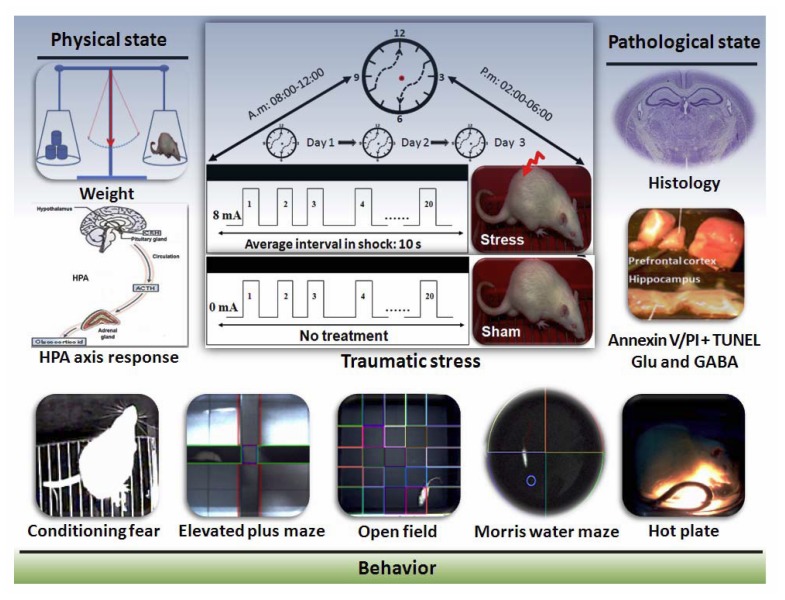
Abstract
Background
People who experience traumatic events have an increased risk of post-traumatic stress disorder (PTSD). However, PTSD-related pathological changes in the hippocampus and prefrontal cortex remain poorly understood.
Material/Methods
We investigated the effect of a PTSD-like animal model induced by severe stress. The experimental rats received 20 inescapable electric foot shocks in an enclosed box for a total of 6 times in 3 days. The physiological state (body weight and plasma corticosterone concentrations), emotion, cognitive behavior, brain morphology, apoptosis, and balance of gamma-aminobutyric acid (GABA) and glutamate in the hippocampus and prefrontal cortex were observed. Cell damages were examined with histological staining (HE, Nissl, and silver impregnation), while apoptosis was analyzed with flow cytometry using an Annexin V and propidium iodide (PI) binding and terminal deoxynucleotidyl transferase mediated-dUTP nick end labeling (TUNEL) method.
Results
In comparison with the sham litter-mates, the stressed rats showed decreased body weight, inhibition of hypothalamic-pituitary-adrenal (HPA) axis activation, increase in freezing response to trauma reminder, hypoactivity and anxiety-like behaviors in elevated plus maze and open field test, poor learning in Morris water maze, and shortened latency in hot-plate test. There were significant damages in the hippocampus but not in the prefrontal cortex. Imbalance between glutamate and GABA was more evident in the hippocampus than in the prefrontal cortex.
Conclusions
These results suggest that neuronal apoptosis in the hippocampus after severe traumatic stress is related to the imbalance between glutamate and GABA. Such modifications may resemble the profound changes observed in PTSD patients.
MeSH Keywords: Apoptosis, Central Nervous System, Stress Disorders, Post-Traumatic
Background
Post-traumatic stress disorder (PTSD) is an anxiety disorder that may develop following exposure to a strongly or extremely traumatic event. Although PTSD is regarded as a long-term, maladaptive stress response in which the central nervous system is not directly affected [1–5], neuroimaging studies have demonstrated that some patients with PTSD show abnormally elevated activity and alterations in gray matter volume and density in the cortex, hippocampus, and corpus callosum [6–8]. The hippocampus and prefrontal cortex, receiving information from the multisensory system and higher cortex, are intrinsically related to learning and memory and play key roles in emotion and behavior. Magnetic resonance imaging studies have shown that patients with PTSD have a smaller hippocampus (up to 8% reduction) compared with normal control, and the degree of reduction is related to the severity of PTSD [9,10]. Some researchers found that PTSD patients had an asymmetric change in activity and increased prefrontal cortex volume, while the others reported that the patients had thinner prefrontal cortex and atrophic white matter [11,12]. Hence, the hippocampus and prefrontal cortex are thought to be partially affected by the stress and to be involved in the pathogenesis of PTSD.
Apoptosis is a process of programmed cell death induced by physiological and pathological stimuli. Converging research showed that psychological stress elevated apoptotic markers in muscle, thymus, and nervous system, which is related to neurochemical changes that may be involved in the initiation of stress disorders. Among them, neuroendocrine substrates and the central neurotransmitters glutamate and gamma-aminobutyric acid (GABA) regulate the neuronal survival and apoptosis [13,14].
Growing evidence shows that stress induces glutamate release, which may be related to the behavioral changes in animal models [15,16]. In the central nervous system, glutamate regulates the opening of N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, inducing the internal flow of Ca2+, activating protein kinase C, and modulating the long-term potentiation [17,18]. Meanwhile, GABA, the principal inhibitory neurotransmitter, is distributed in the central nervous system, especially the limbic system, and shows pervasive inhibition of the neurons. Researchers found that PTSD patients showed significantly higher plasma GABA(A)-antagonistic neurosteroids dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) levels than the control, suggesting that a decreased GABA tone may be related to symptomatology and pathophysiology of PTSD [19,20]. Furthermore, it has been found that the lower level of plasma GABA may be a marker of recovery from PTSD, and the low density of benzodiazepine recognition site of the GABA(A) receptor in the prefrontal cortex may be the basis of PTSD pathogenesis [9,12]. Moreover, GABA derived from glutamate decarboxylase-mediated glutamate catalysis produces a positive feedback to its own release. Such GABA/glutamate imbalance further induces dysfunctional excitatory spasm.
The present study, therefore, hypothesized that glutamate-GABA imbalance may be involved in the mechanism of PTSD by inducing apoptosis in the hippocampus or prefrontal cortex. We use a modified rat model of PTSD induced by short time-current inescapable foot shock in an enclosed box to evaluate the effect of stress input on the changes in apoptosis and balance between glutamate and GABA in the hippocampus and prefrontal cortex, which underlies a variety of PTSD-related behavioral abnormalities.
Materials and Methods
Ethical approval
All animal procedures were approved by the Institutional Animal Care and Use Committee of the Third Military Medical University (Permit No. SCXK (yu) 2007017) and in accordance with the recommendations in the ARRIVE Guidelines for the care and use of experimental animals. We made great efforts to minimize the number of animals used and their suffering.
Animals
A total of 106 male Sprague-Dawley rats weighing 210–236 g (age 4–6 months) were purchased from the Third Military Medical University (Chongqing, China) and given at least 7 days of acclimation. The rats lived in acrylic boxes 44×33×20 cm (4 rats per box) at constant room temperature (23±2°C) and humidity (60%) with a 12h/12h light-dark cycle. Animals (sham: n=10, stress: n=8 per group) were used for weight and behavioral tests such as freezing, pain latency, elevated plus maze (EPM), open field, and water maze tests. Rats, except for those in water maze tests, were randomly assigned for morphological, apoptosis, and amino acid analysis (n=6 per group for morphological and TUNEL analysis, n=7 per group for flow cytometry, and n=6 per group for amino acid analysis). Sixteen rats were used for Dexamethasone suppression test (sham: n=8, stress: n=8 per group).
Procedure for inescapable electric-foot shock in an enclosed box
All experiments were performed between 8: 00 a.m. and 6: 00 p.m. After habituation, each rat was placed individually in an enclosed opaque box (17.0×8.0×40 cm) above a stainless steel grid floor. The stress box was connected to a scrambler controller that delivers shocks to the metallic floor. On Day 1, 2, and 3, rats in the stress group were given stimulus twice a day, with an interval of >4 h. For traumatic stress, rats were confined to an enclosed box (stress box) for 30 min and given 20 inescapable electrical shocks (intensity: 8 mA, duration: 3 s) with a random shock interval ranging from 7.5 to 15 s (Figure 1). The sham-stressed rats were kept in the stress box without delivering the shock.
Figure 1.
Experimental design. Male rats in the stress group began twice-daily inescapable electric shock in an enclosed box on 3 consecutive days. After 1 week of stress, the body weight changes, dexamethasone suppression and behavior in contextual fear conditioning, open field, elevated plus maze, hot plate, and Morris water maze were tested. Meanwhile, tissues from the hippocampus and prefrontal cortex were collected immediately for detection of apoptosis rate, concentration of Glu and GABA and morphological evaluation. HPA: hypothalamic-pituitary-adrenal; Glu: glutamate; GABA: gamma-aminobutyric acid.
