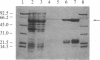
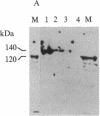
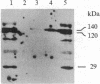
Abstract
Sequence studies of the adenovirus 2 genome have revealed the presence of a large open reading frame (ORF) from 22.9 to 14.2 map units that is believed to encode most of the adenovirus DNA polymerase (Ad Pol). An 838-base-pair fragment (19.6-17.3 map units) containing approximately 25% of this ORF has been cloned and expressed in a beta-galactosidase-chloramphenicol acetyltransferase (lacZ-CAT) expression vector under the control of the trp-lac hybrid promoter. This recombinant vector directed the synthesis of a 58-kDa lacZ-Ad Pol-CAT fusion protein that has CAT activity. This fusion protein was easily purified by affinity chromatography in which chloramphenicol, the substrate for CAT, was covalently bound to a matrix. Antisera were prepared against the purified 58-kDa lacZ-Ad Pol-CAT fusion protein and were found to react specifically with the 140-kDa Ad Pol by ELISA and immunoblot analysis. In addition, these antisera recognized 120- and 29-kDa polypeptides in immunoblot analysis of partially purified terminal protein precursor (pTP)-Ad Pol complex. The exact nature of the 120- and 29-kDa polypeptides is not known, but they may be breakdown products of Ad Pol. Although the lacZ-Ad Pol-CAT fusion protein is not active in any of the Ad Pol enzymatic reactions, antibody against the prokaryotic fusion protein should be useful for screening bacteria harboring plasmids that have been constructed to express the entire Ad Pol ORF.
Full text
PDF

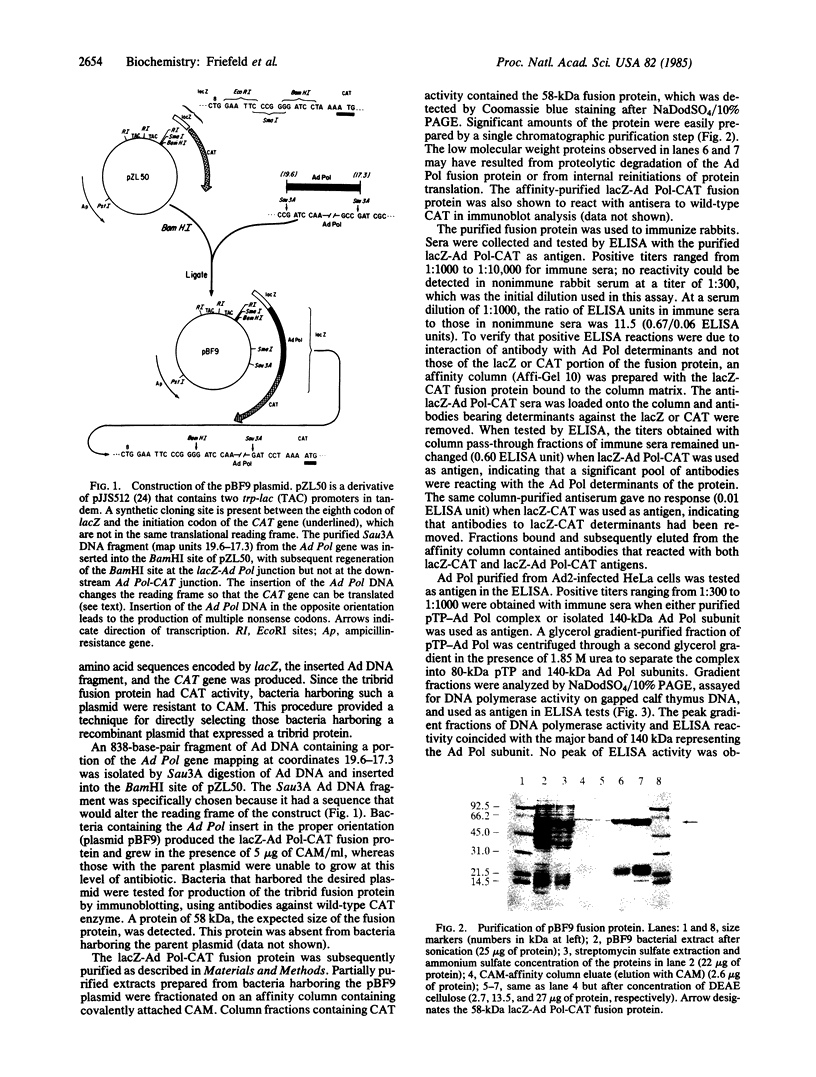

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleström P., Akusjärvi G., Pettersson M., Pettersson U. DNA sequence analysis of the region encoding the terminal protein and the hypothetical N-gene product of adenovirus type 2. J Biol Chem. 1982 Nov 25;257(22):13492–13498. [PubMed] [Google Scholar]
- Aleström P., Stenlund A., Li P., Bellett A., Pettersson U. Sequence homology between avian and human adenoviruses. J Virol. 1982 Apr;42(1):306–310. doi: 10.1128/jvi.42.1.306-310.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariga H., Klein H., Levine A. J., Horwitz M. S. A cleavage product of the adenovirus DNA binding protein is active in DNA replication in vitro. Virology. 1980 Feb;101(1):307–310. doi: 10.1016/0042-6822(80)90510-3. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakel C. L., Blumenthal A. B. Multiple forms of Drosophila embryo DNA polymerase: evidence for proteolytic conversion. Biochemistry. 1977 Jul 12;16(14):3137–3143. doi: 10.1021/bi00633a016. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Gelinas R. E., Broker T. R., Roberts R. J. An amazing sequence arrangement at the 5' ends of adenovirus 2 messenger RNA. Cell. 1977 Sep;12(1):1–8. doi: 10.1016/0092-8674(77)90180-5. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Enomoto T., Lichy J. H., Ikeda J. E., Hurwitz J. Adenovirus DNA replication in vitro: purification of the terminal protein in a functional form. