Abstract
Host immune responses influence follicular lymphoma (FL) outcomes. To test our hypothesis that immune cells in blood reflect that response, we assessed by peripheral blood flow cytometry in 75 untreated FL patients the absolute counts of: lymphocytes (ALC), CD4+T (ACD4C), CD8+T (ACD8C) and natural killer (ANKC) cells. Low ANKC was the only parameter associated with inferior overall survival by univariate analysis (p= 0.02), and trended to significance in multivariable analysis with ACD4C (p= 0.08). Five (24%) patients with low initial ANKC died, while none with normal/high ANKC have died Conclusions: Evaluation of blood ANKC may be a useful indicator of outcome in previously untreated FL patients.
Keywords: Follicular lymphoma, immunology, lymphocyte subsets, NK cells, prognosis
Introduction
The clinical course of follicular lymphoma (FL) is heterogeneous. The Follicular Lymphoma International Prognostic Index (FLIPI) [1] and FLIPI2 [2] predict outcome using clinical, not biologic, parameters. The interaction between microenvironment and FL has been recognized from microarray [3] and immunohistochemical [4] studies. Immune system constituents comprise much of the FL microenvironment and influence outcome, depending on specific treatments [5]. Since most cases of FL are systemic, it is logical to also expect systemic immune alterations, and blood T cell populations are indeed altered in untreated FL [6]. Some reports suggest that higher absolute lymphocyte count (ALC) predicts improved overall survival (OS) [7], longer time to progression, improved response to rituximab [8], and response to radioimmunotherapy in FL patients [9] although this remains controversial [10]. There are as yet limited data regarding lymphocyte and natural killer (NK) cell subsets in the blood of FL patients, and no data on their prognostic value.
Since 1996, when we reported circulating lymphoma cells demonstrable by peripheral blood flow cytometry (PBFCM) in many lymphoma patients, we incorporated PBFCM into most lymphoma protocols. We retrospectively examined our PBFCM data of previously untreated, recently diagnosed FL patients, calculated T lymphocyte subsets and absolute natural killer cell counts (ANKC) and related these to overall survival. We find that low blood ANKC may be associated with inferior outcome in FL.
Materials and Methods
Of 276 consecutive patients with FL evaluated during an 11-year period at Fox Chase Cancer Center (FCCC), 127 had PBFCM performed by multicolor flow cytometry in our laboratory for T, B, NKT and NK cell markers. Of these, this report analyzes 75 who had no prior therapy, most of whom were recently diagnosed. Fifty-two patients previously treated or presenting with transformed disease were excluded from this analysis. All PBFCM studies were performed in our laboratory by multicolor flow cytometry. Lysing and incubation with antibodies were performed according to the manufacturer’s recommendations [BD Biosciences, San Diego, CA]. The most pertinent antibody combinations are (CD19, CD20, CD5, CD10), (CD19, CD20, kappa, lambda) and (CD3, CD4, CD8, CD56 and/or CD16). Blood absolute lymphocyte counts were obtained from the hematology laboratory and the counts of the subtypes were calculated from the percentages obtained by flow cytometry. Absolute NK cells (ANKC) refers to CD3−CD56+ and/or CD16+ per microliter of blood, absolute CD4 cells (ACD4C) to CD3+CD4+ and absolute CD8 cells (ACD8C) to CD3+CD8+ lymphocytes per microliter. All patients’ slides were reviewed by one of us (TAS). Clinical information was gathered from patient charts, approved by the FCCC Institutional Review Board.
OS was calculated from the time of initial PBFCM. The predictors were modeled as both continuous variables and dichotomized at our low normal absolute counts (ANKC: 150, ACD4C: 500, ACD8C:300 and ALC:1500/μl), as well as ALC 1000/μl as suggested in earlier reports. Univariate analysis of these subsets, along with age, stage, gender, performance status, and FLIPI, which incorporates age, stage, LDH, hemoglobin and number of nodal sites of disease, was by Kaplan-Meier estimates. Multivariable analysis was by Cox proportional hazard model. Stepwise selection was used to find the most parsimonious model. Analysis used SAS9-1 software (SAS Institute, Cary, NC).
