at present, but the method merits for further investigation.
Radiation therapy
Radiation therapy kills cells by damaging their DNA. The radiation may be delivered from an external source or from radioactive material placed in or near a cancer, which is called brachytherapy. Radiation therapy is most often used as primary therapy with the intent to cure, but it is also used as an adjunct and for palliation. Radiation therapy utilizes free radical producing effects of ionizing radiation to cause cell death. Improvements in providing a radiation dose include 3D conformal radiation therapy, which permits the dosing to match target shape, and intensity-modulated radiation therapy (IMRT) which improves dose matching to target with concave shapes.
Radiation therapy is used in many different organs, including the prostate, uterus, brain, lung, breast, urinary bladder and others. When used for selected patients with cancer of the prostate, high rates of local control were reported [30,82,140]. When used for early stage lung cancer, the 5 year survival rate was about 11% [80]. A common use is to combine radiation with cytotoxic drug therapy, as has been reported for advanced nasopharyngeal carcinoma [32] and to combine radiation with androgen deprivation in prostate cancer [15]. Radiation injures all dividing cells which leads to co-morbidities like injury, erythema, swelling, local tenderness, ulceration, bleeding, intestinal problems and immunosuppression. Long term effects include fibrosis, vascular angiomas, strictures, chronic infection and secondary malignancy.
DISCUSSION
Surgical excision is the gold standard for most tumors and provides the opportunity to make a thorough histologic examination.
For the percutaneous treatment of small liver tumors, RFA appears to be superior to percutaneous alcohol injection and is the preferred technique used in the United States [3]. Its use in other tumors is not as well developed. In general, focused microwave therapy lacks controlled comparisons with other ablative therapies. Cryosurgery is used in a wide range of benign and malignant diseases. When used for visceral tumors, the technique is competitive with RFA. In the management of kidney tumors, cryosurgery was thought to be somewhat superior to RFA; however, no randomized studies are available. Cryosurgery is well suited to use in cancer of the prostate. In China, the technique has extraordinary wide use in diverse visceral tumors.
Comparing these diverse ablative techniques is difficult for several reasons. Most important is a dearth of randomized studies. Differences in patient and tumor characteristics, staging criteria and lack of standardized treatment procedures make comparison an uncertain task. Protocols for biopsies after ablation and criteria for biochemical recurrence are also not always the same. The use of adjunctive therapy may alter results and affect comparison. The easiest comparison of efficacy of ablative techniques may be between RFA and cryosurgery for liver cancer and kidney cancer, but differences in the selection of patients introduces variations that do not yield to valuable controlled studies. For prostate cancer, improvements in equipment design for HIFU and cryosurgery complicate comparison in studies over many years. Physicians’ bias in selection of treatment is a factor also. For these reasons, the comparative results of different reports are difficult to evaluate. When the clinical benefit of treatment is marginal, the risk of side effects becomes more important. Preferred techniques must also meet today’s requirements of short hospital stays and cost effectiveness.
DIRECTION
Cryosurgical ablation is an effective safe method of the treatment of selected tumors [5]. The potential for further development of the techniques is good. The use of cryosurgery is largely dependent on image-guided control of freezing the tissue, especially when used percutaneously or laparoscopically. In freezing, care must be taken to use the precise freeze-thaw cycles that have been well defined by past experimental and clinical use. Improvement in clinical results is dependent on the use of adjunctive therapeutic agents to enhance the effect of freezing on the cells. Advances in instrumentation may be expected.
In the treatment of malignant tumors, surgical excision remains the standard of care, but diverse competing ablative techniques have yielded reasonable curative or palliative results. Clinical circumstances, especially the stage of disease, compel alternate choices in therapy and affects controlled clinical comparisons. The dearth of comparative studies hinders evaluation of benefit, so physician bias remains an important factor in choice of treatment.
Unique to cryoablation is the consequential suspension and ultimate disruption of the principle hallmarks or characteristics of cancer (Table 1) that support re-growth and secondary mutation. Tumor progression is dependent on the maintenance of a complex microenvironment composed of discrete supportive cells. It is widely accepted that a freeze insult which provides a nadir temperature of <−40°C will destroy all cells with the cancer cell typically demonstrating a greater level of resistance. For example, endothelial cells are completely destroyed over a temperature range of −15 to −25°C with some cancer cells requiring a lower ablative exposure. This sensitivity differential will, in the more distal regions of the freeze zone, yield a hypoxic zone due to vascular disruption with compromised cell survival due to oxygen and therefore ATP deprivation, waste accumulation and local acidosis. Death of tumor associated fibroblasts, which are also highly freeze sensitive, will deny the microenvironment of essential growth factors required for revascularization, cancer cell growth and re-establishment of a local immune cell population.
Following initial freeze rupture of all cell types, the release of cytokines leads to the recruitment of circulating immune cells and local inflammation. A “cryoimmunologic response” is later [109] which may overcome the local immunosuppressive action of tumor-associated immune cells. With destruction of the tumor microenvironment, surviving cancer cells (if any) are deprived of essential support and lose proliferative, and therefore mutagenic, capabilities. As a result, any surviving cells within the freeze target are likely to undergo delayed or secondary necrosis for a period of days to weeks that is observed histologically as a region of coagulative necrosis. It is the sequence and consequences of three ablative processes (i.e. freeze rupture, apoptosis and necrosis) [105] that uniquely support a prolonged tissue destructive outcome.