Physiological test
The body weight
The body weights were recorded before stress, and then the rats were divided into 2 weight-matched groups. The body weight change was calculated according to the following equation: The percent of body change = (body weight after stress 1w – body weight before stress)/body weight before stress% [21].
Neuroendocrine measurements by Dexamethasone suppression test
To determine activation of the hypothalamic-pituitary-adrenal (HPA) axis after stress, plasma corticosterone levels were determined by the dexamethasone suppression test. One week after stress, dexamethasone (0.05 mg/kg; Sigma, St. Louis, MO) or vehicle (saline containing 5% ethanol) was administered s.c. 2 h prior to the second stress exposure (20 inescapable electric-foot shock for 30 min in a stress box) as described above. Each animal was placed in an acrylic rat holder (60×50×220 mm, China) and blood samples were collected from the tail vein at 0 and 30 min after the beginning of the second stress. The tests were performed between 11:00 a.m. and 4:00 p.m. Blood samples were collected in microcentrifuge tubes containing EDTA as the anticoagulant, and kept on ice. Then, blood was centrifuged at 3000 rpm for 15 min. Plasma was frozen (−80°C) until assayed. Corticosterone levels were determined by enzyme-linked immunosorbent assay (ELISA) (GC ELISA Kit, Rapidbio, USA) [22]. Each experiment was conducted in duplicate. The concentration of corticosterone was detected at 450 nm using a microplate reader (Tecan, Infinite 2000, Austria).
Behavioral test
Fear test for exposure to trauma reminder
Freezing is a reliable measure of fear in rats and is tested by a 3-min re-exposure to the stress box without any shock (as reminder) between 9:00 a.m. and 12:00 p.m. in the first week after stress. The test was videotaped with a small CCD camera (Kodak, Rochester, NY, USA). Freezing behavior was defined as the absence of all movements except for those related to respiration. Freezing percentage = freezing periods/total periods ×100 [23].
EPM test
The EPM test was used to detect the emotional responses to stressful external stimuli. The parameters were calculated as the percentage (%) of time spent in the open arms during 5 min in all arms and the percentage (%) of number of entries into the open arms to total number of entries into all arms (Smart video-tracking system, Panlab, Spain).
Open field test
According to the methods described previously, rats were placed at the corner of an open field. For the following 5 minutes, the number of line crossings, the time spent in the central areas, the rearing (upright posture) and grooming behaviors were video-recorded and calculated (Smart video-tracking system, Panlab, Spain). The total crossing, rearing, and grooming represent the activity level of the rats. The ratio between line crossings in the inner area and the total line crossings, and the relative time spent, are considered as the measure of anxiety [24].
Morris water maze test
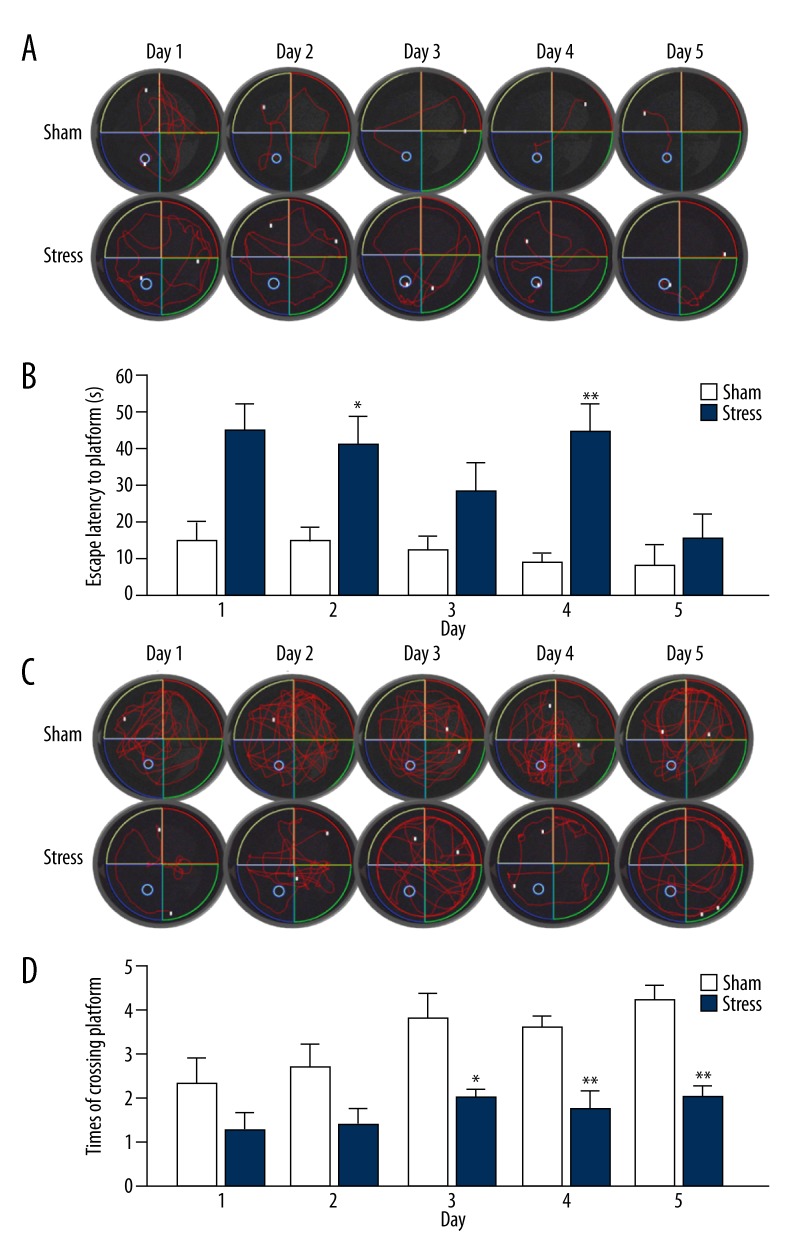
Learning and spatial memory performance was measured using the Morris water maze (MWM) according to the protocol reported [25]. The MWM apparatus consisted of a black-colored pool (160 cm in diameter and 55 cm in height). The pool was housed in a temperature-controlled room and divided into 4 quadrants. For each session of experiments, the pool was filled with 23 cm of 20–23°C water stained with dark ink. A cylindrical dark platform (21 cm in height and 10 cm in diameter) was placed in one of the quadrants (the target quadrant). The platform was submerged about 2 cm below the surface of the water during learning trials. Three extra-maze cues were set on the wall surrounding the pool. A digital video camera was attached to a computer-controlled system and positioned directly above the pool, enabling full recording of the swimming activities in different quadrants (Smart video-tracking system, Panlab, Spain). Animals were initially put in a quadrant without the platform. If animals could not find the escape platform within 60 s, the experimenters gently put animals onto the platform and allowed them to stay for 20 s. For learning performance, animals had four 60-s learning trials daily, each started randomly in 1 of the 4 quadrants, with a 20-s interval between trials. The escape latency to the platform in 60 s was calculated by averaging 4 trial values as indices representing the spacial learning. Each animal was tested for 5 consecutive days for learning performance. After completion of learning performance tests, the platform was removed, and animals were placed in a novel position; the number of target crossings in 120 s was calculated for the spatial probe test.
Hot-plate test
To determine analgesic efficacy in rodents, each rat was placed in a glass beaker on a hot plate (BME-410C, China). A hot-plate analgesia meter, maintained at 52.5°C, was used in this experiment. The latency to flinching or raising hind paws was recorded. To prevent tissue damage, the rats were removed from the hot plate if there was no response within 40 s [25].
Histology
The animals were deeply anesthetized with pentobarbital sodium (60 mg/kg) and perfused with saline and then with ice-cold 4% phosphate-buffered paraformaldehyde. Brains were refixed overnight by immersion in 4% paraformaldehyde. The samples were progressively dehydrated with ethanol and dimethylbenzene and then embedded with paraffin. Coronal sections were cut from the dorsal hippocampus (between 3.14 and 4.16 mm posterior to bregma) and prefrontal cortex (between 0.2 and 1.2 mm posterior to bregma). Sections were prepared for hematoxylin and eosin (HE), Nissl staining, and silver impregnation. The neuron degeneration in the hippocampus and prefrontal cortex was determined by silver impregnation. Briefly, sections were first incubated in the pretreating solution (20% AgNO3), and then in the impregnating solution (boric acid-buffered saline: 11 mL; borax: 9 mL; 1% AgNO3: 0.15 mL; 10% pyridine solution: 1 mL; pH: 8.4) at 37°C for 12 h. After washing (0.1% hydroquinol and 5% anhydrous sodium sulfite, 3 min), the sections were mixed-colored in 0.2% AgCl, developed color in 2% oxalic acid for 30 min, fixed color in sodium hyposulfite, dehydrated, and finally covered with Depex and a coverslip. A CCD camera (Olympus, Tokyo, Japan) was used to project microscopic images onto the screen of computer. The image analysis system was calibrated with a microscopic ruler (Olympus, Tokyo, Japan). For quantitative assessment of brain injury, cells were counted in 5 non-overlapping regions of interest (ROI) in the hippocampus (CA1, CA3, and DG) and prefrontal cortex, as described previously [26]. Neurons were classified as necrotic when they exhibited pyknosis, karyorrhexis, karyolysis, and cytoplasmic eosinophilia. The neuronal damage evaluated from silver impregnation was based on semiquantitative analysis. The scores for neuronal damage were 0 = normal, no damage; 1 = damage up to 10%; 2 = 10–50%; 3 = over 50% of the cells were irreversibly damaged [27].
