Abstract
Background
The study aimed to compare the efficacy and safety of nebulized steroid (NS) with systemic corticosteroids (SC) and to determine optimal NS dose in the treatment of patients with COPD exacerbations requiring hospitalization.
Material/Methods
The study was a randomized, parallel design trial. Eligible patients (n=86) were randomly allocated to 1 of the 3 treatment groups: parenteral corticosteroid (PS) (n=33), 4 mg (NB) (n=27), or 8 mg NB (n=26). Partial pressure of arterial oxygen (PaO2), carbon dioxide (PaCO2), pH, and oxygen saturation (SaO2) were evaluated at baseline, 24 h, 48 h, and discharge. Airway obstruction (forced vital capacity [FVC] and forced expiratory volume 1 s [FEV1]) was evaluated at admission and discharge.
Results
There were no significant differences between the groups for all parameters at all time periods, except for higher FEV1 value in the 8-mg NB group at baseline. In groups, significant differences were determined for FVC, FEV1, PaO2, and SaO2 (p<0.001), but not for PaCO2 and pH, in comparison to their baseline values. As adverse events, hyperglycemia and oral moniliasis were observed in the PS group (n=4) and in the NB groups (n=5), respectively, and treatment change was required in 9 patients (2 patients in the PS group and 7 patients in the NB groups) (p=0.57).
Conclusions
Nebulized budesonide may be used as an alternative to SC because of its equal effectiveness and lesser systemic adverse effects. The choice of optimal dosage needs to be evaluated carefully because adverse effect and dropout rates varied according to dosage. However, there is a need for further studies including more severe cases and evaluating long-term outcomes or relapses comparing the 3 arms.
MeSH Keywords: Budesonide - therapeutic use, Methylprednisolone - therapeutic use, Pulmonary Disease, Chronic Obstructive - drug therapy
Background
Chronic obstructive pulmonary disease (COPD) is a common disease that has a chronic and progressive course. In patients with COPD, exacerbation history is an important component in therapeutic decision-making. The number of exacerbations is important because of increased morbidity, mortality, and healthcare costs [1].
Systemic corticosteroids (SC) are recommended by all international guidelines in the management of exacerbations of COPD as well as bronchodilator, oxygen, and antibacterial treatment [2,3]. However, there are still some concerns about systemic corticosteroid use because COPD patients tend to be older, relatively immobilized, and prone to development of steroid-related complications. Exacerbation rate is significantly higher in COPD patients, and these patients need higher amounts of SC to control their exacerbation [4,5]. Because of its potential and frequent use, SC results in adverse effects such as osteoporosis and bone fractures, thinning of the skin, posterior subcapsular cataract formation, glucose intolerance, and myopathy [6–11]. Thus, this condition leads clinicians to seek alternative options such as nebulized steroid use. However, there are few studies showing that nebulized steroids (NS) are as effective as SC in controlling exacerbations of COPD [13–18] and the optimal NS dose is still uncertain.
We aimed to compare the efficacy and safety of NS with SC and determine optimal NS dose in the treatment of patients with COPD exacerbations requiring hospitalization.
Material and Methods
Study population
One hundred patients with moderate or severe COPD exacerbation who were older than 40-years-old, had a smoking history of at least 10-pack-years, and who required hospitalization were included in the study. COPD diagnosis was based on clinical evaluation as defined by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) [2]. The patients were excluded if they had asthma, allergic rhinitis, atopy, or any systemic disease (such as diabetes mellitus or hypertension); were exposed to systemic corticosteroids in the preceding month; used more than 1500 microg/d of inhaled beclomethasone equivalent; were admitted to the intensive care unit (pH <7.30 and/or PaCO2 >70 mm Hg, and/or PaO2 <50 mm Hg despite supplemental oxygen); or if a specific cause for the exacerbation, such as pneumonia, pneumothorax, or heart failure, was diagnosed.