SUMMARY
Cryoablation is a well-established therapeutic regime for the treatment of numerous cancers. While often thought of as a simple ablative mechanism relying on primarily physical destruction of cancer cells, cryoablation is now understood to be a sophisticated, combinatorial therapy involving a complex cascade of destructive stresses which include extra- and intra-cellular ice crystal formation, initial post-thaw necrosis due to partial cellular damage from ice, the activation of a rapid membrane based apoptotic response within the core of a cryogenic lesion, by a delayed mitochondrial-based apoptotic response in the periphery of the iceball due, in part, to severe oxidative stress, secondary necrosis due to hypoxia and then coagulative necrosis due to vascular stasis. Destruction due to physical events is immediate. Freezing based cell stress related physiological-based destruction (apoptosis) occurs over hours to days. Further, the prolonged cytotoxic effects of vascular damage and inflammation may occur over many days to weeks.
While the hallmarks of effective cryoablation are well known (i.e. fast freezing to a lethal nadir temperature, slow thawing, and repetition of the freeze-thaw cycle) the incidence of persistent disease following freezing suggests that application of these principles requires continued investigation and optimization. This has resulted in the exploration of various adjunctive therapeutic strategies (molecular-based therapy) to enhance and assure cancer destruction throughout the entire frozen tissue mass. While research and optimization remain ongoing, today cryoablation is a highly effective and practical means of treating numerous cancers with the long term studies (5 and 10 year follow-up) demonstrating outcomes equivalent to or better than those achieved with other ablative techniques such as RFA and radiation therapy. As a more in-depth understanding of the molecular mechanisms involved in cryogenic injury and adjunct therapy evolves, further enhancement of the efficacy of cryosurgical technique is anticipated.
Acknowledgments
This report was supported in part from grants from the NIH and CPSI Biotech. The authors wish to express their appreciation to Sara E. Palmer for her diligent efforts in the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abern MR, Tsivian M, Polascik TJ. Focal therapy of prostate cancer: Evidence-based analysis for modern selection criteria. Curr Urol Rep. 2012;13:160–9. doi: 10.1007/s11934-012-0241-5. [DOI] [PubMed] [Google Scholar]
- 2.Akimov AB, Seregin VE, Rusanov KV, Tyurina EG, Glushko TA, Nevzorov VP, et al. Nd: Yag interstitial laser thermotherapy in the treatment of breast cancer. Lasers Surg Med. 1998;22:257–67. doi: 10.1002/(sici)1096-9101(1998)22:5<257::aid-lsm1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 3.Ansari D, Andersson R. Radiofrequency ablation or percutaneous ethanol injection for the treatment of liver tumors. World J Gastroenterol. 2012;18:1003–8. doi: 10.3748/wjg.v18.i10.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atwell TD, Carter RE, Schmit GD, Carr CM, Boorjian SA, Curry TB, et al. Complications following 573 percutaneous renal radiofrequency and cryoablation procedures. J Vasc Interv Radiol. 2012;23:48–54. doi: 10.1016/j.jvir.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Babaian RJ, Donnelly B, Bahn D, Baust JG, Dineen M, Ellis D, et al. Best practice statement on cryosurgery for the treatment of localized prostate cancer. J Urol. 2008;180:1993–2004. doi: 10.1016/j.juro.2008.07.108. [DOI] [PubMed] [Google Scholar]
- 6.Bang HJ, Littrup PJ, Currier BP, Goodrich DJ, Aoun HD, Klein LC, et al. Percutaneous cryoablation of metastatic lesions from non-small-cell lung carcinoma: Initial survival, local control, and cost observations. J Vasc Interv Radiol. 2012;23:761–9. doi: 10.1016/j.jvir.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baust JG. Concepts in biopreservation. In: Baust JG, Baust JM, editors. Advances in Biopreservation. CRC Press; Boca Raton: 2007. [Google Scholar]
- 8.Baust JG, Gage AA. Progress toward optimization of cryosurgery. Technol Cancer Res Treat. 2004;3:95–101. doi: 10.1177/153303460400300202. [DOI] [PubMed] [Google Scholar]
- 9.Baust JG, Gage AA. The molecular basis of cryosurgery. BJU Int. 2005;95:1187–1191. doi: 10.1111/j.1464-410X.2005.05502.x. [DOI] [PubMed] [Google Scholar]
- 10.Baust JG, Gage AA, Clarke D, Baust JM, Van Buskirk R. Cryosurgery--a putative approach to molecular-based optimization. Cryobiology. 2004;48:190–204. doi: 10.1016/j.cryobiol.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Baust JG, Gage AA, Robilotto AT, Baust JM. The pathophysiology of thermoablation: Optimizing cryoablation. Curr Opin Urol. 2009;19:127–32. doi: 10.1097/MOU.0b013e328323f654. [DOI] [PubMed] [Google Scholar]