Analysis of apoptosis
Annexin V/Propidium Iodide analysis of apoptosis by flow cytometry
The anesthetized rats were decapitated and dissected for the hippocampus and prefrontal cortex, which were then put individually into cold PBS, made into single-cell suspension, and adjusted to the concentration to 1×104 cells/mL. A 1-μL microliter suspension was centrifuged at 3000 rpm for 5 min at 4°C. After removing the supernatant, cells were resuspended in 1 mL of cold PBS with gently shaking, washed 2 more times with PBS and 3 more times with binding buffer, and incubated with 5 μL Annexin V and Propidium iodide on ice for 10 min. Finally, cells were resuspended in 300 μL of binding buffer and analyzed by flow cytometry (Becton Dickinson, USA) within 1 h. About 1×104 cells were analyzed for each group.
In situ cell death detection analysis of apoptosis by TUNEL
To detect DNA fragmentation in cell nuclei, TUNEL reaction was applied to the paraffin sections by using a Kit (Roche, Germany). After deparaffinization, the sections were treated with 0.1% Triton X-100 and 0.1% sodium citrate for 8 min on ice. After treatment with 0.3% H2O2 in methanol for 10 min, the sections were incubated with the TUNEL reaction mixture for 60 min at 37°C. Further incubation with horseradish peroxidase (POD, 1:500) was performed for 30 min at 37°C. The sections were stained with diaminobenzidine (DAB) solution for 2 min at room temperature and then counterstained with hematoxylin. The apoptotic cells evaluation was performed with a modified technique originally described by Sidhu [28]. The apoptotic cells and dying cells were identified from among those cells having intensely eosinophilic cytoplasm and small, dense nuclei. The number of apoptotic cells in the hippocampus (CA1, CA3, and DG) and prefrontal cortex was counted in 6 serial sections from each animal, using an image analyzer. About 200 cells were counted per slide. The TUNEL-positive cells rate = (number of TUNEL-positive cells/total cells) ×100% [29,30].
Glutamate and GABA analysis
The brain tissues in the hippocampus and prefrontal cortex were individually put into cold PBS and homogenized. The homogenate was deproteinized with 10% sulfosalicylic acid, centrifuged at 3000 rpm for 20 min at 4°C, and stored at −70°C until analysis. Just before the analysis, the supernatant was diluted with LiS buffer containing an internal standard of S-2-aminoethyl-L-cysteine and filtered through a Durapore-PVDF membrane. The amino acid concentration in the filtrate was determined by ion-exchange chromatography using a Beckman 6300 Amino Acid Analyzer (Beckman Instruments, Palo Alto, CA).
Statistical analyses
The data obtained from behavioral tests were recorded with small video CCD cameras and analyzed with the Smart animal behavior observation system. All the results were expressed as mean ±SEM and analyzed by paired t-test, independent samples t-test, and repeated measures 2-way ANOVA (group and day of water maze test) using the statistical software package SPSS 13.0 for Windows (SPSS Inc., Chicago, IL, USA). Statistical differences were tested 2-sided and considered significant when p < 0.05.
Results
Effect of stress on body weight change
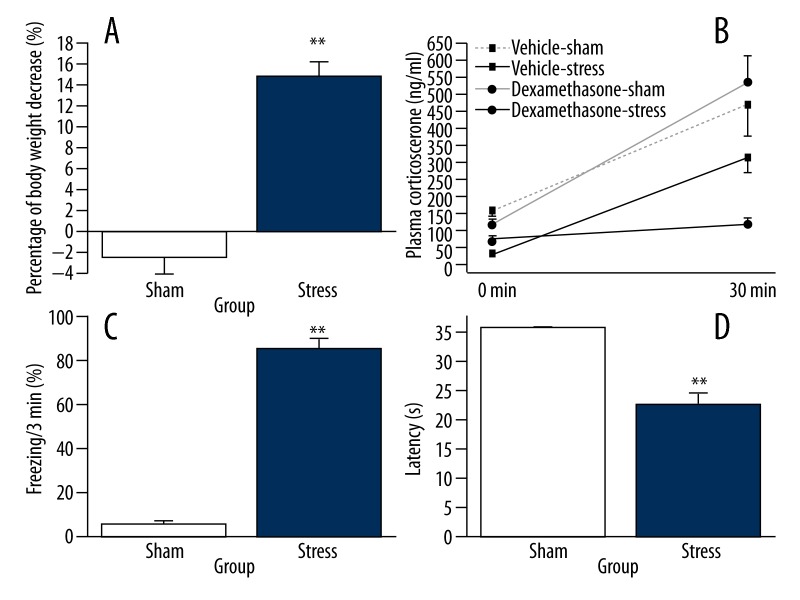
The body weight of animals in the stress group decreased significantly (paired t-test, t=9.355, df=7, p=0.001), whereas it was only slightly reduced in the sham group (paired t-test, t=1.018, df=9, p=0.335). Compared to that in the sham group, the change of body weight was more evident in the stress group [t (16)=8.638, p=0.001, Figure 2A].
Figure 2.
Effects of stress exposure on changes of body weight, dexamethasone suppression test, freezing behavior, and latency to flinch or raise hind paws in hot-plate test. Rats were randomly assigned to 2 groups and subjected to an enclosed box with or without electric foot shock stress a total of 6 times during 3 days. (A) Rats were weighed before and after the stress. The body weight changes were calculated as percentages of change over the body weight before stress (stress group: n=8, sham group: n=10). (B) Enhancement of HPA axis inhibition after stress. The dexamethasone suppression test indicated significantly enhanced inhibition of the plasma corticosterone increase in the stressed rats (n=8 for each group). Vehicle-sham, no stress exposure with vehicle (saline containing 5% ethanol) injection. (C) After 1 week of stress, rats of all groups were re-exposed to the stress context for 3 min without stimulus presentation (stress group: n=8, sham group: n=10). (D) Comparison of latency to flinch or raise hind paws in hot-plate test (stress group: n=8, sham group: n=10). Data were normalized to respective observation periods. The results are expressed as mean ±SEM. * p<0.05, ** p<0.01 compared with the sham group.
Effect of stress on the inhibition of the HPA axis
The changes in plasma corticosterone concentrations in response to foot-shock exposure are shown in Figure 2B. Baseline corticosterone levels differed between sham and stress groups with or without Dexamethasone (F (3, 28)=17.302, p=0). At the end of stress, Dexamethasone-induced inhibition of corticosterone secretion in the stressed rats was significantly potentiated compared with the non-stressed rats (1-way ANOVA, F (3, 28)=7.827, p=0.001).
Effect of trauma reminder on freezing behavior
To determine the behavioral changes induced by severe stress experience, rats were reexposed to an enclosed box without shock for 3 minutes. As assessed by unpaired t-test, the animals in the stress group continuously showed a freezing response with longer period of immobility compared to those in the sham group [t (16)=16.278, p=0.0001, Figure 2C].
Hot plate test
In the hot plate test, unpaired t-test revealed a significant effect of stress [t (16)=10.299, p=0.0001] on the latency to flinching or raising hind paws (Figure 2D), and the latency in the stress group was significantly shorter than that in the sham group.