Study design
The study was as a randomized, parallel design trial. The randomization order was determined using a computer-generated list of random numbers. Eligible patients were randomly allocated to 1 of the 3 treatment groups: parenteral corticosteroid (PS), 4 mg nebulized budesonide (NB), or 8 mg NB. The efficacy of the study medications was assessed at hospitalization, 24 h, 48 h, and discharge. Patients were monitored during the hospitalization. Patients were withdrawn from the study if they required intubation and were managed in the intensive care unit. The Karadeniz Technical University Faculty of Medicine Ethics Board approved the protocol of the study (IRB. 74. KTU. 0.02.012/148). Informed consent was obtained from all the patients at the beginning of the study.
Treatments
Treatment in the PS group consisted of methylprednisolone 40 mg (intravenous ampoule); treatment in the NB groups consisted of nebulized budesonide suspension (Pulmicort Nebuampul® 0.5 mg/ml; Astra-Zeneca Pharmaceutical Production) during hospitalization. Budesonide were given as 2 mg twice daily or 4 mg twice daily; methylprednisolone were given intravenously once daily.
Nebulization procedures were performed by using a jet nebulizer (Porta Neb® Ventstream® 1803; Medic-Aid) with 80% of output of less than 5 microns. Patients received standard treatment with a nebulized β-agonist (salbutamol 3.01 mg) and anticholinergic (ipratropium bromide 0.5 mg) combination every 6 h and intravenous aminophylline (0.5 mg/kg/h). Supplementary oxygen therapy was used to maintain oxygen saturation (SaO2) >90%.
Measurements
Patients were assessed every 12 h during the acute phase (from H0 to H48), and at hospital discharge. Arterial blood samples were taken at baseline, 24 h, 48 h, and discharge for the determination of PaO2, PaCO2, and pH, when the patient was on room air. Post-bronchodilator spirometry (Sensor Medics, Vmax22) was performed according to ATS standards [19]. Dyspnea was assessed according to the modified Borg scale [20]. Complete blood cell counts were obtained at entry, and blood glucose, sodium, potassium were measured at H0 and H48.
Endpoints
The primary endpoint was to assess treatment efficacy by the change of arterial blood gases (ABG) from H0 to H24, H48, and before discharge. Secondary endpoints included the changes in FEV1 (forced expiratory volume in 1 s), dyspnea score, duration of hospitalization, and occurrence of adverse events. An adverse event was defined as any medical event reported by the attending physician or events resulting in discontinuation of study medication and/or treatment change or that prolonged hospitalization.
Definitions (modified from Burge S and Wedzicha JA) [21]
COPD exacerbation
An acute event characterized by a worsening of the patient’s respiratory symptoms that is beyond normal day-to-day variations and leads to a change in medication [2].
Moderate exacerbation
A respiratory failure with mild hypoxemia (PO2: 60–80 mmHg) but no carbon dioxide retention or acidosis; PCO2 <45 mmHg.
Severe exacerbation
A respiratory failure with moderate hypoxemia (PO2: 40–60 mmHg) but no carbon dioxide retention or acidosis; PCO2 <45 mmHg.
Very severe exacerbation
A respiratory failure with carbon dioxide retention or acidosis; PCO2 >45 mmHg and pH >7.35.
Statistical analysis
Statistical analysis was performed with SPSS for Windows version 17.0 (SPSS Inc., Chicago, USA). The sample size of 22 subjects per treatment arm was selected to provide 80% power to detect an increase of PaO2 of 5 mmHg in each group assuming a 2-sided test and type I error rate of 5%. All analyses were done on an intent-to-treat basis, including all data available on patients who received at least 1 dose of study drugs, irrespective of discontinuation of study drug or treatment change from the trial. For comparison of changes in continuous variables between and within the groups, ANOVA, paired t-test, and univariate analysis were used, respectively. Pearson’s chi-square test was used to compare categorical variables between the groups. If p-value is <0.05, was considered to be statistically significant.