Results
The majority of this cohort consisted of recently diagnosed patients. Median time from initial diagnosis to PBFCM was 1.1 months (range 0–81). Median follow-up from PBFCM was 26 months (range 1–138). Median age was 58 years (range 31–88), and male/female ratio 37/38.
Seventy-four patients had grade 1/2 FL (1 with grade 3A). Thirty-three had stage I/II disease, and 42 stage III/IV. The relatively high frequency of early stage patients likely reflects a bias to analyze PBFCM in this population where it might influence treatment decisions. FLIPI was low in 42 patients, intermediate or high in 31 and unknown in 2.
Asymptomatic patients were observed. Those symptomatic at presentation or becoming symptomatic during follow-up were treated. Mean time from PBFCM to treatment for the 40 (53%) patients who were treated was 7 months, with a median time to treatment of 1 month (range 0–120). Early stage patients were treated initially by radiation alone if localized (11 patients), or with rituximab (N=1). More advanced disease was treated initially by single-agent rituximab (N=15), or with non-anthracycline based chemotherapy plus rituximab (N=5) or without (N=4). Only 4 patients subsequently received anthracycline-containing regimens plus rituximab, either for transformation or patient/oncologist preference. Two patients lost to follow-up had survival identified through the tumor registry, but their management was unknown.
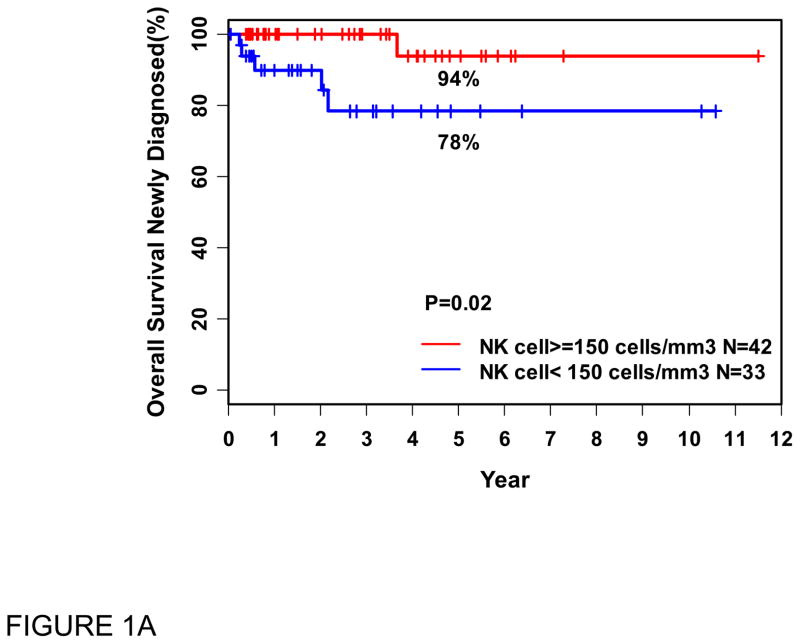
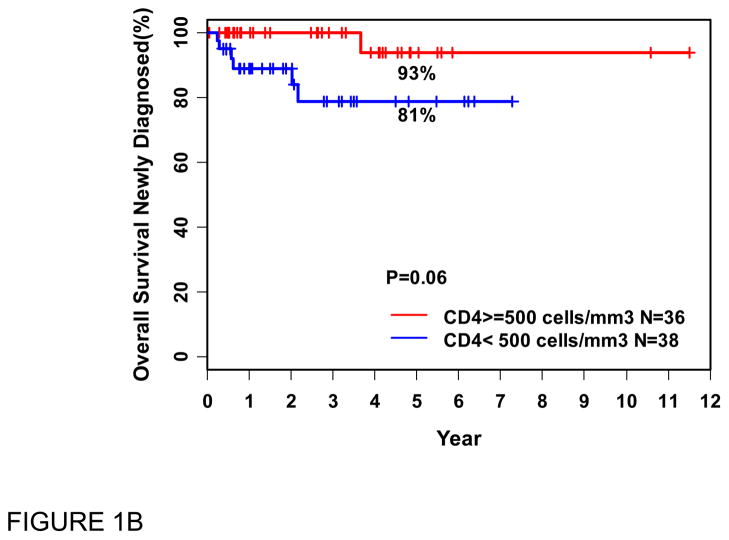
At the time of last available follow-up, 69 patients were alive and 6 had died. By univariate analysis, the only factors associated with inferior OS were low ANKC (<150/μl), p=0.02, with a trend toward significance for low ACD4C (<500/μL), p=0.06 (Figure 1). The FLIPI and ACD8C had no effect on survival. Low ALC, whether defined as <1500/μl, or <1000/μl reportedly associated with inferior survival by others [7], did not have prognostic significance for OS in our series. By multivariable analysis, only low ANKC trended towards an association with inferior OS, independent of ACD4C; hazard ratio was 6.73 (95% Cl: 0.76–59) with p = 0.08. This did not reach statistical significance, probably due to small sample size and/or relatively short follow-up. In the same analysis, ACD4C (<500) showed no significant effects in this cohort (p = 0.19).
