Abstract
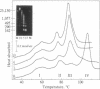
Differential scanning calorimetry revealed that chromatin melts in four distinct transitions in intact HeLa nuclei at 60 degrees C, 76 degrees C, 88 degrees C, and 105 degrees C. Calorimetry of whole cells was characterized by the same four transitions along with another at 65 degrees C, which was probably RNA. Isolated chromatin, however, melted in only two transitions at 72 degrees C and 85 degrees C. Very brief digestion of HeLa nuclei with either micrococcal nuclease or DNase I resulted in the conversion of a structure that melted at 105 degrees C to one that melted at 88 degrees C. Further digestion with micrococcal nuclease to the level of the mononucleosome did not result in any further substantial changes in either enthalpy or melting temperatures. In contrast, further DNase I digestion that resulted in cleavage within the nucleosome produced a pronounced shift in melting temperatures to broad transitions at 62 degrees C and 78 degrees C.
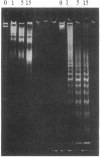
Full text
PDF
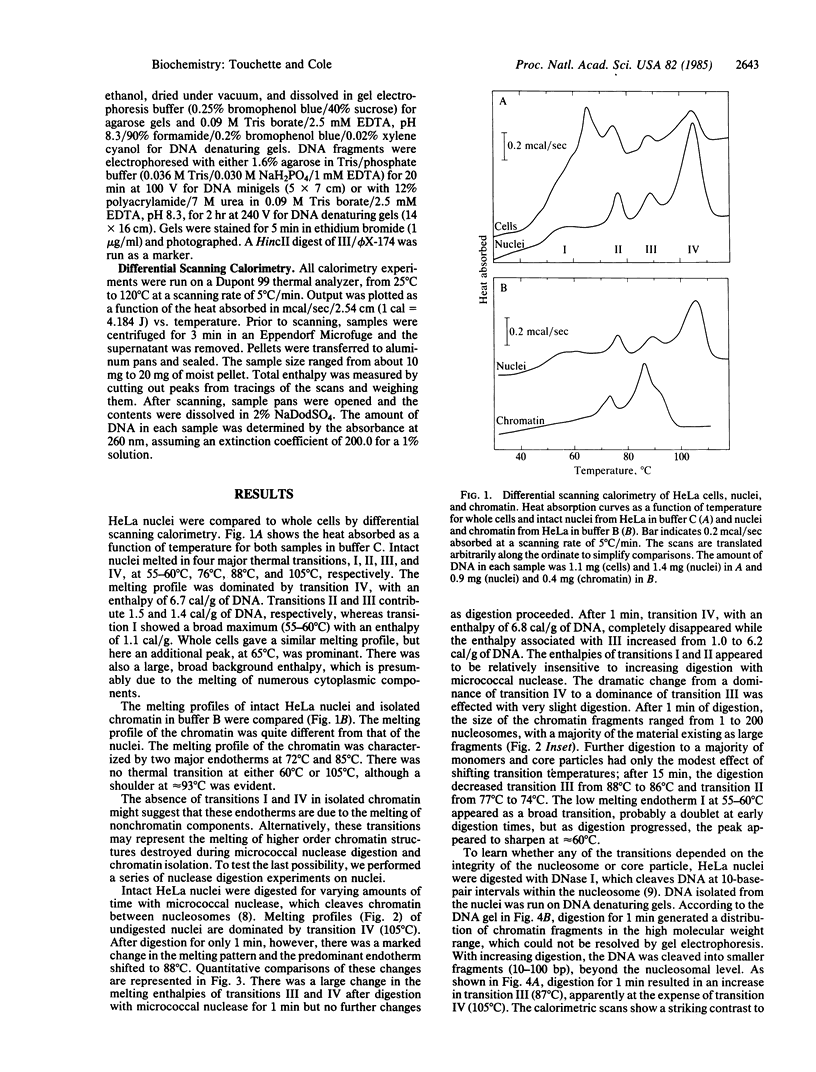
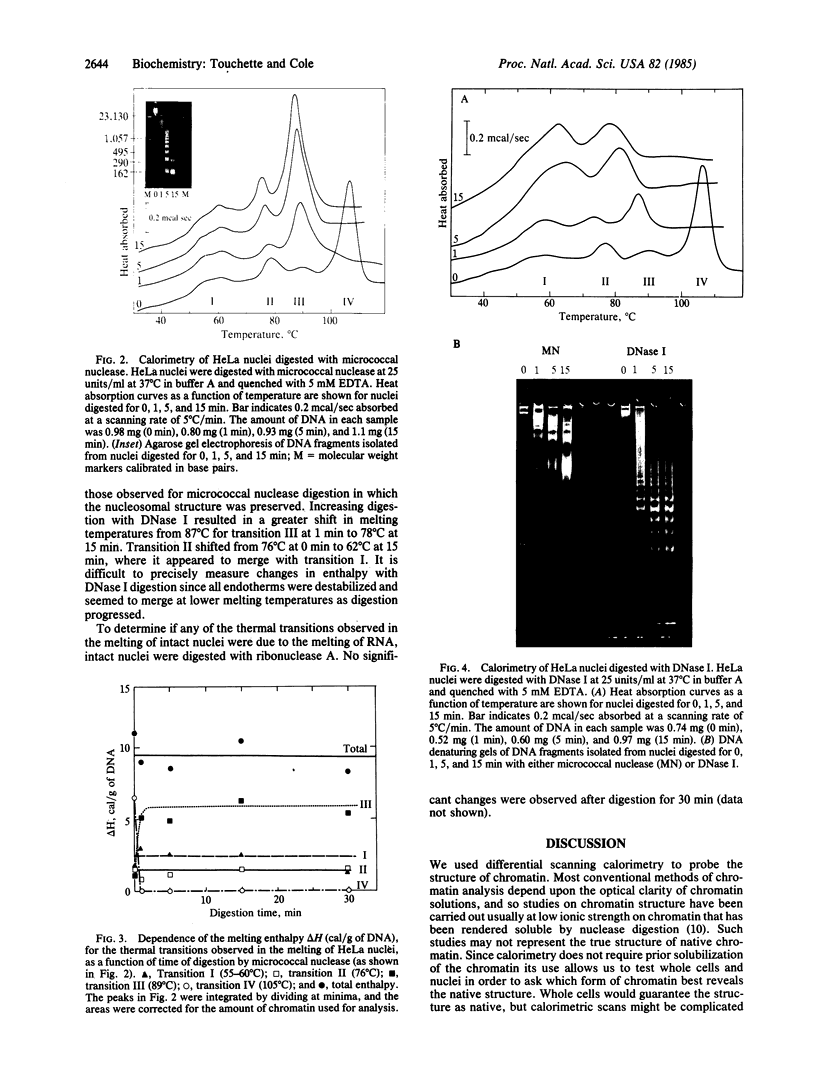


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adolphs K. W., Cheng S. M., Paulson J. R., Laemmli U. K. Isolation of a protein scaffold from mitotic HeLa cell chromosomes. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4937–4941. doi: 10.1073/pnas.74.11.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak A. L., Zeuthen J., Crick F. H. Higher-order structure of human mitotic chromosomes. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1595–1599. doi: 10.1073/pnas.74.4.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyajati C., Worcel A. Isolation, characterization, and structure of the folded interphase genome of Drosophila melanogaster. Cell. 1976 Nov;9(3):393–407. doi: 10.1016/0092-8674(76)90084-2. [DOI] [PubMed] [Google Scholar]