Results
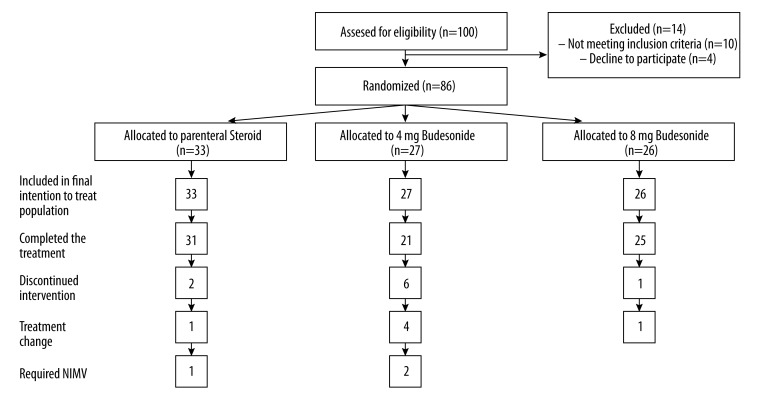
A total of 86 patients (71 male and 15 female) with an average age 67.5±9.3 were randomized into 3 groups. Thirty-three patients were randomly allocated to PS, 27 to 4-mg NB, and 26 to 8-mg NB (Figure 1). The characteristics of patients are summarized in Table 1. The groups were similar except for FEV1, which was higher in the 8-mg NB group (49.0±14.7 vs. 41.0±13.4 and 39.4±11.3 in the 4-mg NB group and the PS group, respectively).
Figure 1.
Disposition of patients by treatment groups.
Table 1.
Patient characteristics at baseline#.
| Group | PS group (n=33) | 4 mg NB (n=27) | 8 mg NB (n=26) |
|---|---|---|---|
| Age, yr. | 66.6 (9.6) | 66.7 (9.7) | 69.6 (8.5) |
| Sex (F/M) | 9/24 | 2/25 | 4/22 |
| Current smoker, n(%) | 25 (78%) | 21 (78%) | 22 (85%) |
| Mean of pack year | 51.3 (26.1) | 47.0 (23.6) | 56.1 (34.2) |
| Post-bronchodilator FEV1 | 39.4 (11.3) | 41.0 (13.4) | 49.0 (14.7) |
| pH | 7.38 (0.06) | 7.38 (0.05) | 7.39 (0.04) |
| PaCO2* | 44.6 (10.1) | 42.8 (8.4) | 40.9 (7.1) |
| PaO2* | 43.8 (11.1) | 44.5 (10.1) | 46.0 (9.4) |
| SaO2 (%) | 76.9 (11.8) | 77.9 (8.4) | 79.8 (9.6) |
Values are mean (SD) or number (%); PS – parenteral steroid; NB – nebulized budesonide; PaCO2 – arterial partial pressure of carbon dioxide; PaO2 – arterial partial pressure of oxygen;
mmHg; SaO2 – arterial oxygen saturation.