- 12.Baust JM, Klossner DP, Robilotto A, Vanbuskirk RG, Gage AA, Mouraviev V, et al. Vitamin d(3) cryosensitization increases prostate cancer susceptibility to cryoablation via mitochondrial-mediated apoptosis and necrosis. BJU international. 2012;109:949–58. doi: 10.1111/j.1464-410X.2011.10408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baust JM, Vogel MJ, Van Buskirk R, Baust JG. A molecular basis of cryopreservation failure and its modulation to improve cell survival. Cell Transplant. 2001;10:561–71. [PubMed] [Google Scholar]
- 14.Blana A, Rogenhofer S, Ganzer R, Lunz JC, Schostak M, Wieland WF, et al. Eight years’ experience with high-intensity focused ultrasonography for treatment of localized prostate cancer. Urology. 2008;72:1329–34. doi: 10.1016/j.urology.2008.06.062. [DOI] [PubMed] [Google Scholar]
- 15.Bolla M, de Reijke TM, Van Tienhoven G, Van den Bergh AC, Oddens J, Poortmans PM, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516–27. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- 16.Bomers JG, Sedelaar JP, Barentsz JO, Futterer JJ. Mri-guided interventions for the treatment of prostate cancer. AJR Am J Roentgenol. 2012;199:714–20. doi: 10.2214/AJR.12.8725. [DOI] [PubMed] [Google Scholar]
- 17.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–7. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 18.Bozzini G, Colin P, Nevoux P, Villers A, Mordon S, Betrouni N. Focal therapy of prostate cancer: Energies and procedures. Urol Oncol. 2013;31:155–67. doi: 10.1016/j.urolonc.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Brunello F, Veltri A, Carucci P, Pagano E, Ciccone G, Moretto P, et al. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: A randomized controlled trial. Scand J Gastroenterol. 2008;43:727–35. doi: 10.1080/00365520701885481. [DOI] [PubMed] [Google Scholar]
- 20.Chen HW, Lai EC, Zhen ZJ, Cui WZ, Liao S, Lau WY. Ultrasound-guided percutaneous cryotherapy of hepatocellular carcinoma. Int J Surg. 2011;9:188–91. doi: 10.1016/j.ijsu.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Chennat J, Waxman I. Endoscopic treatment of barrett’s esophagus: From metaplasia to intramucosal carcinoma. World J Gastroenterol. 2010;16:3780–5. doi: 10.3748/wjg.v16.i30.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clarke DM, Baust JM, Van Buskirk RG, Baust JG. Chemo-cryo combination therapy: An adjunctive model for the treatment of prostate cancer. Cryobiology. 2001;42:274–285. doi: 10.1006/cryo.2001.2333. [DOI] [PubMed] [Google Scholar]
- 23.Clarke DM, Baust JM, Van Buskirk RG, Baust JG. Addition of anticancer agents enhances freezing-induced prostate cancer cell death: Implications of mitochondrial involvement. Cryobiology. 2004;49:45–61. doi: 10.1016/j.cryobiol.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Cohen JK, Miller RJ, Jr, Ahmed S, Lotz MJ, Baust J. Ten-year biochemical disease control for patients with prostate cancer treated with cryosurgery as primary therapy. Urology. 2008;71:515–8. doi: 10.1016/j.urology.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 25.Cooper IS. Cryobiology as viewed by the surgeon. Cryobiology. 1964;1:44–51. doi: 10.1016/0011-2240(64)90019-7. [DOI] [PubMed] [Google Scholar]
- 26.Cooper IS. Cryogenic surgery for cancer. Fed Proc. 1965;24:S237–40. [PubMed] [Google Scholar]
- 27.Corn PG. The tumor microenvironment in prostate cancer: Elucidating molecular pathways for therapy development. Cancer Manag Res. 2012;4:183–93. doi: 10.2147/CMAR.S32839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coventry BJ, Ashdown ML. Complete clinical responses to cancer therapy caused by multiple divergent approaches: A repeating theme lost in translation. Cancer Manag Res. 2012;4:137–49. doi: 10.2147/CMAR.S31887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Da Rosa MR, Trachtenberg J, Chopra R, Haider MA. Early experience in mri-guided therapies of prostate cancer: Hifu, laser and photodynamic treatment. Cancer Imaging. 2011;11(SpecA):S3–8. doi: 10.1102/1470-7330.2011.9003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demanes DJ, Brandt D, Schour L, Hill DR. Excellent results from high dose rate brachytherapy and external beam for prostate cancer are not improved by androgen deprivation. Am J Clin Oncol. 2009;32:342–7. doi: 10.1097/COC.0b013e31818cd277. [DOI] [PubMed] [Google Scholar]
- 31.Dowlatshahi K, Francescatti DS, Bloom KJ. Laser therapy for small breast cancers. Am J Surg. 2002;184:359–63. doi: 10.1016/s0002-9610(02)00942-x. [DOI] [PubMed] [Google Scholar]
- 32.Du C, Ying H, Zhou J, Hu C, Zhang Y. Experience with combination of docetaxel, cisplatin plus 5-fluorouracil chemotherapy, and intensity-modulated radiotherapy for locoregionally advanced nasopharyngeal carcinoma. Int J Clin Oncol. 2012 doi: 10.1007/s10147-012-0403-y. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 33.Duffey B, Nguyen V, Lund E, Koopmeiners JS, Hulbert J, Anderson JK. Intermediate-term outcomes after renal cryoablation: Results of a multi-institutional study. J Endourol. 2012;26:15–20. doi: 10.1089/end.2011.0179. [DOI] [PubMed] [Google Scholar]
- 34.El Dib R, Touma NJ, Kapoor A. Cryoablation vs radiofrequency ablation for the treatment of renal cell carcinoma: A meta-analysis of case series studies. BJU international. 2012;110:510–6. doi: 10.1111/j.1464-410X.2011.10885.x. [DOI] [PubMed] [Google Scholar]