- Doty P., Boedtker H., Fresco J. R., Haselkorn R., Litt M. SECONDARY STRUCTURE IN RIBONUCLEIC ACIDS. Proc Natl Acad Sci U S A. 1959 Apr;45(4):482–499. doi: 10.1073/pnas.45.4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch J. T., Klug A. Solenoidal model for superstructure in chromatin. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1897–1901. doi: 10.1073/pnas.73.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P., Walker I. O. The partial dissociation of nucleohistone by salts. Circular dichroism and denaturation studies. Eur J Biochem. 1970 Nov;16(3):524–531. doi: 10.1111/j.1432-1033.1970.tb01112.x. [DOI] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem Biophys Res Commun. 1973 May 15;52(2):504–510. doi: 10.1016/0006-291x(73)90740-7. [DOI] [PubMed] [Google Scholar]
- Jackson J. B., Rill R. L. Circular dichroism, thermal denaturation, and deoxyribonuclease I digestion studies of nucleosomes highly enriched in high mobility group proteins HMG 1 and HMG 2. Biochemistry. 1981 Feb 17;20(4):1042–1046. doi: 10.1021/bi00507a060. [DOI] [PubMed] [Google Scholar]
- Krishinan K. S., Brandts J. F. Scanning calorimetry. Methods Enzymol. 1978;49:3–14. doi: 10.1016/s0076-6879(78)49003-2. [DOI] [PubMed] [Google Scholar]
- Lawrence J. J., Chan D. C., Piette L. H. Conformational state of DNA in chromatin subunits. Circular dichroism, melting, and ethidium bromide binding analysis. Nucleic Acids Res. 1976 Nov;3(11):2879–2893. doi: 10.1093/nar/3.11.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P. N. A thermal denaturation study of chromatin and nuclease-produced chromatin fragments. Can J Biochem. 1977 Jul;55(7):736–746. doi: 10.1139/o77-106. [DOI] [PubMed] [Google Scholar]
- Li H. J., Chang C., Weiskopf M. Helix-coil transition in nucleoprotein-chromatin structure. Biochemistry. 1973 Apr 24;12(9):1763–1772. doi: 10.1021/bi00733a016. [DOI] [PubMed] [Google Scholar]