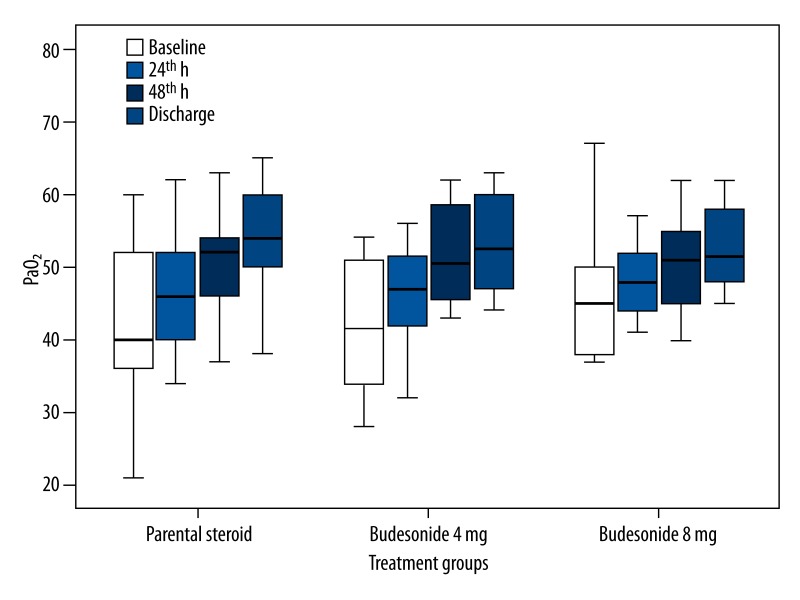
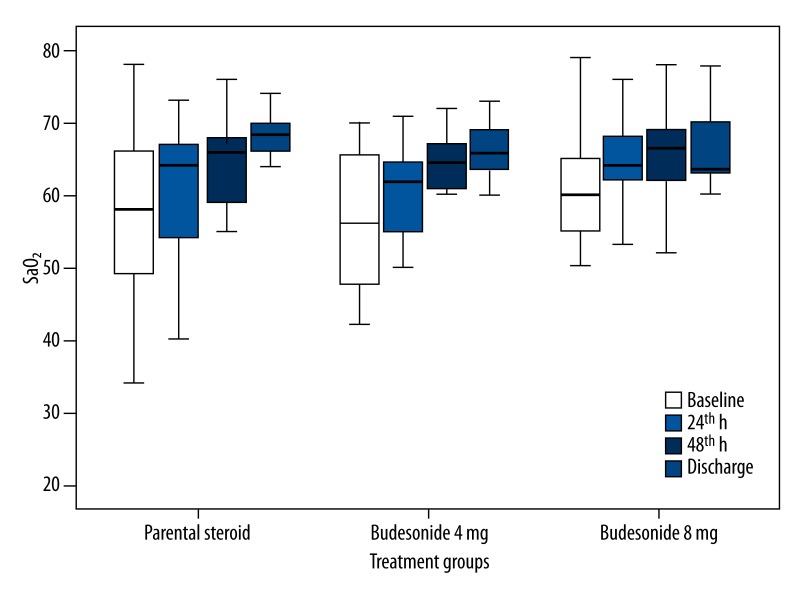
Mean values of PaO2, SaO2, pH, PaCO2 (H0 to H24, H48, and discharge day), FEV1, and FVC (H0 and discharge day) are shown in Table 2. We found that increases in PaO2, SaO2, FEV1, and FVC (forced vital capacity) values within the groups were statistically significant compared to baseline values (p<0.001 for all parameters). However, the changes in pH and PaCO2 values in each group were not statistically significant (p>0.05). In the comparison of the groups, the changes of PaO2 (Figure 2), SaO2 (Figure 3), and FVC, but not FEV1, between the groups were not statistically significant in all assessment periods (p=0.68, p=0.90, p=0.14 and p=0.04 for PaO2, SaO2, FVC, and FEV1, respectively). Because baseline FEV1 values were different between the groups, we performed univariate analysis and the difference was not statistically significant (p=0.13).
Table 2.
Mean values in the groups at different follow-up times.