- 35.Faddegon S, Cadeddu JA. Does renal mass ablation provide adequate long-term oncologic control? Urol Clin North Am. 2012;39:181–90. doi: 10.1016/j.ucl.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Gage A, Baust J, Baust J. Bone tumors- adjunctive cryosurgery. Low Temperature Medicine. 2011;37:92–99. [Google Scholar]
- 37.Gage A, Snyder K, Baust JM. Selective cryotherapy: Preservation-ablation. In: Baust J, Baust J, editors. Advances in biopreservation. CRC Press; Boca Raton, FL: 2007. p. 4. [Google Scholar]
- 38.Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998;37:171–86. doi: 10.1006/cryo.1998.2115. [DOI] [PubMed] [Google Scholar]
- 39.Gage AA, Baust JG. Cryosurgery - a review of recent advances and current issues. Cryo Letters. 2002;23:69–78. [PubMed] [Google Scholar]
- 40.Gage AA, Baust JG. Cryosurgery for tumors. J Am Coll Surg. 2007;205:342–356. doi: 10.1016/j.jamcollsurg.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 41.Gage AA, Baust JM, Baust JG. Experimental cryosurgery investigations in vivo. Cryobiology. 2009;59:229–43. doi: 10.1016/j.cryobiol.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaitanis G, Nomikos K, Vava E, Alexopoulos EC, Bassukas ID. Immunocryosurgery for basal cell carcinoma: Results of a pilot, prospective, open-label study of cryosurgery during continued imiquimod application. J Eur Acad Dermatol Venereol. 2009;23:1427–31. doi: 10.1111/j.1468-3083.2009.03224.x. [DOI] [PubMed] [Google Scholar]
- 43.Germani G, Pleguezuelo M, Gurusamy K, Meyer T, Isgro G, Burroughs AK. Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocelullar carcinoma: A meta-analysis. J Hepatol. 2010;52:380–8. doi: 10.1016/j.jhep.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 44.Gervais DA, Arellano RS. Percutaneous tumor ablation for hepatocellular carcinoma. AJR Am J Roentgenol. 2011;197:789–94. doi: 10.2214/AJR.11.7656. [DOI] [PubMed] [Google Scholar]
- 45.Ghisolfi L, Keates AC, Hu X, Lee DK, Li CJ. Ionizing radiation induces stemness in cancer cells. PLoS One. 2012;7:e43628. doi: 10.1371/journal.pone.0043628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giorgio A, Di Sarno A, De Stefano G, Scognamiglio U, Farella N, Mariniello A, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma compared to percutaneous ethanol injection in treatment of cirrhotic patients: An italian randomized controlled trial. Anticancer Res. 2011;31:2291–5. [PubMed] [Google Scholar]
- 47.Goel R, Anderson K, Slaton J, Schmidlin F, Vercellotti G, Belcher J, et al. Adjuvant approaches to enhance cryosurgery. J Biomech Eng. 2009;131:074003. doi: 10.1115/1.3156804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goel RK, Kaouk JH. Probe ablative treatment for small renal masses: Cryoablation vs. Radio frequency ablation. Current opinion in urology. 2008;18:467–73. doi: 10.1097/MOU.0b013e32830a735b. [DOI] [PubMed] [Google Scholar]
- 49.Greenwald BD, Dumot JA. Cryotherapy for barrett’s esophagus and esophageal cancer. Curr Opin Gastroenterol. 2011;27:363–7. doi: 10.1097/MOG.0b013e328347bae8. [DOI] [PubMed] [Google Scholar]
- 50.Hanahan D, Coussens LM. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–22. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 51.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 52.Hanai A, Yang WL, Ravikumar TS. Induction of apoptosis in human colon carcinoma cells ht29 by sublethal cryo-injury: Mediation by cytochrome c release. Int J Cancer. 2001;93:526–33. doi: 10.1002/ijc.1359. [DOI] [PubMed] [Google Scholar]
- 53.Healey TT, Dupuy DE. Radiofrequency ablation: A safe and effective treatment in nonoperative patients with early-stage lung cancer. Cancer J. 2011;17:33–7. doi: 10.1097/PPO.0b013e318209176f. [DOI] [PubMed] [Google Scholar]
- 54.Hoffmann NE, Bischof JC. The cryobiology of cryosurgical injury. Urology. 2002;60:40–9. doi: 10.1016/s0090-4295(02)01683-7. [DOI] [PubMed] [Google Scholar]
- 55.Hollister WR, Mathew AJ, Baust JG, Van Buskirk RG. Effects of freezing on cell viability and mechanisms of cell death in a human prostate cell line. Mol Urol. 1998;2:13–18. [Google Scholar]
- 56.Hong JS, Rubinsky B. Patterns of ice formation in normal and malignant breast tissue. Cryobiology. 1994;31:109–20. doi: 10.1006/cryo.1994.1015. [DOI] [PubMed] [Google Scholar]
- 57.Hutcheson KA, Jantharapattana K, Barringer DA, Lewin JS, Holsinger FC. Functional and oncological outcomes of primary versus salvage transoral laser microsurgery for supraglottic carcinoma. Ann Otol Rhinol Laryngol. 2012;121:664–70. doi: 10.1177/000348941212101007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang J, Goel R, Schmechel S, Vercellotti G, Forster C, Bischof J. Pre-conditioning cryosurgery: Cellular and molecular mechanisms and dynamics of tnf-alpha enhanced cryotherapy in an in vivo prostate cancer model system. Cryobiology. 2010;61:280–8. doi: 10.1016/j.cryobiol.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73:2013–26. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 60.Khairy P, Chauvet P, Lehmann J, Lambert J, Macle L, Tanguay JF, et al. Lower incidence of thrombus formation with cryoenergy versus radiofrequency catheter ablation. Circulation. 2003;107:2045–50. doi: 10.1161/01.CIR.0000058706.82623.A1. [DOI] [PubMed] [Google Scholar]