Figure 1.
Overall survival (OS in years) from the time of blood flow cytometry (PBFCM), in patients with recently diagnosed FL, related to absolute natural killer cell (ANKC) (A) and absolute CD4 cell (ACD4C) counts (B).
N: Number of patients
We analyzed therapy and outcome for this cohort of 75 patients. Forty have required therapy, 21 of 33 (64%) with low baseline NK count and 19 of 42 (45%) with normal/high ANKC (p=0.11). Treatment regimens were similar in both cohorts, however, 5 (24%) of those with low initial ANKC died, while none with normal/high ANKC have died (p=0.02 for survival). Survival curves (Figure 1) illustrate the significant effect of ANKC on OS. Clinical characteristics of patients with low vs. normal/high ANKC are similar (Table 1).
Table 1.
Newly diagnosed FL patients with known absolute natural killer cell count (ANKC) by ANKC <150/μl vs ≥150/μl. Clinical parameters are similar in both cohorts; p value > 0.05 for all variables.
| VARIABLE | ANKC < 150/μl (n=33) | ANKC ≥ 150/μl (n=42) |
|---|---|---|
|
| ||
| Median Age (range) years | 57 (35–79) | 58 (31–88) |
|
| ||
| Stage | ||
| I | 8 (24%) | 12(29%) |
| II | 6 (18%) | 7 (17%) |
| III | 8 (24%) | 11 (26%) |
| IV | 11(33%) | 12(29%) |
|
| ||
| Gender | ||
| M | 15 (45%) | 23 (55%) |
| F | 18 (55%) | 19 (45%) |
|
| ||
| FLIPI* | ||
| 1 | 17 (51%) | 25 (60%) |
| 2 | 12 (36%) | 12 (29%) |
| 3 | 3 (9%) | 4 (10%) |
|
| ||
| Number of Extranodal Sites* | ||
| 0 | 19 (58%) | 27 (66%) |
| 1 | 11 (33%) | 12 (29%) |
| 2 | 2 (6%) | 2 (5%) |
|
| ||
| Number of Nodal Sites | ||
| Median (range) | 2 (0–6) | 2 (0–6) |
|
| ||
| Performance Status | ||
| 0 | 22 (67%) | 24 (57%) |
| 1 | 11 (33%) | 17 (40%) |
| 2 | 1 (3%) | 1 (2%) |
|
| ||
| LDH | ||
| Increased | 3 (9%) | 3 (7%) |
| Normal | 29 (88%) | 38 (90%) |
| Unknown | 1 (3%) | 1 (2%) |
|
| ||
| Hemoglobin | ||
| Decreased | 2 (6%) | 3 (7%) |
| Normal | 30 (91%) | 37 (88%) |
| Unknown | 1 (3%) | 2 (5%) |
Two patients did not have complete data.