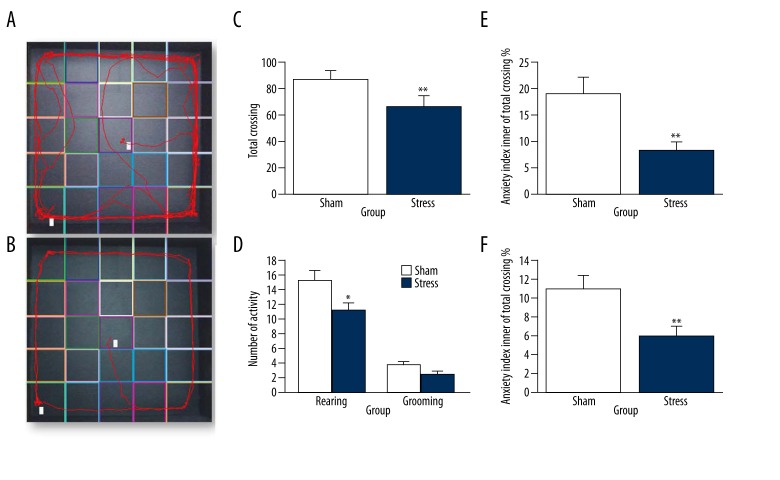
Effects of stress on behavior in open field test
The open field test was conducted after the exposure to foot shock stress in an enclosed box (Figure 3). The activity level was measured by total line crossings, and the t-test revealed a significant decrease (~30%) in activity in the stress group compared to the sham group [t (16)=2.504, p=0.024, Figure 3C]. The behaviors of rearing and grooming were slightly lower in the stress group than in the sham group [t (16)=2.866, p=0.012; t (16)=2.142, p=0.050, Figure 3D]. The ratio between the number of line crossings in the peripheral part of the open field and the total line crossings is considered as a measure of the anxiety, and the t-test revealed that exposure to stress increased (~9%) the anxiety level in the stress group compared to that in the sham group. The percentage [t (16)=4.516, p=0.001] and the ratio between the time spent in the inner part and total time were also decreased [~5%, t (16)=4.353, p=0.001, Figure 3E and 3F].
Figure 3.
The anxiety-like behavior and locomotor activity after exposure to electric foot shock stress in enclosed box in rats. (A) The path track of sham group in the open field. (B) The path track of stressed group in the open field. (C–F) Measured 1 week after the exposure to stress, rats in the stressed group showed a decrease in locomotor activity and an increase in anxiety index. Data were normalized to the respective observation periods. * p<0.05 compared with the sham group (stress group: n=8, sham group: n=10). The results are expressed as mean ±SEM. * p<0.05, ** p<0.01 compared with the sham group.
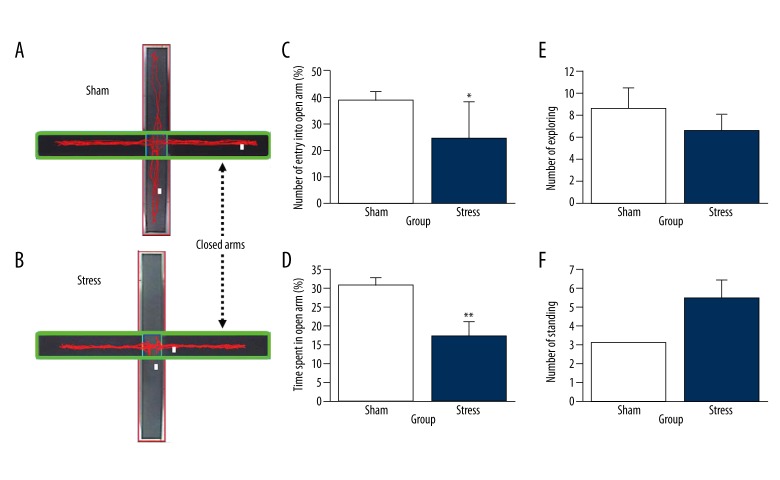
Emotional responses to external stress
As shown in Figure 4, independent t-test analyses revealed that the differences in time spent in open arms [t (16)=4.336, p=0.001, Figure 4C] and in percentage of number of entries into open arms (Figure 4D) were significant between groups [t (16)=3.003, p=0.008]. No differences were found in number of protected head dipping [t (16)=0.925, p=0.369, Figure 4E] and standing behavior [t (16)=2.102, p=0.052. Figure 4F].
Figure 4.
Anxiety-like effects of stress on the behavior in EPM test. (A): The path track of the sham group in the EPM test. (B): The path track of the stressed group in the EPM test. (C): Anxiety-like effects of stress on the percentage of entries into open arms (% number entry into open entries). (D): Anxiety-like effects of stress on the percentage of time spent (% open arm time). (E) Anxiety-like effects of stress on the number of rearings. (F) Anxiety-like effects of stress on the number of head dips. Data were collected from 2 groups (stress group: n=8, sham group: n=10). The results are expressed as mean ±SEM. * p<0.05 compared with the sham group.
Morris water maze test
There was a significant effect of stress and time on the escape latency in the water maze test [F (1, 16)=15.654, p=0.001, Figure 5B]. The time spent on finding the hidden platform in the sham group (escape latency) was shorter than that in the stress group. There were differences between groups on Day 2 and 4 of the trial (Day 2: t (16)=2.891, p=0.011; day 4: t (16)=4.280, p=0.003), but not on Day 1 (t (16)=0.197, p=0.846), 3 (t (16)=1.449, p=0.167), and 5 (t (16)=1.453, p=0.186).
Figure 5.
Comparison of escape latency and spatial probe test between animals in stress and sham groups during Morris water maze test. Rats were tested for 5 consecutive days. (A) The path track of escape latency to the platform for the rats of sham and stressed groups during 5 days. (B) Comparison of the escape latency to the platform in 60 s for the animals in stress and sham groups for 5 days for escape test. (C) The path track of target crossing for the rats of sham and stress groups during 5 days after removing the platform. (D) Comparison of the times of crossing platform in 120 s after removing the target in stress and sham groups for 5 days in spatial probe test. The results are expressed as mean ±SEM. * p<0.05, ** p<0.01 compared with the sham group (stress group: n=8, sham group: n=10).
When the platform was removed in the spatial probe test, there was also a significant effect of stress and time on the number of platform crossings. The rats exposed to traumatic stress had a lower average number of crossings overall compared to those in the sham group [F (1, 16)=19.457, p<0.0001, Figure 5D]. Differences were found between groups on Day 3 (t (16)=2.894, p=0.015), 4 (t (16)=3.912, p=0.001), and 5 (t (16)=5.747, p=0), but not on Day 1 (t (16)=1.757, p=0.098) and 2 (t (16)=1.917, p=0.073).
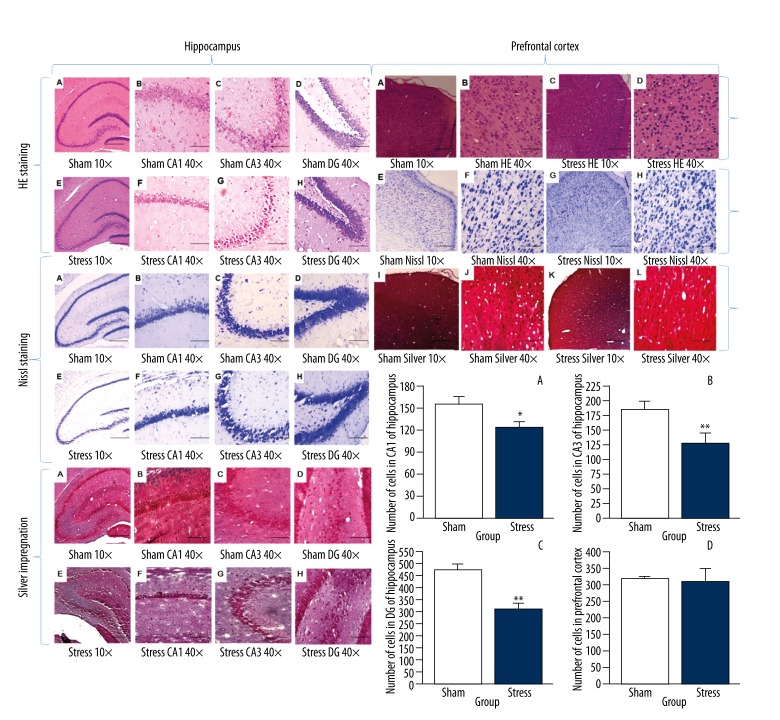
Stress-induced morphological changes in hippocampus and prefrontal cortex tissue
The morphological changes in CA1, CA3, and DG regions in the hippocampus 1 week after the stress exposure are shown in Figure 6. Normal morphological features of HE staining in CA1, CA3, and DG pyramidal neurons were observed in the sham group (Figure 6A–6D), where the pyramidal layer was thick with high cell density, and cells line up serriedly. The structure was intact, with a clear borderline. However, in the stress group, under HE and Nissl staining, 2 out of 9 hippocampal neurons were obviously shrunken and pyknotic and some of them also displayed irregular shape and a tangled appearance (Figure 6E–6H). Morphometric analysis revealed that the total count of intact cells in CA1, CA3, and DG regions significantly decreased in the stress group [CA1:120.00±9.01, t (10)=2.535, p=0.030; CA3: 116.33±6.26, t (16)=6.004, p=0.0001, DG: 294.00±4.83, t (10)=10.255, p=0.0001] compared to that in the sham group (CA1:154.67±10.29, CA3: 187.50±10.06, DG: 482.33±17.72; Figure 6B–D). The number of cells in the prefrontal cortex decreased sporadically, but the morphological features were similar in both groups [t (10)=0.613, p=0.554; Figure 6A–6H].