| Parameter | Baseline | 24th h | 48th h | Discharge day |
|---|---|---|---|---|
| PaO2 | ||||
| PS | 43.8 | 47.7 | 49.8 | 54.3 |
| 4 mg NB | 44.5 | 49.1 | 52.7 | 56.0 |
| 8 mg NB | 46.0 | 50.0 | 52.6 | 53.6 |
| SaO2 | ||||
| PS | 76.9 | 82.0 | 83.5 | 87.0 |
| 4 mg NB | 77.9 | 82.3 | 84.5 | 86.8 |
| 8 mg NB | 79.8 | 83.5 | 86.4 | 86.6 |
| pH | ||||
| PS | 7.38 | 7.39 | 7.39 | 7.40 |
| 4 mg NB | 7.38 | 7.39 | 7.38 | 7.39 |
| 8 mg NB | 7.39 | 7.40 | 7.40 | 7.41 |
| PaCO2 | ||||
| PS | 44.6 | 44.2 | 45.1 | 43.0 |
| 4 mg NB | 42.8 | 43.2 | 46.2 | 42.0 |
| 8 mg NB | 40.9 | 40.3 | 40.0 | 40.4 |
| FVC | ||||
| PS | 64.9 | 69.1 | ||
| 4 mg NB | 66.6 | 76.5 | ||
| 8 mg NB | 74.8 | 79.4 | ||
| FEV1 | ||||
| PS | 39.4 | 44.5 | ||
| 4 mg NB | 41.0 | 50.7 | ||
| 8 mg NB | 49.8 | 54.8 | ||
PS – parenteral corticosteroid group; NB – nebulized budesonide group; PaO2 – arterial partial pressure of oxygen, SaO2 – arterial oxygen saturation, PaCO2 – arterial partial pressure of carbon dioxide; FVC – forced vital capacity; FEV1 – forced expiratory volume in 1 second.
Figure 2.
Partial pressure of arterial oxygen (PaO2): mean values, 95% CIs, minimum and maximum values (whiskers) for the 3 groups (parenteral steroid, budesonide 4 mg and budesonide 8 mg).
Figure 3.
Arterial oxygen saturation (SaO2): mean values, 95% CIs, minimum and maximum values (whiskers) for the three groups ( parenteral steroid, budesonide 4 mg and budesonide 8 mg).
The reduction in Borg scale ratings was statistically significant in each group (p<0.001). However, in the comparison of the groups, there was no statistically significant difference (p=0.34). Mean duration of hospitalization was 9.3±4.5 and the difference between the groups was not statistically significant (p=0.74).
During the study period, non-invasive mechanical ventilation (NIMV) or discontinuation of study medication and/or treatment change was required in 9 patients. Three patients required NIMV and 6 patients required discontinuation of study medication and/or treatment change, as adverse events (hyperglycemia and oral moniliasis) developed in 4 and 5 cases, respectively. However, the overall occurrence of adverse events over the study period was not statistically significant (p=0.57).
Discussion
Our study findings show that both dosages of nebulized steroid can be as effective as PS in the management of COPD exacerbation. Adverse events, including hyperglycemia and oral moniliasis, were determined in the PS group and both NB groups, respectively. In the comparison of NB groups, discontinuation study medication and/or treatment change was higher in the 4-mg NB group, but frequency of oral moniliasis was higher in the 8-mg NB group without reaching statistical significance. Discontinuation of study medication and/or treatment change was higher in the 4-mg NB group compared to the other groups. However, there was no statistically significant difference between the groups.
Systemic steroids (SS) have long been used in the treatment of COPD exacerbation [22]. Recent guidelines suggest using 30–40 mg prednisone or an equivalent SS in addition to the treatment for COPD exacerbation including bronchodilator, antibiotics, and oxygen [2,3]. Exacerbations in COPD patients result in rapid decline of respiratory function, frequent hospitalization, poor quality of life, several comorbidities, and mortality [4,23–25]. In patients with an exacerbation, frequent SS use may cause complications such as hyperglycemia, weight gain, osteoporosis, insomnia, anxiety, and depression, which increase treatment costs and jeopardize life. The development of hyperglycemia requiring treatment in the PS groups of our study also supports this conclusion. Thus, choosing NB may be appropriate in patients with COPD who are either at risk for the development of hyperglycemia or who have diabetes mellitus.
Nebulized steroids (NS) have been available for the past 2 decades. They have a high level of topical anti-inflammatory activity and a low level of systemic activity. They are safely used when necessary as a substitute for inhaled steroids in patients with bronchial asthma and stable COPD. Previous studies [26–28] have shown that nebulized steroids may be beneficial during both stable asthma and asthma exacerbation, which suggests that they may also be used for COPD exacerbation. Considering the findings of our study and previous ones [13–18], NB can be used as an alternative for patients with COPD exacerbations.