- 61.Kimura M, Rabbani Z, Mouraviev V, Tsivian M, Caso J, Satoh T, et al. Role of vitamin d(3) as a sensitizer to cryoablation in a murine prostate cancer model: Preliminary in vivo study. Urology. 2010;76(764):e14–20. doi: 10.1016/j.urology.2010.03.041. [DOI] [PubMed] [Google Scholar]
- 62.Klossner DP, Baust JM, Van Buskirk RG, Gage AA, Baust JG. Cryoablative response of prostate cancer cells is influenced by androgen receptor expression. BJU Int. 2008;101:1310–1316. doi: 10.1111/j.1464-410X.2008.07499.x. [DOI] [PubMed] [Google Scholar]
- 63.Klossner DP, Robilotto AT, Clarke DM, Van Buskirk RG, Baust JM, Gage AA, et al. Cryosurgical technique: Assessment of the fundamental variables using human prostate cancer model systems. Cryobiology. 2007;55:189–199. doi: 10.1016/j.cryobiol.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 64.Kuang M, Lu MD, Xie XY, Xu HX, Xu ZF, Liu GJ, et al. Ethanol ablation of hepatocellular carcinoma up to 5.0 cm by using a multipronged injection needle with high-dose strategy. Radiology. 2009;253:552–61. doi: 10.1148/radiol.2532082021. [DOI] [PubMed] [Google Scholar]
- 65.Kuflik EG. Cryosurgery for skin cancer: 30-year experience and cure rates. Dermatol Surg. 2004;30:297–300. doi: 10.1111/j.1524-4725.2004.30090.x. [DOI] [PubMed] [Google Scholar]
- 66.Landstrom FJ, Nilsson CO, Crafoord S, Reizenstein JA, Adamsson GB, Lofgren LA. Electroporation therapy of skin cancer in the head and neck area. Dermatol Surg. 2010;36:1245–50. doi: 10.1111/j.1524-4725.2010.01617.x. [DOI] [PubMed] [Google Scholar]
- 67.Le Pivert PJ, Morrison DR, Haddad RS, Renard M, Aller A, Titus K, et al. Percutaneous tumor ablation: Microencapsulated echo-guided interstitial chemotherapy combined with cryosurgery increases necrosis in prostate cancer. Technol Cancer Res Treat. 2009;8:207–16. doi: 10.1177/153303460900800305. [DOI] [PubMed] [Google Scholar]
- 68.Lee SM, Won JY, Lee DY, Lee KH, Lee KS, Paik YH, et al. Percutaneous cryoablation of small hepatocellular carcinomas using a 17-gauge ultrathin probe. Clin Radiol. 2011;66:752–9. doi: 10.1016/j.crad.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 69.Liang P, Wang Y, Yu X, Dong B. Malignant liver tumors: Treatment with percutaneous microwave ablation--complications among cohort of 1136 patients. Radiology. 2009;251:933–40. doi: 10.1148/radiol.2513081740. [DOI] [PubMed] [Google Scholar]
- 70.Littrup PJ, Jallad B, Chandiwala-Mody P, D’Agostini M, Adam BA, Bouwman D. Cryotherapy for breast cancer: A feasibility study without excision. J Vasc Interv Radiol. 2009;20:1329–41. doi: 10.1016/j.jvir.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, et al. Hepatocellular carcinoma: Radio-frequency ablation of medium and large lesions. Radiology. 2000;214:761–8. doi: 10.1148/radiology.214.3.r00mr02761. [DOI] [PubMed] [Google Scholar]
- 72.Long CJ, Canter DJ, Smaldone MC, Li T, Simhan J, Rozenfeld B, et al. Role of tumor location in selecting patients for percutaneous versus surgical cryoablation of renal masses. Can J Urol. 2012;19:6417–22. [PMC free article] [PubMed] [Google Scholar]
- 73.Long JP, Bahn D, Lee F, Shinohara K, Chinn DO, Macaluso JN., Jr Five-year retrospective, multi-institutional pooled analysis of cancer-related outcomes after cryosurgical ablation of the prostate. Urology. 2001;57:518–23. doi: 10.1016/s0090-4295(00)01060-8. [DOI] [PubMed] [Google Scholar]
- 74.Mack MG, Straub R, Eichler K, Sollner O, Lehnert T, Vogl TJ. Breast cancer metastases in liver: Laser-induced interstitial thermotherapy--local tumor control rate and survival data. Radiology. 2004;233:400–9. doi: 10.1148/radiol.2332030454. [DOI] [PubMed] [Google Scholar]
- 75.Mala T. Cryoablation of liver tumours -- a review of mechanisms, techniques and clinical outcome. Minim Invasive Ther Allied Technol. 2006;15:9–17. doi: 10.1080/13645700500468268. [DOI] [PubMed] [Google Scholar]
- 76.Mathew AJ, Baust JM, Van Buskirk RG, Baust JG. Cell preservation in reparative and regenerative medicine: Evolution of individualized solution composition. Tissue Eng. 2004;10:1662–71. doi: 10.1089/ten.2004.10.1662. [DOI] [PubMed] [Google Scholar]
- 77.Matthiessen LW, Chalmers RL, Sainsbury DC, Veeramani S, Kessell G, Humphreys AC, et al. Management of cutaneous metastases using electrochemotherapy. Acta Oncol. 2011;50:621–9. doi: 10.3109/0284186X.2011.573626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McWilliams JP, Yamamoto S, Raman SS, Loh CT, Lee EW, Liu DM, et al. Percutaneous ablation of hepatocellular carcinoma: Current status. J Vasc Interv Radiol. 2010;21:S204–13. doi: 10.1016/j.jvir.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 79.Mertyna P, Hines-Peralta A, Liu ZJ, Halpern E, Goldberg W, Goldberg SN. Radiofrequency ablation: Variability in heat sensitivity in tumors and tissues. J Vasc Interv Radiol. 2007;18:647–54. doi: 10.1016/j.jvir.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 80.Milano MT, Zhang H, Usuki KY, Singh DP, Chen Y. Definitive radiotherapy for stage i nonsmall cell lung cancer: A population-based study of survival. Cancer. 2012;118:5572–9. doi: 10.1002/cncr.27589. [DOI] [PubMed] [Google Scholar]