Figure 6.
Morphological evaluation of HE staining, Nissl staining, and silver impregnation in CA1, CA3, and DG subfields of hippocampus and prefrontal cortex after stress. In HE staining of hippocampus, normal histology and morphology (A–D) of hippocampus were seen in the sham group (magnification: 100× and 400×, respectively). No injured neurons were observed. In the stress group (E–H), injured neurons in CA1, CA3, and DG subfields in the hippocampus were noted after stress. In hippocampal morphological evaluation of Nissl staining in CA1, CA3, and DG subfields, normal histology and morphology (A–D) of the hippocampus in the sham group (magnification 100× and 400×, respectively). No injured neurons were observed. In the stress group (E–H), damaged neurons in CA1, CA3, and DG subfields of the hippocampus were noted after stress. In the silver-impregnated sections of the hippocampus, healthy cells of sham rats (A–D) are stained as deep brown, and the nerves are in a line (B, D). The stressed rats show dark argyrophilia of pyramidal cells, mainly in the CA3 subfield (G). In addition, some degenerating cells were seen in the DG and CA1 subregions (F, H). The array of fiber is tangled (E, H). While in morphological evaluation of the HE staining, Nissl staining, and silver impregnation in prefrontal cortex, normal histology and morphology (A, B, E, F, I, J) of neurons were seen in the sham group, with no injured neurons (magnification 100× and 400 ×, respectively). In the stress group (C–D, G–H, K–L), damaged neurons are seen spreading after stress. Scale bars =200 μm. The neuronal density in 1-mm sections of hippocampal CA1, CA3, and DG subfields and prefrontal cortex were also calculated after stress. A: CA1, B: CA3, C: DG, D: prefrontal cortex. (n=6 per group, * p<0.05 compared with the sham group). The results are expressed as mean ± SEM. * p<0.05, ** p<0.01 compared with the sham group.
Under silver impregnation, while different cells were observed in the hippocampus of the sham group (Figure 6A–6D), shrunken, argyrophilic, and degenerating cells were found in all stressed animals (Figure 6E–6H). The most heavily damaged area was the subfield of CA3, where one-third of the pyramidal cells were darkly stained or had already disappeared, so that only glial cells occupied most of the CA3 layers. The CA1 and DG areas had also undergone degeneration, but to a lesser extent compared to CA3 subfield. In these areas, accumulation of glial cells and loss of large nuclei indicated structural damage. Degeneration of the nerve terminals in the hippocampus was also seen in CA3 in the stress group, while an array of fibers was tangled (Figure 6G). No degeneration of fiber was observed in the prefrontal cortex (Figure 6I–6L).
Analysis of apoptosis
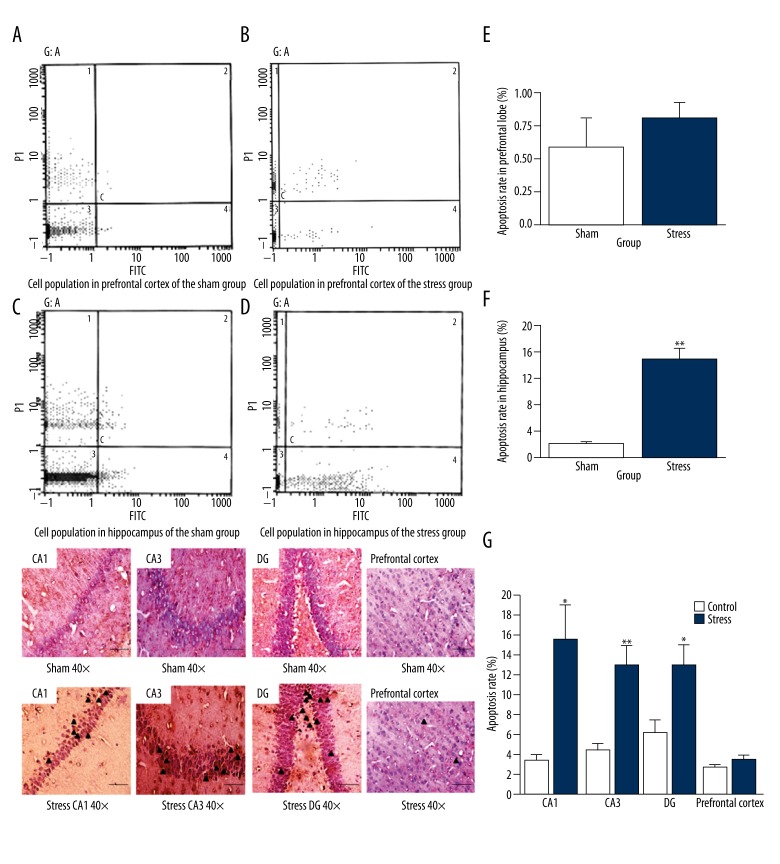
Analysis of apoptosis by flow cytometry
Apoptotic cells were detected by Annexin V/PI staining in all groups. The apoptosis rate in prefrontal cortex of the stress group was 0.831±0.107%, a little higher than that of the sham group (0.609±0.200%, Figure 7A and 7B), but without statistical significance [t (12)=0.982, p=0.351, Figure 7E]. In the stress group, there were many apoptotic cells in the hippocampus (Figure 7C). The hippocampal cells appeared to be sensitive to the stress (Figure 7F). These data indicate that stress made hippocampal cells prone to apoptosis, and the apoptosis rate in the hippocampus of the stress group was significantly higher than that of the sham group [15.251±0.917% vs. 2.381±0.284%, t (12)=13.410, p=0.0001, Figure 7C, 7D and 7F].
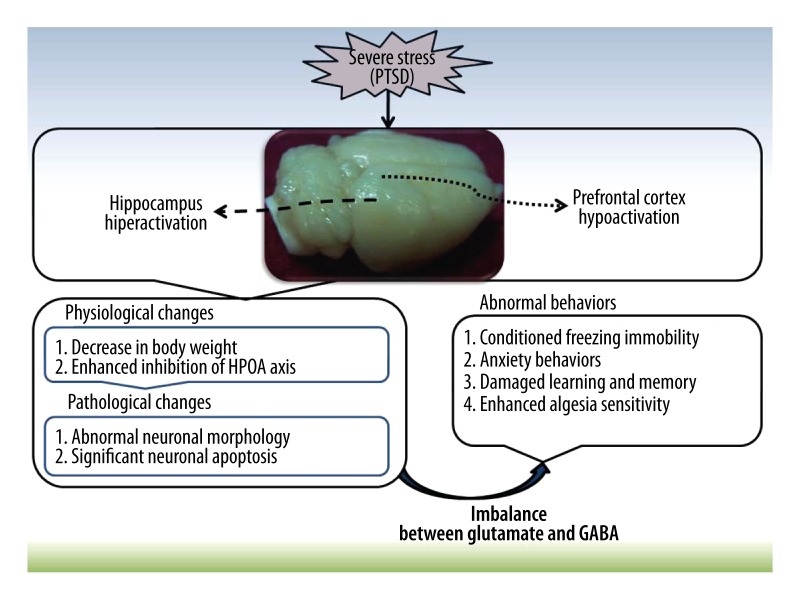
Figure 7.
Schematic view of the role of physiological, pathological, and neurochemical changes in abnormal behaviors after severe stress in the present study. The severe stress induced hippocampal hyperactivation and prefrontal cortex hypoactivation, which resulted in the abnormal behaviors in rats. The possible mechanisms mainly refer to neuronal injury and apoptosis caused by the imbalance of glutamate and GABA.
Analysis of apoptosis by TUNEL
The positive cells showed the typical morphological features of apoptosis, such as the chromatin condensation, cytoplasmic budding, and apoptotic bodies. In the sham group, few cells with fragmented DNA labeled by TUNEL staining were found in the prefrontal cortex and hippocampus. In comparison with the sham group, the rate of TUNEL-positive cells in the prefrontal cortex increased, but without significance (t(10)=1.090, p=0.323), but the stressed group increased in the rate of TUNEL-positive cells in the CA1, CA3, and DG of the hippocampus compared with the sham group (t(10)=5.152, p=0.19; t (10)=3.811, p=0.003; t(10)=3.048, p=0.019, respectively, Figure 7G).