Discussion
The indolent natural history of FL and documented spontaneous remissions suggest an interaction with host immunity [11]. In fact, gene expression signatures of non-neoplastic immune cells predict FL survival [3]. There is bi-directional cross-talk between FL cells and the immune system [12]. Immunohistochemical and flow cytometric studies of intra-tumoral immune cells revealed contradictory results in terms of FL behavior, perhaps depending on specific treatments [5]. More recently, attention is being directed to systemic immune mechanisms. Bone marrow immune cells are altered when lymphoma is present [13], and FL cells alter the microenvironment, for example converting CD4 helper cells into regulatory T cells [14]. Our data suggest that cellular immune constituents assayed in blood may predict OS in FL. Prior studies correlating blood immune cells with outcome in lymphoid malignancies have reported that blood NK cell count is associated with clinical outcome in patients with high-risk diffuse large B-cell lymphoma [15], low lymphocyte:monocyte ratio is predictive in diffuse large B-cell lymphoma [16], immunosuppressive monocytes correlate with lymphoma outcome [12] and both NK and T cell ratio to tumor cells are independent predictors of time to treatment in chronic lymphocytic leukemia [17]. Within the FL microenvironment, CD56+ NK cells are scarce; however, any correlation of NK cells in FL microenvironment with clinical outcome is as yet undefined. FL cells express high levels of HLA class I [18], so may not be recognized by NK cells as missing self-targets. Nonetheless, expression of NKG2D ligands on HLA-class I positive cells restores NK cell-mediated cytotoxicity despite HLA matching. Thus, the potential role of NK cells in controlling HLA-class I positive malignancies, including FL, requires reevaluation, particularly since low NK levels are associated with inferior outcome in our cohort.
Since the majority of our patients who eventually needed treatment received rituximab or rituximab-containing regimens, an association between ANKC and rituximab efficacy is possible. The relative importance of various mechanisms of rituximab action is still not fully elucidated. Low NK cell number and/or altered phenotype may decrease rituximab mediated antibody dependent cellular cytotoxicity (ADCC) [8]. NK cells are heterogeneous, for example CD56bright and CD56dim differ functionally [19]. Quantitation of those subtypes as well as their activation markers in FL may contribute to understanding NK-lymphoma interaction in the presence or absence of rituximab. Alternative treatment strategies for FL patients with low ANKC may be appropriate. For example, as lenalidomide enhances NK-mediated ADCC in rituximab-treated CD20+ lymphoma [20] and overcomes FL inhibition of the immune synapse [21], this would be a rational combination to explore.
Conclusion
In our retrospective analysis, baseline blood ANKC < 150/μl and, to a lesser extent CD4 cells < 500/μl, are associated with inferior OS in patients newly diagnosed with FL. T and NK cell numbers and function in blood may be a systemic reflection of FL microenvironment and/or be an independent measure of systemic host immune response. Regardless, detailed prospective evaluations of immune cell subsets and function in blood, as well as within the tumor microenvironment, with specific attention to effects of therapeutic regimens are warranted.
Acknowledgments
We thank Dr. Kerry Campbell for his critical review of the manuscript and Barbara Lankford and Lori Smith for secretarial assistance.
Funding:
Supported in part by NCI P30 CA006927. Additional funding was from the Janice Charach Epstein Lymphoma Research Fund.
Footnotes
Contribution of each author:
D.S., A.R. and H.B. collected the clinical material from patient charts and organized them. D.S. drafted the first version of the manuscript. M.R.S. and M.M.M. supervised the clinical management and assisted in the study design. T.L. and S.L. performed the statistical analysis. T.A-S. designed the studies, interpreted the pathology and the flow cytometry and drafted the final version of the manuscript. M.R.S. critically revised the manuscript and all authors approved final version submitted.