- 81.Minami Y, Kudo M. Review of dynamic contrast-enhanced ultrasound guidance in ablation therapy for hepatocellular carcinoma. World J Gastroenterol. 2011;17:4952–9. doi: 10.3748/wjg.v17.i45.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mottet N, Peneau M, Mazeron JJ, Molinie V, Richaud P. Addition of radiotherapy to long-term androgen deprivation in locally advanced prostate cancer: An open randomised phase 3 trial. Eur Urol. 2012;62:213–9. doi: 10.1016/j.eururo.2012.03.053. [DOI] [PubMed] [Google Scholar]
- 83.Mu F, Niu L, Li H, Liao M, Li L, Liu C, et al. Percutaneous comprehensive cryoablation for metastatic hepatocellular cancer. Cryobiology. 2013;66:76–80. doi: 10.1016/j.cryobiol.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 84.Muldrew K, Rewcastle J, Donnelly BJ, Saliken JC, Liang S, Goldie S, et al. Flounder antifreeze peptides increase the efficacy of cryosurgery. Cryobiology. 2001;42:182–9. doi: 10.1006/cryo.2001.2321. [DOI] [PubMed] [Google Scholar]
- 85.Nahta R, Esteva FJ. Bcl-2 antisense oligonucleotides: A potential novel strategy for the treatment of breast cancer. Semin Oncol. 2003;30:143–9. doi: 10.1053/j.seminoncol.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 86.Narayanan G, Froud T, Lo K, Barbery KJ, Perez-Rojas E, Yrizarry J. Pain analysis in patients with hepatocellular carcinoma: Irreversible electroporation versus radiofrequency ablation-initial observations. Cardiovasc Intervent Radiol. 2012;36:176–82. doi: 10.1007/s00270-012-0426-9. [DOI] [PubMed] [Google Scholar]
- 87.Nazario J, Hernandez J, Tam AL. Thermal ablation of painful bone metastases. Tech Vasc Interv Radiol. 2011;14:150–9. doi: 10.1053/j.tvir.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 88.Neel HB, Ketcham AS, Hammond WG. Requisites for successful cryogenic surgery of cancer. Arch Surg. 1971;102:45–8. doi: 10.1001/archsurg.1971.01350010047012. [DOI] [PubMed] [Google Scholar]
- 89.Ng KM, Chua TC, Saxena A, Zhao J, Chu F, Morris DL. Two decades of experience with hepatic cryotherapy for advanced colorectal metastases. Ann Surg Oncol. 2012;19:1276–83. doi: 10.1245/s10434-011-2025-4. [DOI] [PubMed] [Google Scholar]
- 90.Niu L, Xu K, Mu F. Cryosurgery for lung cancer. J Thorac Dis. 2012;4:408–19. doi: 10.3978/j.issn.2072-1439.2012.07.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ohno K, Nelson LR, Mitooka K, Bourne WM. Transplantation of cryopreserved human corneas in a xenograft model. Cryobiology. 2002;44:142–9. doi: 10.1016/s0011-2240(02)00016-0. [DOI] [PubMed] [Google Scholar]
- 92.Olweny EO, Park SK, Tan YK, Best SL, Trimmer C, Cadeddu JA. Radiofrequency ablation versus partial nephrectomy in patients with solitary clinical t1a renal cell carcinoma: Comparable oncologic outcomes at a minimum of 5 years of follow-up. Eur Urol. 2012;61:1156–61. doi: 10.1016/j.eururo.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 93.Onik G. The male lumpectomy: Rationale for a cancer targeted approach for prostate cryoablation. A review. Technol Cancer Res Treat. 2004;3:365–70. doi: 10.1177/153303460400300406. [DOI] [PubMed] [Google Scholar]
- 94.Onik G, Mikus P, Rubinsky B. Irreversible electroporation: Implications for prostate ablation. Technol Cancer Res Treat. 2007;6:295–300. doi: 10.1177/153303460700600405. [DOI] [PubMed] [Google Scholar]
- 95.Orlando A, Leandro G, Olivo M, Andriulli A, Cottone M. Radiofrequency thermal ablation vs. Percutaneous ethanol injection for small hepatocellular carcinoma in cirrhosis: Meta-analysis of randomized controlled trials. Am J Gastroenterol. 2009;104:514–24. doi: 10.1038/ajg.2008.80. [DOI] [PubMed] [Google Scholar]
- 96.Pathak S, Jones R, Tang JM, Parmar C, Fenwick S, Malik H, et al. Ablative therapies for colorectal liver metastases: A systematic review. Colorectal Dis. 2011;13:e252–65. doi: 10.1111/j.1463-1318.2011.02695.x. [DOI] [PubMed] [Google Scholar]
- 97.Peikert JM. Prospective trial of curettage and cryosurgery in the management of non-facial, superficial, and minimally invasive basal and squamous cell carcinoma. Int J Dermatol. 2011;50:1135–8. doi: 10.1111/j.1365-4632.2011.04969.x. [DOI] [PubMed] [Google Scholar]
- 98.Pfleiderer SO, Marx C, Camara O, Gajda M, Kaiser WA. Ultrasound-guided, percutaneous cryotherapy of small (< or = 15 mm) breast cancers. Invest Radiol. 2005;40:472–7. doi: 10.1097/01.rli.0000166935.56971.ff. [DOI] [PubMed] [Google Scholar]
- 99.Pham L, Dahiya R, Rubinsky B. An in vivo study of antifreeze protein adjuvant cryosurgery. Cryobiology. 1999;38:169–75. doi: 10.1006/cryo.1999.2158. [DOI] [PubMed] [Google Scholar]
- 100.Piro LD. Apoptosis, bcl-2 antisense, and cancer therapy. Oncology (Williston Park) 2004;18:5–10. [PubMed] [Google Scholar]