Analysis of glutamate and GABA
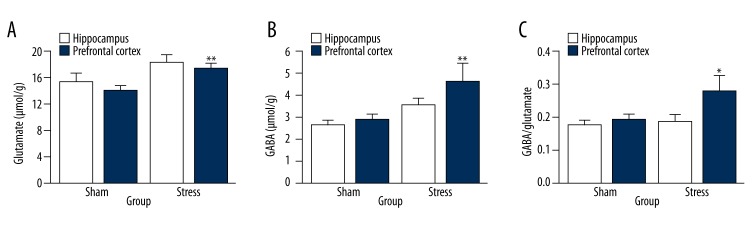
Compared with those in the sham group, the concentrations of glutamate in the stress group did not change in the prefrontal cortex [t (10)=2.129, p=0.055] but increased in the hippocampus [t (10)=3.875, p=0.003, Figure 8A]. There were no significant changes in GABA level in the prefrontal cortex between stress and sham groups [t (10)=1.824, p=0.098], whereas the concentration of GABA in the stress group showed a trend of increase in the hippocampus [t (10)=4.660, p=0.001, Figure 8B]. The GABA/glutamate ratio in the prefrontal cortex showed no difference between sham and stress groups [t (10)=0.147, p=0.886, Figure 8C], while its increase was found in the hippocampus in the stress group compared to that of the sham group [t (10)=2.444, p=0.035, Figure 8C].
Figure 8.
Analysis of the apoptotic rate in prefrontal cortex and hippocampus by flow cytometry and TUNEL after stress. Flow cytometry pictographs: viable cell population (Annexin V−/PI−) is in region 3; early apoptotic cell population (Annexin V+/PI−) is in region 4; late apoptotic and necrotic cell populations (Annexin V+/PI+) are in region 2; and necrotic cell population (Annexin V−/PI+) is in region 1. (A) Cell population in the prefrontal cortex of sham group. (B) Cell population in the prefrontal cortex of stress group. (C) Cell population in the hippocampus of sham group. (D) Cell population in the hippocampus of stress group. (E) Comparison of the apoptotic rate in prefrontal cortex between stress and sham groups (* p<0.05 compared with the sham group). (F) Comparison of the apoptotic rate in the hippocampus between stress and sham groups (n=7 per group, * p<0.05). (G) Photomicrograph of neuronal apoptotic cells (TUNEL-positive cells) in the hippocampus (CA1, CA3, DG) and prefrontal cortex between stress and sham groups (see triangles) (n=6 per group, * p<0.05, ** p<0.01). The results are expressed as mean ±SEM. (Bar size 200 μm, magnification ×400).
Discussion
PTSD is a severe, long-term clinical mental and body syndrome that may develop after violent personal assaults, natural or human-caused disasters, accidents, or military combat [1,2,5,31]. Although studies of PTSD pathogenesis are mainly focused on abnormal changes in the limbic system and related structures and on various neurotransmitters and possible downstream pathways, a detailed mechanism of PTSD remains controversial. The present study uses inescapable high-intensity electric foot shock in an enclosed box as traumatic stress to investigate the aspects of learning and memory disorder, anxiety, and algesia hypersensitivity in the rat model of PTSD. Our results indicate that disequilibrium between excitatory (glutamate) and inhibitory (GABA) transmitters may promote the apoptosis of hippocampal neurons and play an important role in PTSD pathogenesis.
Several validated models developed for PTSD using foot shock stress [32], tail shock stress [33], predator stress [34], single prolonged stress [30], and water immersion restraint stress [35] to induce behavioral changes similar to that in PTSD patients. However, according to the criteria of PTSD animal models, the predator stress model, although it exhibits reduced open arm activity and reduced risk assessment behavior in the EPM test, does not show bidirectional behaviors characteristic to PTSD patients, such as numbing and pain sensitivity [37]. Similarly, in the single prolonged stress model, which consisted of restraint for 2 h, followed by forced swimming for 20 min and subsequent ether anesthesia, although the stressed animals exhibited behaviors and endocrinal feathers similar to PTSD patients, it is inappropriate for constructive validity of the effect of ether and it is not easy to mimic the re-experience [38]. There are also some reports about the modified single prolonged stress (SPS) model, which accompanied by foot shock of different intensities [39,40]. The water immersion restraint stress model usually is accompanied by acute gastric mucosal injury; therefore, it is hard to discriminate the behavioral changes caused by the initial stress from those induced by the physical injury [41]. Fear conditioning in animals with inescapable electric shock has been used as a PTSD model [42]. However, it was found that only 5–20% of rats develop learned helplessness following 20 min of 0.8 mA foot shock [43]. The researchers also found that fear behaviors were proportionately affected by the intensity and number of shocks [44]. We modified the stress paradigm with short time-current and prolonged period. After exposure to electric foot shock in an enclosed stress box, rats re-exposed to a traumatic stress reminder displayed freezing behavior and decreased exploratory behavior in the inner part of the open field, indicating that high-level anxiety-like behaviors result from the stress of limited space and non-predicted, unavoidable, long-term electric stimulation. Pijlman et al. reported that rats given electric foot stimulation showed immobility and significantly decreased amount of activity in the open field [45]. Also, in the stress group, the percentage of time and entry in an open arm in EPM decreases significantly; meanwhile, the algesia response latency in the hot plate test is shortened, indicating that stress obviously affected the pain tolerance and increased the somatic pain sensitivity, as often seen in PTSD patients. In the MWM test, the latency to locating the underwater platform was significantly prolonged in stressed animals compared to sham animals. The number of target crossings for the spatial probe test in the stress group were also less than those in the sham group. These results indicate that intensified stress significantly impaired spatial memory. As to the effect of stress on the HPA axis, dexamethasone-induced inhibition of HPA axis activation in the stressed rats was significantly blunted compared with the non-stressed rats, which was found in PTSD patients. All these phenomena are similar to the symptoms of PTSD patients: avoidance of the stimuli related to the trauma, general low response or emotional numbing, and sensitive response to novel stressful stimuli [3]. Severe trauma induces the arousal and anxiety-related behavioral inhibition, which forces patients to suffer from re-experience. Such characteristic information processing and behavioral activity have been solidified, and subsequent stressors in a similar stress field stimulate the successive abnormal responses [46,47]. Therefore, the present stress model has successfully imitated the main symptoms of PTSD patients.
Brain imaging analysis of PTSD patients shows that the hippocampal volume is reduced, and the reduction is relevant to the severity of exposure to dangerous environments [48,49]. In addition, abnormal iconography data are observed in the prefrontal cortex and corpus callosum [5]. All this evidence sheds light on why the long-term distress in chronic PTSD patients is difficult to cure. Until now, we could not rule out the possibility that individual structural difference may promote the abnormality of prefrontal cortex and hippocampus relevant to the high sensitivity of PTSD patients. The results in this study indicate that the apoptosis rate of hippocampal neurons in PTSD rats is significantly higher than that in the sham group, implying that the hippocampus is involved in emotional regulation, learning, and memory. Clinical brain imaging analysis results suggest that hippocampal atrophy is very likely caused by severe stress. Meanwhile, patho-morphological investigation shows that the number of neurons in CA1, CA3, and dentate gyrus of hippocampus decreases as evidenced by the disappearance of Nissl bodies or pale dying. Mossy fiber stained by silver impregnation is irregularly aligned or even disrupted, which is in line with the significant apoptosis of hippocampal neurons. These results indicate that these pathological changes occur in the early phase of PTSD-like stress, and considered collectively, the apoptosis of hippocampal neurons is related to the PTSD symptom. On the other hand, as a key structure related to higher brain function, the prefrontal cortex shows sporadic neuronal apoptosis in the stress group, but was insignificant compared to the sham group. Few neurons were dyed a pale color; Nissl bodies were seldom lost, and the fibers were regularly arranged in silver staining, indicating a low-grade injury in the prefrontal cortex. Actually, the parameters of cerebral blood flow, metabolism, and functional abnormality cannot directly reflect the pathological changes in clinical studies. However, the thickness of the prefrontal cortex was shown to be decreased in 15 veterans with PTSD [12], while other researchers showed that prefrontal cortex volume in PTSD children is enlarged [6]. All these changes may be related to the cognition dysfunction. Some discrepancies may have resulted from the insensitive statistical parameter and excessive differences among groups. Also, a 7-day time interval after stress may not be long enough to see the apoptosis of prefrontal cortex neurons in this model. Further investigation is necessary to clarify these differences.