Conflicts of Interest: None to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Solal-Céligny P, Roy P, Colombat P, White J, Armitage JO, Arranz-Saez R, Au WY, Bellei M, Brice P, Caballero D, Coiffier B, Conde-Garcia E, Doyen C, Federico M, Fisher RI, Garcia-Conde JF, Guglielmi C, Hagenbeek A, Haïoun C, LeBlanc M, Lister AT, Lopez-Guillermo A, McLaughlin P, Milpied N, Morel P, Mounier N, Proctor SJ, Rohatiner A, Smith P, Soubeyran P, Tilly H, Vitolo U, Zinzani P-L, Zucca E, Montserrat E. Follicular lymphoma international prognostic index. Blood. 2004;104:1258–1265. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- 2.Federico M, Bellei M, Marcheselli L, Luminari S, Lopez-Guillermo A, Vitolo U, Pro B, Pileri S, Pulsoni A, Soubeyran P, Cortelazzo S, Martinelli G, Martelli M, Rigacci L, Arcaini L, Di Raimondo F, Merli F, Sabattini E, McLaughlin P, Solal-Céligny P. Follicular lymphoma international prognostic index 2: A new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. Journal of Clinical Oncology. 2009;27:4555–4562. doi: 10.1200/JCO.2008.21.3991. [DOI] [PubMed] [Google Scholar]
- 3.Dave SS, Wright G, Tan B, Rosenwald A, Gascoyne RD, Chan WC, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. New England Journal of Medicine. 2004;351:2159–2169. doi: 10.1056/NEJMoa041869. [DOI] [PubMed] [Google Scholar]
- 4.Farinha P, Masoudi H, Skinnider BF, Shumansky K, Spinelli JJ, Gill K, Klasa R, Voss N, Connors JM, Gascoyne RD. Analysis of multiple biomarkers shows that lymphoma-associated macrophage content is an independent predictor of survival in follicular lymphoma. Blood. 2005;106:2169–74. doi: 10.1182/blood-2005-04-1565. [DOI] [PubMed] [Google Scholar]
- 5.de Jong D, Koster A, Hagenbeek A, Raemaekers J, Veldhuizen D, Heisterkamp S, de Boer JP, van Glabbeke M. Impact of the tumor microenvironment on prognosis in follicular lymphoma is dependent on specific treatment protocols. Haematologica. 2009;94:70–77. doi: 10.3324/haematol.13574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christopoulos P, Pfeifer D, Bartholomé K, Follo M, Timmer J, Fisch P, Veelken H. Definition and characterization of the systemic T-cell dysregulation in untreated indolent B-cell lymphoma and very early CLL. Blood. 2011;117:3836–3846. doi: 10.1182/blood-2010-07-299321. [DOI] [PubMed] [Google Scholar]
- 7.Siddiqui M, Ristow K, Markovic SN, Witzig TE, Habermann TM, Colgan JP, Inwards DJ, White WL, Ansell SM, Micallef IN, Johnston PB, Call TG, Porrata LF. Absolute lymphocyte count predicts overall survival in follicular lymphomas. Br J Haematol. 2006;134:596–601. doi: 10.1111/j.1365-2141.2006.06232.x. [DOI] [PubMed] [Google Scholar]
- 8.Behl D, Ristow K, Markovic SN, Witzig TE, Habermann TM, Colgan JP, Inwards DJ, White WL, Ansell SM, Micallef IN, Johnston PB, Porrata LF. Absolute lymphocyte count predicts therapeutic efficacy of rituximab therapy in follicular lymphomas. British Journal of Haematology. 2007;137:409–415. doi: 10.1111/j.1365-2141.2007.06596.x. [DOI] [PubMed] [Google Scholar]
- 9.Porrata LF, Ristow K, Witzig TE, Tuinistra N, Habermann TM, Inwards DJ, Ansell SM, Micallef IN, Johnston PB, Markovic SN. Absolute lymphocyte count predicts therapeutic efficacy and survival at the time of radioimmunotherapy in patients with relapsed follicular lymphomas. Leukemia. 2007;21:2554–6. doi: 10.1038/sj.leu.2404819. [DOI] [PubMed] [Google Scholar]