Previous studies have shown that use of nebulized steroid has similar or better effect on the parameters of spirometry or arterial blood gases with acceptable aadverse effects (Table 3). Morice et al. [13] studied the role of 4-mg NB in exacerbation of COPD by comparing it with 30-mg oral prednisolone. They found a similar increase of FEV1 in SS and NB groups during a 5-day course of treatment; the biochemical markers associated with corticosteroid adverse effects were higher in the PS group, but urinary steroid metabolites were higher in the NB group. Maltais et al. [14] showed that 8-mg NB has beneficial effects comparable to 60-mg oral prednisolone in the first 72 h of COPD exacerbation and NB was associated with fewer adverse effects, in contrast to SS, which was associated with higher incidence of hyperglycemia. The evaluated parameters in their study were ABG, FEV1, change in dyspnea score, duration of hospitalization, and adverse effects; they found no statistically significant differences between the treatment groups in any of the study parameters. Mirici et al. [15] compared the efficacy of 8-mg NB with parenteral 40-mg prednisolone in the treatment of COPD exacerbation. They evaluated peak expiratory flow rate and ABG changes between the groups. They found similar clinical efficacy and no adverse effects. Gunen et al. [16] studied the role of 6-mg NB in the treatment of COPD exacerbation by comparing it with 40-mg oral prednisolone. They showed that NB achieved significant improvement in spirometry parameters and PaO2. The relapse and re-hospitalization rates were reduced by half in the NB group and oral prednisolone was associated with hyperglycemia. Gaude et al. [18] compared the role of 4-mg NB with 100-mg parenteral hydrocortisone every 6 h in the treatment of COPD exacerbation. They found spirometry variables and saturation improvements were similar in both groups and there were no adverse effects.
Table 3.
Studies showing results of utilization of nebulized corticosteroids in COPD exacerbation.
| Authors | Number of patients | Treatment given | Primary outcome | Results | Side effects |
|---|---|---|---|---|---|
| Morice et al. [6] | 19 | Nebulized budesonide – 4 mg daily Oral prednisolone – 30 mg |
To compare the FEV1 increase and biochemical parameters between the groups | Similar clinical efficacy in both groups | Urinary steroid metabolites were higher in budesonide group |
| Maltais et al. [7] | 199 | Nebulized budesonide – 8 mg daily Oral prednisolone – 60 mg Placebo |
To compare the changes in FEV1 between the groups | FEV1 improvement was similar to oral prednisolone Nebulized budesonide was associated less side effects |
Higher incidence of hyperglycemia with oral prednisolone |
| Mirici et al. [8] | 40 | Nebulized budesonide – 8 mg Daily IV prednisolone – 40 mg |
To compare the FEV1, PEF and ABG changes between the groups | Similar clinical efficacy as parenteral steroids in PEF, ABG parameters | No adverse effects |
| Gunen et al. [9] | 159 | Nebulized budesonide – 6 mg Oral prednisolone – 40 mg Standard bronchodilator therapy |
To compare the FEV1 and ABG changes between the groups | Significant improvement in FEV1 and PaO2 in budesonide group | Hyperglycemia in oral prednisolone group |
| Wei et al. [10] | 60 | Nebulized budesonide Oral prednisolone Control group |
To compare dyspnea score, FEV1 and ABG changes between the groups | Dyspnea score, FEV1 and improvement in ABG were significantly better in budesonide group | Minimal side effects |
| Gaude and Nemagouda.[11] | 125 | Nebulized budesonide – 4 mg Daily IV Hydrocortisone – 400 mg |
To compare the Spirometry variables and saturation between the groups | Spirometry variables and saturation similar in both groups | Minimal side effects |
| Our study | 86 | Nebulized budesonide – 4 mg Nebulized budesonide – 8 mg IV prednisolone – 40 mg |
To compare the PaO2 and FEV1 changes between the groups | PaO2 and FEV1 improvement similar between the groups. 8 mg seems to be first choice | Treatment failure Oral moniliazis Hyperglycemia |
FEV1 – forced expiratory volume in 1 second; ABG – arterial blood gases; PEF – peak expiratory flow; PaO2 – arterial partial pressure of oxygen.