Neuronal apoptosis may be initiated by various internal and external agents. Apoptotic DNA fragmentation requires the activation of internal Ca2+ and Mg2+-dependent DNA endonuclease [50], and the excitatory and inhibitory amino acids may also involve in the apoptosis of neurons. A growing body of evidence has shown that glutamate and GABA directly act in drug abuse, alcohol addiction, and chronic stress-induced neuronal apoptosis via respective receptors [51–53]. Our results further show that the elevated glutamate level in the hippocampus implies the involvement of stress-initiated hippocampal glutamate outflow in PTSD pathogenesis. The elevated glutamate may then induce neuronal apoptosis; excessive glutamate accumulation also directly exerts lethal effects on sensitive neurons. In addition, considering the important role of glutamate in memory coding, it is reasonable to speculate that the pathogenesis of PTSD may be related to the excessive responses of glutamate receptors, and the formation of long-term memory via a large amount of Ca2+ inflow into the postsynaptic neuron, which could increase vulnerability to cell death and may be a potential physiological mechanism of intrusive re-experience in PTSD patients. Meanwhile, glutamate is the primary excitatory neurotransmitter in the brain, acting in concert with GABA to control the balance of excitation and inhibition in many brain circuits. The inhibition of GABA may cause hypervigilance [16,54]. The level of GABA was significantly elevated in the stress group. Based on previous data, the inhibitory role of GABA can be deduced as follows: 1) anti-anxiety effect, barbiturates have an anti-anxiety role via the GABA-A receptor; 2) in anti-convulsive effect, all kinds of convulsion are mostly related to GABA reduction in the brain, and GABA inhibitors may initiate convulsion; 3) in analgesic effect, the level of GABA in the brain is related to the pain relief of morphine; 4) in the morphine regulatory effect, intra-cerebral injection of GABA inhibits the release of corticotropin-releasing hormone; GABA increase may be due to a self-feedback protection, which is similar to the process of cell apoptosis. In this study, the levels of glutamate and GABA in the prefrontal cortex in the stress group are similar to that in the sham group, but are inconsistent with the brain imaging results in patients, which may be due to the different responses to PTSD-like stress. During the early stage after severe stress, the prefrontal cortex exhibits hypoactivation, whereas the temporal cortex (the hippocampus) shows a hyperactivation, which is of great significance for both the encoding and retrieval phases of memory processing or the performance in a work task.
Conclusions
In summary, the present study has successfully imitated the PTSD symptom using enclosed and electric foot shock stress in rats. The animal model clearly exhibits inhibition of the HPA axis, conditioned freezing immobility, enhanced algesia sensitivity, damaged learning and memory, and anxiety behaviors. The increased neuronal apoptosis in the hippocampus and abnormal hippocampal morphology may be related to imbalanced glutamate and GABA levels and be synergistically involved in the pathogenesis of PTSD (Figure 9), which provides new theoretical evidence for clinical treatment of PTSD based on glutamate-GABA equilibrium.
Figure 9.
The changes in concentration of Glu and GABA and ratio of GABA/Glu in hippocampus and prefrontal cortex after the stress. (A) Glu analysis. (B) GABA analysis (C) Ratio of GABA/Glu (n=6 per group, * p<0.05). The results are expressed as mean ±SEM. * p<0.05, ** p<0.01 compared with the sham group.
Acknowledgements
The authors would like to thank Professor Weihong Liao, Baoming Wu, Associate Professor Qinghua He, Gui Jin, Zhongxing Zhao, and Zhenwei Du for providing important advice.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Source of support: This work was partly supported by the grants from Natural Science Foundation of China (No.30800299, 81372105), the Special Funds for Major State Basic Research Projects (2012CB518102), and Medical Research Funding of PLA (ASW11J008)
References
- 1.Donnelly CL, Amaya-Jackson L, March JS. Psychopharmacology of pediatric posttraumatic stress disorder. J Child Adolesc Psychopharmacol. 1999;9:203–20. doi: 10.1089/cap.1999.9.203. [DOI] [PubMed] [Google Scholar]
- 2.Lanius RA, Williamson PC, Densmore M, et al. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am J Psychiatry. 2001;158:1920–22. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- 3.Davidson JR, Payne VM, Connor KM, et al. Trauma, resilience and saliostasis: effects of treatment in post-traumatic stress disorder. Int Clin Psychopharmaco. 2005;20:43–48. doi: 10.1097/00004850-200501000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Susan K, David AA. Epidemiology and presentation of post-traumatic disorders. Psychiatry. 2009;8:282–87. [Google Scholar]
- 5.Daniels JK, McFarlane AC, Bluhm RL, et al. Switching between executive and default mode networks in posttraumatic stress disorder: alterations in functional connectivity. J Psychiatry Neurosci. 2010;35:258–66. doi: 10.1503/jpn.090175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrion VG, Weems CF, Watson C. Converging evidence for abnormalities of the prefrontal cortex and evaluation of midsagittal structures in pediatric posttraumatic stress disorder: an MRI study. Psychiatry Res. 2009;172:226–34. doi: 10.1016/j.pscychresns.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw ME, Moores KA, Clark RC, et al. Functional connectivity reveals inefficient working memory systems in post-traumatic stress disorder. Psychiatry Res. 2009;172:235–41. doi: 10.1016/j.pscychresns.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Tsolaki M, Eleftheriou M, Karavida N. Alzheimer’s dementia and post-traumatic stress disorder differences and similarities in neuroimaging. Hell J Nucl Med. 2009;12:41–46. [PubMed] [Google Scholar]
- 9.Bremner JD, Innis RB, Southwick SM, et al. Decreased benzodiazepine receptor binding in prefrontal cortex in combat-related posttraumatic stress disorder. Am J Psychiatry. 2000;157:1120–26. doi: 10.1176/appi.ajp.157.7.1120. [DOI] [PubMed] [Google Scholar]
- 10.Lee T, Jarome T, Li SJ, et al. Chronic stress selectively reduces hippocampal volume in rats: a longitudinal magnetic resonance imaging study. Neuroreport. 2009;20:1554–58. doi: 10.1097/WNR.0b013e328332bb09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richert KA, Carrion VG, Karchemskiy A, Reiss AL. Regional differences of the prefrontal cortex in pediatric PTSD: an MRI study. Depress Anxiety. 2006;23:17–25. doi: 10.1002/da.20131. [DOI] [PubMed] [Google Scholar]
- 12.Geuze E, Westenberg HG, Heinecke A, et al. Thinner prefrontal cortex in veterans with posttraumatic stress disorder. Neuroimage. 2008;41:675–81. doi: 10.1016/j.neuroimage.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Engelbrecht AM, Smith C, Neethling I, et al. Daily brief restraint stress alters signaling pathways and induces atrophy and apoptosis in rat skeletal muscle. Stress. 2009;13:132–41. doi: 10.3109/10253890903089834. [DOI] [PubMed] [Google Scholar]
- 14.Zhang YM, Bhavnani BR. Glutamate-induced apoptosis in neuronal cells is mediated via caspase-dependent and independent mechanisms involving calpain and caspase-3 proteases as well as apoptosis inducing factor (AIF) and this process is inhibited by equine estrogens. BMC Neurosci. 2006;7:49. doi: 10.1186/1471-2202-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chambers RA, Bremner JD, Moghaddamv B. Glutamate and post-traumatic stress disorder: toward a psychobiology of dissociation. Semin Clin Neuropsychiatry. 1999;4:274–81. doi: 10.153/SCNP00400274. [DOI] [PubMed] [Google Scholar]
- 16.Nair J, Singh AS. The role of the glutamatergic system in posttraumatic stress disorder. CNS Spectr. 2008;13:585–91. doi: 10.1017/s1092852900016862. [DOI] [PubMed] [Google Scholar]
- 17.Refojo D, Schweizer M, Kuehne C, et al. Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1. Science. 2011;333:1903–7. doi: 10.1126/science.1202107. [DOI] [PubMed] [Google Scholar]
- 18.Gafford GM, Guo JD, Flandreau EI, et al. Cell-type specific deletion of GABA (A) α1 in corticotrophin-releasing factor-containing neurons enhances anxiety and disrupts fear extinction. Proc Natl Acad Sci. 2012;109:16330–35. doi: 10.1073/pnas.1119261109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spivak B, Maayan R, Kotler M, et al. Elevated circulatory level of GABA(A) – antagonistic neurosteroids in patients with combat-related post-traumatic stress disorder. Psychol Med. 2000;30:1227–31. doi: 10.1017/s0033291799002731. [DOI] [PubMed] [Google Scholar]