- 10.Bishton MJ, Hicks RJ, Prince HM, Ritchie DS, Wolf M, Seymour JF. Claimed association of absolute lymphocyte count with therapeutic efficacy of radio-immunotherapy in patients with indolent lymphoma cannot be verified in an independent data set. Leukemia. 2008;22:2259–2260. doi: 10.1038/leu.2008.116. [DOI] [PubMed] [Google Scholar]
- 11.de Jong D. Molecular pathogenesis of follicular lymphoma: A cross talk of genetic and immunologic factors. J Clin Oncol. 2005;23:6358–63. doi: 10.1200/JCO.2005.26.856. [DOI] [PubMed] [Google Scholar]
- 12.Mittal S, Marshall NA, Duncan L, Culligan DJ, Barker RN, Vickers MA. Local and systemic induction of CD4+CD25+ regulatory T-cell population by non-Hodgkin lymphoma. Blood. 2008;111:5359–5370. doi: 10.1182/blood-2007-08-105395. [DOI] [PubMed] [Google Scholar]
- 13.Wahlin BE, Sander B, Christensson B, Ostenstad B, Holte H, Brown PD, Sundstrom C, Kimby E. Entourage: The immune microenvironment following follicular lymphoma. Blood Cancer J. 2012;2:e52. doi: 10.1038/bcj.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ai WZ, Hou J-Z, Zeiser R, Czerwinski D, Negrin RS, Levy R. Follicular lymphoma B cells induce the conversion of conventional CD4+ T cells to T-regulatory cells. International Journal of Cancer. 2009;124:239–244. doi: 10.1002/ijc.23881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plonquet A, Haioun C, Jais JP, Debard AL, Salles G, Bene MC, Feugier P, Rabian C, Casasnovas O, Labalette M, Kuhlein E, Farcet JP, Emile JF, Gisselbrecht C, Delfau-Larue MH. Peripheral blood natural killer cell count is associated with clinical outcome in patients with AA-IPI 2-3 diffuse large B-cell lymphoma. Ann Oncol. 2007;18:1209–15. doi: 10.1093/annonc/mdm110. [DOI] [PubMed] [Google Scholar]
- 16.Li Z-M, Huang J-J, Xia Y, Sun J, Huang Y, Wang Y, Zhu Y-J, Li Y-J, Zhao W, Wei W-X, Lin T-Y, Huang H-Q, Jiang W-Q. Blood lymphocyte-to-monocyte ratio identifies high-risk patients in diffuse large B-cell lymphoma treated with R-CHOP. PLoS ONE. 2012;7:e41658. doi: 10.1371/journal.pone.0041658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmer S, Hanson CA, Zent CS, Porrata LF, Laplant B, Geyer SM, Markovic SN, Call TG, Bowen DA, Jelinek DF, Kay NE, Shanafelt TD. Prognostic importance of T and NK-cells in a consecutive series of newly diagnosed patients with chronic lymphocytic leukaemia. Br J Haematol. 2008;141:607–14. doi: 10.1111/j.1365-2141.2008.07070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schultze JL, Cardoso AA, Freeman GJ, Seamon MJ, Daley J, Pinkus GS, Gribben JG, Nadler LM. Follicular lymphomas can be induced to present alloantigen efficiently: A conceptual model to improve their tumor immunogenicity. Proc Natl Acad Sci U S A. 1995;92:8200–4. doi: 10.1073/pnas.92.18.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu L, Adams M, Carter T, Chen R, Muller G, Stirling D, Schafer P, Bartlett JB. Lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res. 2008;14:4650–7. doi: 10.1158/1078-0432.CCR-07-4405. [DOI] [PubMed] [Google Scholar]
- 21.Ramsay AG, Clear AJ, Kelly G, Fatah R, Matthews J, MacDougall F, Lister TA, Lee AM, Calaminici M, Gribben JG. Follicular lymphoma cells induce T-cell immunologic synapse dysfunction that can be repaired with lenalidomide: Implications for the tumor microenvironment and immunotherapy. Blood. 2009;114:4713–4720. doi: 10.1182/blood-2009-04-217687. [DOI] [PMC free article] [PubMed] [Google Scholar]