- Nicolini C., Trefiletti V., Cavazza B., Cuniberti C., Patrone E., Carlo P., Brambilla G. Quaternary and quinternary structures of native chromatin DNA in liver nuclei: differential scanning calorimetry. Science. 1983 Jan 14;219(4581):176–178. doi: 10.1126/science.6849127. [DOI] [PubMed] [Google Scholar]
- Noll M. Internal structure of the chromatin subunit. Nucleic Acids Res. 1974 Nov;1(11):1573–1578. doi: 10.1093/nar/1.11.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll M., Kornberg R. D. Action of micrococcal nuclease on chromatin and the location of histone H1. J Mol Biol. 1977 Jan 25;109(3):393–404. doi: 10.1016/s0022-2836(77)80019-3. [DOI] [PubMed] [Google Scholar]
- Paulson J. R., Laemmli U. K. The structure of histone-depleted metaphase chromosomes. Cell. 1977 Nov;12(3):817–828. doi: 10.1016/0092-8674(77)90280-x. [DOI] [PubMed] [Google Scholar]
- Seligy V. L., Poon N. H. Alteration in nucleosome structure induced by thermal denaturation. Nucleic Acids Res. 1978 Jul;5(7):2233–2252. doi: 10.1093/nar/5.7.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subirana J. A. Studies on the thermal denaturation of nucleohistones. J Mol Biol. 1973 Mar 5;74(3):363–386. doi: 10.1016/0022-2836(73)90378-1. [DOI] [PubMed] [Google Scholar]
- Tsai Y. H., Ansevin A. T., Hnilica L. S. Association of tissue-specific histones with deoxyribonucleic acid. Thermal denaturation of native, partially dehistonized, and reconstituted chromatins. Biochemistry. 1975 Mar 25;14(6):1257–1265. doi: 10.1021/bi00677a026. [DOI] [PubMed] [Google Scholar]
- Weischet W. O., Tatchell K., Van Holde K. E., Klump H. Thermal denaturation of nucleosomal core particles. Nucleic Acids Res. 1978 Jan;5(1):139–160. doi: 10.1093/nar/5.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittig B., Wittig S. Nucleosome mono, di, tri-, and tetramers from chicken embryo chromatin. Nucleic Acids Res. 1977 Nov;4(11):3901–3917. doi: 10.1093/nar/4.11.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]