- 20.Pinna G, Rasmusson AM. Up-regulation of neurosteroid biosynthesis as a pharmacological strategy to improve behavioural deficits in a putative mouse model of post-traumatic stress disorder. J Neuroendocrinol. 2012;24:102–16. doi: 10.1111/j.1365-2826.2011.02234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris RB, Zhou J, Youngblood BD, et al. Effect of repeated stress on body weight and body composition of rats fed low- and high-fat diets. Am J Physiol. 1998;275:R1928–38. doi: 10.1152/ajpregu.1998.275.6.R1928. [DOI] [PubMed] [Google Scholar]
- 22.Dagyte G, Van der Zee EA, Postema F, et al. Chronic but not acute foot-shock stress leads to temporary suppression of cell proliferation in rat hippocampus. Neuroscience. 2009;162:904–13. doi: 10.1016/j.neuroscience.2009.05.053. [DOI] [PubMed] [Google Scholar]
- 23.Tamaki K, Yamada K, Nakamichi N, et al. Transient suppression of progenitor cell proliferation through NMDA receptors in hippocampal dentate gyrus of mice with traumatic stress experience. J Neurochem. 2008;105:1642–55. doi: 10.1111/j.1471-4159.2008.05253.x. [DOI] [PubMed] [Google Scholar]
- 24.Imanaka A, Morinobu S, Toki S, Yamawaki S. Importance of early environment in the development of post-traumatic stress disorder-like behaviors. Behav Brain Res. 2006;173:129–37. doi: 10.1016/j.bbr.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 25.Avital A, Ram E, Maayan R, et al. Effects of early-life stress on behavior and neurosteroid levels in the rat hypothalamus and entorhinal cortex. Brain Res Bull. 2006;68:419–24. doi: 10.1016/j.brainresbull.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 26.Gouirand AM, Matuszewich L. The effects of chronic unpredictable stress on male rats in the water maze. Physiol Behav. 2005;86:21–31. doi: 10.1016/j.physbeh.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 27.Kotti T, Halonen T, Sirviö J, et al. Comparison of NADPH diaphorase histochemistry, somatostatin immunohistochemistry, and silver impregnation in detecting structural and functional impairment in experimental status epilepticus. Neuroscience. 1997;80:105–17. doi: 10.1016/s0306-4522(97)00128-0. [DOI] [PubMed] [Google Scholar]
- 28.Dowling P, Husar W, Menonna J, et al. Cell death and birth in multiple sclerosis brain. J Neurol Sci. 1997;149:1–11. doi: 10.1016/s0022-510x(97)05213-1. [DOI] [PubMed] [Google Scholar]
- 29.Han JM, Chang BJ, Li TZ, et al. Protective effects of ascorbic acid against lead-induced apoptotic neurodegeneration in the developing rat hippocampus in vivo. Brain Res. 2007;1185:68–74. doi: 10.1016/j.brainres.2007.09.044. [DOI] [PubMed] [Google Scholar]
- 30.Ding J, Han F, Shi Y. Single-prolonged stress induces apoptosis in the amygdala in a rat model of post-traumatic stress disorder. J Psychiatr Res. 2010;44:48–55. doi: 10.1016/j.jpsychires.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Lewitus GM, Cohen H, Schwartz M. Reducing post-traumatic anxiety by immunization. Brain Behav Immun. 2008;22:1108–14. doi: 10.1016/j.bbi.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Li Z, Zhou Q, Li L, et al. Effects of unconditioned and conditioned aversive stimuli in an intense fear conditioning paradigm on synaptic plasticity in the hippocampal CA1 area in vivo. Hippocampus. 2005;15:815–24. doi: 10.1002/hipo.20104. [DOI] [PubMed] [Google Scholar]
- 33.Rau V, DeCola JP, Fanselow MS. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci Biobehav Rev. 2005;29:1207–23. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 35.Liberzon I, Krstov M, Young EA. Stress-restress: effects on ACTH and fast feedback. Psychoneuroendocrinology. 1997;22:443–53. doi: 10.1016/s0306-4530(97)00044-9. [DOI] [PubMed] [Google Scholar]
- 36.Tamaki K, Yamada K, Nakamichi N, et al. Transient suppression of progenitor cell proliferation through NMDA receptors in hippocampal dentate gyrus of mice with traumatic stress experience. J Neurochem. 2008;105:1642–55. doi: 10.1111/j.1471-4159.2008.05253.x. [DOI] [PubMed] [Google Scholar]
- 37.Adamec RE, Blundell J, Burton P. Phosphorylated cyclic AMP response element binding protein expression induced in the periaqueductal gray by predator stress: its relationship to the stress experience, behavior and limbic neural plasticity. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1243–67. doi: 10.1016/j.pnpbp.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 38.Rau V, Oh I, Laster M, et al. Isoflurane suppresses stress-enhanced fear learning in a rodent model of post-traumatic stress disorder. Anesthesiology. 2009;110:487–95. doi: 10.1097/ALN.0b013e3181974f3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, Zuo D, He B, et al. Conditioned fear stress combined with single-prolonged stress: a new PTSD mouse model. Neurosci Res. 2012;73:142–52. doi: 10.1016/j.neures.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Wang W, Liu Y, Zheng H, et al. A modified single-prolonged stress model for post-traumatic stress disorder. Neurosci Lett. 2008;441:237–41. doi: 10.1016/j.neulet.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 41.keuchi K, Furukawa O, Okada M, et al. Influences of stress on gastric alkaline secretion in rats. J Pharmacol Exp Ther. 1990;252:1228–33. [PubMed] [Google Scholar]
- 42.Maier SF. Exposure to the stressor environment prevents the temporal dissipation of behavioral depression/learned helplessness. Biol Psychiatry. 2001;49:763–73. doi: 10.1016/s0006-3223(00)01095-7. [DOI] [PubMed] [Google Scholar]
- 43.Shumake J, Barrett D, Gonzalez-Lima F. Behavioral characteristics of rats predisposed to learned helplessness: reduced reward sensitivity, increased novelty seeking, and persistent fear memories. Behav Brain Res. 2005;164:222–30. doi: 10.1016/j.bbr.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 44.Vinuta R, Joseph PD, Michael SF. Stress-induced enhancement of fear learning: An animal model of posttraumatic stress disorder. Neurosci Biobehav Rev. 2005;29:1207–23. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 45.Pijlman FT, Herremans AH, van de Kieft J, et al. Behavioural changes after different stress paradigms: prepulse inhibition increased after physical, but not emotional stress. Eur Neuropsychopharmacol. 2003;13:369–80. doi: 10.1016/s0924-977x(03)00040-3. [DOI] [PubMed] [Google Scholar]
- 46.Dudai Y. Neurobiology: Fear thou not. Nature. 2003;421:325–27. doi: 10.1038/421325a. [DOI] [PubMed] [Google Scholar]
- 47.Seedat S, Stein MB, Oosthuizen PP, et al. Linking posttraumatic stress disorder and psychosis: a look at epidemiology, phenomenology, and treatment. J Nerv Ment Dis. 2003;191:675–81. doi: 10.1097/01.nmd.0000092177.97317.26. [DOI] [PubMed] [Google Scholar]
- 48.De Bellis MD, Hall J, Boring AM, et al. A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol Psychiatry. 2001;50:305–9. doi: 10.1016/s0006-3223(01)01105-2. [DOI] [PubMed] [Google Scholar]
- 49.Lee AL, Ogle WO, Sapolsky RM. Stress and depression: possible links to neuron death in the hippocampus. Bipolar Disord. 2002;4:117–28. doi: 10.1034/j.1399-5618.2002.01144.x. [DOI] [PubMed] [Google Scholar]
- 50.Yu SP, Yeh C, Strasser U, et al. NMDA receptor-mediated K+ efflux and neuronal apoptosis. Science. 1999;284:336–39. doi: 10.1126/science.284.5412.336. [DOI] [PubMed] [Google Scholar]
- 51.Desfeux A, El Ghazi F, Jégou S, et al. Dual effect of glutamate on GABAergic interneuron survival during cerebral cortex development in mice neonates. Cereb Cortex. 2010;20:1092–108. doi: 10.1093/cercor/bhp181. [DOI] [PubMed] [Google Scholar]
- 52.Llorens-Martin M, Trejo JL. Mifepristone prevents stress-induced apoptosis in newborn neurons and increases AMPA receptor expression in the dentate gyrus of C57/BL6 mice. PLoS One. 2011;6:e28376. doi: 10.1371/journal.pone.0028376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lussier AL, Romay-Tallón R, Caruncho HJ, Kalynchuk LE. Altered GABAergic and glutamatergic activity within the rat hippocampus and amygdala in rats subjected to repeated corticosterone administration but not restraint stress. Neuroscience. 2013;231C:38–48. doi: 10.1016/j.neuroscience.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 54.Rossi S, De Capua A, Tavanti M, et al. Dysfunctions of cortical excitability in drug-naïve posttraumatic stress disorder patients. Biol Psychiatry. 2009;66:54–61. doi: 10.1016/j.biopsych.2009.03.008. [DOI] [PubMed] [Google Scholar]