- 101.Pompili M, Nicolardi E, Abbate V, Miele L, Riccardi L, Covino M, et al. Ethanol injection is highly effective for hepatocellular carcinoma smaller than 2 cm. World J Gastroenterol. 2011;17:3126–32. doi: 10.3748/wjg.v17.i26.3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rebersek M, Cufer T, Cemazar M, Kranjc S, Sersa G. Electrochemotherapy with cisplatin of cutaneous tumor lesions in breast cancer. Anticancer Drugs. 2004;15:593–7. doi: 10.1097/01.cad.0000132234.30674.df. [DOI] [PubMed] [Google Scholar]
- 103.Ren H, Liang P, Yu X, Wang Y, Lu T, Li X. Treatment of liver tumours adjacent to hepatic hilum with percutaneous microwave ablation combined with ethanol injection: A pilot study. Int J Hyperthermia. 2011;27:249–54. doi: 10.3109/02656736.2011.552086. [DOI] [PubMed] [Google Scholar]
- 104.Rhim H, Lim HK, Kim YS, Choi D, Lee WJ. Radiofrequency ablation of hepatic tumors: Lessons learned from 3000 procedures. J Gastroenterol Hepatol. 2008;23:1492–500. doi: 10.1111/j.1440-1746.2008.05550.x. [DOI] [PubMed] [Google Scholar]
- 105.Robilotto AT, Baust JM, Van Buskirk RG, Gage AA, Baust JG. Temperature-dependent activation of differential apoptotic pathways during cryoablation in a human prostate cancer model. Prostate Cancer Prostatic Dis. 2013;16:41–9. doi: 10.1038/pcan.2012.48. [DOI] [PubMed] [Google Scholar]
- 106.Robinson DS, Parel JM, Denham DB, Gonzalez-Cirre X, Manns F, Milne PJ, et al. Interstitial laser hyperthermia model development for minimally invasive therapy of breast carcinoma. J Am Col Surgeons. 1998;186:284–92. doi: 10.1016/s1072-7515(97)00152-x. [DOI] [PubMed] [Google Scholar]
- 107.Sabel MS. Cryo-immunology: A review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology. 2009;58:1–11. doi: 10.1016/j.cryobiol.2008.10.126. [DOI] [PubMed] [Google Scholar]
- 108.Sabel MS, Kaufman CS, Whitworth P, Chang H, Stocks LH, Simmons R, et al. Cryoablation of early-stage breast cancer: Work-in-progress report of a multi-institutional trial. Ann Surg Oncol. 2004;11:542–9. doi: 10.1245/ASO.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 109.Sabel MS, Su G, Griffith KA, Chang AE. Rate of freeze alters the immunologic response after cryoablation of breast cancer. Ann Surg Oncol. 2010;17:1187–93. doi: 10.1245/s10434-009-0846-1. [DOI] [PubMed] [Google Scholar]
- 110.Salhab M, Canelo R. An overview of evidence-based management of hepatocellular carcinoma: A meta-analysis. J Cancer Res Ther. 2011;7:463–75. doi: 10.4103/0973-1482.92023. [DOI] [PubMed] [Google Scholar]
- 111.Santucci KL, Snyder KK, Baust JM, Van Buskirk RG, Mouraviev V, Polascik TJ, et al. Use of 1,25alpha dihydroxyvitamin d3 as a cryosensitizing agent in a murine prostate cancer model. Prostate Cancer Prostatic Dis. 2011;14:97–104. doi: 10.1038/pcan.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys. 1984;10:787–800. doi: 10.1016/0360-3016(84)90379-1. [DOI] [PubMed] [Google Scholar]
- 113.Schmitz AC, Gianfelice D, Daniel BL, Mali WP, van den Bosch MA. Image-guided focused ultrasound ablation of breast cancer: Current status, challenges, and future directions. Eur Radiol. 2008;18:1431–41. doi: 10.1007/s00330-008-0906-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sharma R, Wagner JL, Hwang RF. Ablative therapies of the breast. Surg Oncol Clin N Am. 2011;20:317–39. doi: 10.1016/j.soc.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 115.Simanovski D, Sarkar M, Irani A, O’Connell-Rodwell C, Contag C, Schwettman HA, et al. Cellular tolerance to pulsed heating. Proc SPIE 5695 Optical Interactions with Tissue and Cells XVI. 2005;5695:254–259. [Google Scholar]
- 116.Simo KA, Sereika SE, Newton KN, Gerber DA. Laparoscopic-assisted microwave ablation for hepatocellular carcinoma: Safety and efficacy in comparison with radiofrequency ablation. J Surg Oncol. 2011;104:822–9. doi: 10.1002/jso.21933. [DOI] [PubMed] [Google Scholar]
- 117.Smith V, Walton S. Treatment of facial basal cell carcinoma: A review. J Skin Cancer. 2011;2011:1–7. doi: 10.1155/2011/380371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sosef MN, Baust JM, Sugimachi K, Fowler A, Tompkins RG, Toner M. Cryopreservation of isolated primary rat hepatocytes: Enhanced survival and long-term hepatospecific function. Ann Surg. 2005;241:125–33. doi: 10.1097/01.sla.0000149303.48692.0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Staren ED, Sabel MS, Gianakakis LM, Wiener GA, Hart VM, Gorski M, et al. Cryosurgery of breast cancer. Arch Surg. 1997;132:28–34. doi: 10.1001/archsurg.1997.01430250030005. [DOI] [PubMed] [Google Scholar]
- 120.Stylianou J, Vowels M, Hadfield K. Novel cryoprotectant significantly improves the post-thaw recovery and quality of hsc from cb. Cytotherapy. 2006;8:57–61. doi: 10.1080/14653240500501021. [DOI] [PubMed] [Google Scholar]
- 121.Sun Y, Campisi J, Higano C, Beer TM, Porter P, Coleman I, et al. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through wnt16b. Nat Med. 2012;18:1359–68. doi: 10.1038/nm.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sun Y, Wang Y, Ni X, Gao Y, Shao Q, Liu L, et al. Comparison of ablation zone between 915- and 2,450-mhz cooled-shaft microwave antenna: Results in in vivo porcine livers. AJR Am J Roentgenol. 2009;192:511–4. doi: 10.2214/AJR.07.3828. [DOI] [PubMed] [Google Scholar]