Our study evaluated ABG, FEV1, change in dyspnea score, duration of hospitalization, and adverse effects and we found no statistically significant differences between the study groups. Although it was not statistically significant, in the comparison of NB groups, discontinuation of study medication, and/or treatment change was higher in the 4-mg NB group and oral moniliasis was higher in the 8-mg NB group; however, only 1 case required treatment change due to oral moniliasis. In previous studies there is no consistency in NB dosage or data, suggesting an optimal NB dose. In the current study, treatment with 4-mg and 8-mg NB were compared and found to be about equally effective.
Our study not only confirmed the findings of previous studies showing the equivalency of NB use to SC use in the treatment of COPD exacerbations, but also contributes to determining the optimal dose of NB by comparing 2 doses of NB. Because we did not include any patients with very severe COPD exacerbation, we cannot claim that nebulized steroids can be used as an alternative to PS in the treatment of all COPD patients with exacerbation. The fact that this was not a double-blind a study with a placebo group makes it difficult to generalize the results of the study.
Conclusions
Nebulized budesonide may be used as an alternative to SC because of its equal effectiveness and lesser systemic adverse effects. The choice of optimal dosage need to be evaluated carefully because adverse effect and dropout rates were varied according to dosage (i.e., high dropout rate and lesser adverse effects with 4 mg or low dropout rate and higher adverse effects with 8 mg). However, further studies are required that include more severe cases and that evaluate long-term outcomes or relapses comparing the 3 arms.
Acknowledgments/disclosure
The authors received no financial support for the research and/or authorship of this article. The authors declare that they have no conflict of interest in the publication of this article.
Footnotes
Source of support: Departmental sources
References
- 1.Rosenberg SR, Kalhan R. An integrated approach to the medical treatment of chronic obstructive pulmonary disease. Med Clin N Am. 2012;96:811–26. doi: 10.1016/j.mcna.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. [Date last accessed; December, 27, 2013]. (Updated 2010). Available at: http://www.goldcopd.org.
- 3.National Clinical Guideline Centre (UK) Chronic Obstructive Pulmonary Disease: Management of Chronic Obstructive Pulmonary Disease in Adults in Primary and Secondary Care [Internet] 2010. [Google Scholar]
- 4.Donaldson GC, Seemungal TA, Bhownik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–52. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donaldson GC, Seemungal TA, Patel IS, et al. Airway and systemic inflammation and decline in lung function in patients with COPD. Chest. 2005;128:1995–2004. doi: 10.1378/chest.128.4.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwong FK, Sue MA, Klaustermeyer WB. Corticosteroid complications in respiratory disease. Ann Allergy. 1987;58:326. [PubMed] [Google Scholar]
- 7.McEvoy CE, Ensrud KE, Bender E, et al. Association between corticosteroid use and vertebral fractures in older men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:704. doi: 10.1164/ajrccm.157.3.9703080. [DOI] [PubMed] [Google Scholar]
- 8.Decramer M, Lacquet LM, Fagard R, Rogiers P. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am J Respir Crit Care Med. 1994;150:11. doi: 10.1164/ajrccm.150.1.8025735. [DOI] [PubMed] [Google Scholar]
- 9.Dujovne CA, Azarnoff DL. Clinical complications of corticosteroid therapy. A selected review. Med Clin North Am. 1973;57:1331. doi: 10.1016/s0025-7125(16)32233-7. [DOI] [PubMed] [Google Scholar]