- 123.Tanagho YS, Roytman TM, Bhayani SB, Kim EH, Benway BM, Gardner MW, et al. Laparoscopic cryoablation of renal masses: Single-center long-term experience. Urology. 2012;80:307–14. doi: 10.1016/j.urology.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 124.Taniguchi M, Kim SR, Imoto S, Ikawa H, Ando K, Mita K, et al. Long-term outcome of percutaneous ethanol injection therapy for minimum-sized hepatocellular carcinoma. World J Gastroenterol. 2008;14:1997–2002. doi: 10.3748/wjg.14.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Telfer NR, Colver GB, Morton CA. Guidelines for the management of basal cell carcinoma. Br J Dermatol. 2008;159:35–48. doi: 10.1111/j.1365-2133.2008.08666.x. [DOI] [PubMed] [Google Scholar]
- 126.Thomson KR, Cheung W, Ellis SJ, Federman D, Kavnoudias H, Loader-Oliver D, et al. Investigation of the safety of irreversible electroporation in humans. J Vasc Interv Radiol. 2011;22:611–21. doi: 10.1016/j.jvir.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 127.Tiong L, Maddern GJ. Systematic review and meta-analysis of survival and disease recurrence after radiofrequency ablation for hepatocellular carcinoma. Br J Surg. 2011;98:1210–24. doi: 10.1002/bjs.7669. [DOI] [PubMed] [Google Scholar]
- 128.Van Buskirk RG, Snyder KK, Baust JM, Mathew AJ, Baust JG. Hypothermic storage and cryopreservation- the issues of successful short-term and long term preservation of cells and tissues. BioProcess International. 2004;2:42–49. [Google Scholar]
- 129.Van der Kwast T. Histological effects of prostate therapies. Montréal Conference on Focal Therapy for Prostate Cancer; Montreal, Canada. 2012. [Google Scholar]
- 130.van Esser S, Stapper G, van Diest PJ, van den Bosch MA, Klaessens JH, Mali WP, et al. Ultrasound-guided laser-induced thermal therapy for small palpable invasive breast carcinomas: A feasibility study. Ann Surg Oncol. 2009;16:2259–63. doi: 10.1245/s10434-009-0544-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Villers A. Hifu (ablaterm): The french hemi-ablation study. Montréal Conference on Focal Therapy for Prostate Cancer; Montreal, Canada. 2012. [Google Scholar]
- 132.Vogl TJ, Naguib NN, Gruber-Rouh T, Koitka K, Lehnert T, Nour-Eldin NE. Microwave ablation therapy: Clinical utility in treatment of pulmonary metastases. Radiology. 2011;261:643–51. doi: 10.1148/radiol.11101643. [DOI] [PubMed] [Google Scholar]
- 133.Vogl TJ, Straub R, Zangos S, Mack MG, Eichler K. Mr-guided laser-induced thermotherapy (litt) of liver tumours: Experimental and clinical data. Int J Hyperthermia. 2004;20:713–24. doi: 10.1080/02656730400007212. [DOI] [PubMed] [Google Scholar]
- 134.Wang W, Shi J, Xie WF. Transarterial chemoembolization in combination with percutaneous ablation therapy in unresectable hepatocellular carcinoma: A meta-analysis. Liver Int. 2010;30:741–9. doi: 10.1111/j.1478-3231.2010.02221.x. [DOI] [PubMed] [Google Scholar]
- 135.Warmuth M, Johansson T, Mad P. Systematic review of the efficacy and safety of high-intensity focussed ultrasound for the primary and salvage treatment of prostate cancer. Eur Urol. 2010;58:803–15. doi: 10.1016/j.eururo.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 136.Wolf FJ, Grand DJ, Machan JT, Dipetrillo TA, Mayo-Smith WW, Dupuy DE. Microwave ablation of lung malignancies: Effectiveness, ct findings, and safety in 50 patients. Radiology. 2008;247:871–9. doi: 10.1148/radiol.2473070996. [DOI] [PubMed] [Google Scholar]
- 137.Xu KC, Niu LZ, Hu YZ, He WB, He YS, Li YF, et al. A pilot study on combination of cryosurgery and (125)iodine seed implantation for treatment of locally advanced pancreatic cancer. World J Gastroenterol. 2008;14:1603–11. doi: 10.3748/wjg.14.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yan K, Chen MH, Yang W, Wang YB, Gao W, Hao CY, et al. Radiofrequency ablation of hepatocellular carcinoma: Long-term outcome and prognostic factors. Eur J Radiol. 2008;67:336–47. doi: 10.1016/j.ejrad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 139.Yang WL, Addona T, Nair DG, Qi L, Ravikumar TS. Apoptosis induced by cryo-injury in human colorectal cancer cells is associated with mitochondrial dysfunction. Int J Cancer. 2003;103:360–9. doi: 10.1002/ijc.10822. [DOI] [PubMed] [Google Scholar]
- 140.Zaorsky NG, Li T, Devarajan K, Horwitz EM, Buyyounouski MK. Assessment of the american joint committee on cancer staging (sixth and seventh editions) for clinically localized prostate cancer treated with external beam radiotherapy and comparison with the national comprehensive cancer network risk-stratification method. Cancer. 2012;118:5535–43. doi: 10.1002/cncr.27597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhao Z, Wu F. Minimally-invasive thermal ablation of early-stage breast cancer: A systemic review. Eur J Surg Oncol. 2010;36:1149–55. doi: 10.1016/j.ejso.2010.09.012. [DOI] [PubMed] [Google Scholar]