- 10.Henzen C, Suter A, Lerch E, et al. Suppression and recovery of adrenal response after short-term, high-dose glucocorticoid treatment. Lancet. 2000;355:542–45. doi: 10.1016/S0140-6736(99)06290-X. [DOI] [PubMed] [Google Scholar]
- 11.Walsh LJ, Wong CA, Oborne J, et al. Adverse effects of oral corticosteroids in relation to dose in patients with lung disease. Thorax. 2001;56:279–84. doi: 10.1136/thorax.56.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walters JA, Gibson PG, Wood-Baker R, et al. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2009:CD001288. doi: 10.1002/14651858.CD001288.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Morice AH, Morris D, Lawson-Matthew P. Acomparison of nebulized budesonide with oral prednisolone in the treatment of exacerbations of obstructive pulmonary disease. Clin Pharmacol Ther. 1996;60:675–78. doi: 10.1016/S0009-9236(96)90216-7. [DOI] [PubMed] [Google Scholar]
- 14.Maltais F, Ostinelli J, Bourbeau J, et al. Comparison of nebulized budesonide and oral prednisolone with placebo in the treatment of acute exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med. 2002;165:698–703. doi: 10.1164/ajrccm.165.5.2109093. [DOI] [PubMed] [Google Scholar]
- 15.Mirici A, Meral M, Akgun M. Comparison of the efficacy of nebulized budesonide with parenteral corticosteroids in the treatment of acute exacerbations of chronic obstructive pulmonary disease. Clin Drug Invest. 2003;23:55–62. doi: 10.2165/00044011-200323010-00007. [DOI] [PubMed] [Google Scholar]
- 16.Gunen H, Hacievliyagil SS, Yetkin O, et al. The role of nebulized budesonide in the treatment of exacerbations of COPD. Eur Respir J. 2007;29:660–67. doi: 10.1183/09031936.00073506. [DOI] [PubMed] [Google Scholar]
- 17.Wei H, Xin Z. Nebulised budesonide in the treatment of acute exacerbations of chronic obstructive pulmonary disease. Practical Clin Med Mag. 2004. p. 3. [Last cited 2004]; Available from: http://www.scholar.ilib.cn/A-jslcyxzz200402003.html.
- 18.Gaude GS, Nemagouda S. Clinical efficacy of nebulized budesonide with parenteral/oral steroids in patients with acute exacerbation of COPD: A prospective study in tertiary care hospital. Lung India. 2009;26:S11–12. [Google Scholar]
- 19.American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 20.Borg G. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–81. [PubMed] [Google Scholar]
- 21.Burge S, Wedzicha JA. COPD exacerbations: definitions and classifications. Eur Respir J. 2003;21:46–53. doi: 10.1183/09031936.03.00078002. [DOI] [PubMed] [Google Scholar]
- 22.Niewoehner D, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1999;340:1941–47. doi: 10.1056/NEJM199906243402502. [DOI] [PubMed] [Google Scholar]
- 23.Seemungal TA, Donaldson GC, Paul EA, et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1418–22. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Aymerich J, Farrero E, Felez MA, Izquierdo J, et al. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax. 2003;58:100–5. doi: 10.1136/thorax.58.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–31. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levy ML, Stevenson C, Maslen T. Comparison of short courses of oral prednisolone and fluticasone propionate in the treatment of adults with acute exacerbations of asthma in primary care. Thorax. 1996;51:1087–92. doi: 10.1136/thx.51.11.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodrigo GJ. Comparison of inhaled fluticasone with intravenous hydrocortisone in the treatment of adult acute asthma. Am J Respir Crit Care Med. 2005;171:1231–36. doi: 10.1164/rccm.200410-1415OC. [DOI] [PubMed] [Google Scholar]
- 28.Belda J, Margarit G, Martinez C, et al. Anti-inflamatory effects of high-dose inhaled fluticasone versus oral prednisone in asthma exacerbations. Eur Respir J. 2007;30:1143–49. doi: 10.1183/09031936.00050306. [DOI] [PubMed] [Google Scholar]