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6779–6783. doi: 10.1073/pnas.78.11.6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T. J., Shaw W. V. Chloramphenicol acetyltransferases specified by fi minus R factors. Antimicrob Agents Chemother. 1973 Jan;3(1):99–104. doi: 10.1128/aac.3.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friefeld B. R., Lichy J. H., Hurwitz J., Horwitz M. S. Evidence for an altered adenovirus DNA polymerase in cells infected with the mutant H5ts149. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1589–1593. doi: 10.1073/pnas.80.6.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galos R. S., Williams J., Binger M. H., Flint S. J. Location of additional early gene sequences in the adenoviral chromosome. Cell. 1979 Aug;17(4):945–956. doi: 10.1016/0092-8674(79)90334-9. [DOI] [PubMed] [Google Scholar]
- Gingeras T. R., Sciaky D., Gelinas R. E., Bing-Dong J., Yen C. E., Kelly M. M., Bullock P. A., Parsons B. L., O'Neill K. E., Roberts R. J. Nucleotide sequences from the adenovirus-2 genome. J Biol Chem. 1982 Nov 25;257(22):13475–13491. [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. S. Temperature-sensitive replication of H5ts125 adenovirus DNA in vitro. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4291–4295. doi: 10.1073/pnas.75.9.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Mathews M. B. Control of adenovirus early gene expression: a class of immediate early products. Cell. 1980 Aug;21(1):303–313. doi: 10.1016/0092-8674(80)90138-5. [DOI] [PubMed] [Google Scholar]
- Lichy J. H., Field J., Horwitz M. S., Hurwitz J. Separation of the adenovirus terminal protein precursor from its associated DNA polymerase: role of both proteins in the initiation of adenovirus DNA replication. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5225–5229. doi: 10.1073/pnas.79.17.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichy J. H., Horwitz M. S., Hurwitz J. Formation of a covalent complex between the 80,000-dalton adenovirus terminal protein and 5'-dCMP in vitro. Proc Natl Acad Sci U S A. 1981 May;78(5):2678–2682. doi: 10.1073/pnas.78.5.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichy J. H., Nagata K., Friefeld B. R., Enomoto T., Field J., Guggenheimer R. A., Ikeda J. E., Horwitz M. S., Hurwitz J. Isolation of proteins involved in the replication of adenoviral DNA in vitro. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):731–740. doi: 10.1101/sqb.1983.047.01.084. [DOI] [PubMed] [Google Scholar]
- Longiaru M., Ikeda J. E., Jarkovsky Z., Horwitz S. B., Horwitz M. S. The effect of aphidicolin on adenovirus DNA synthesis. Nucleic Acids Res. 1979 Jul 25;6(10):3369–3386. doi: 10.1093/nar/6.10.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrove J. M., Rosenfeld P., Williams J., Kelly T. J., Jr In vitro complementation as an assay for purification of adenovirus DNA replication proteins. Proc Natl Acad Sci U S A. 1983 Feb;80(4):935–939. doi: 10.1073/pnas.80.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson U., Tibbetts C., Philipson L. Hybridization maps of early and late messenger RNA sequences on the adenovirus type 2 genome. J Mol Biol. 1976 Mar 15;101(4):479–501. doi: 10.1016/0022-2836(76)90241-2. [DOI] [PubMed] [Google Scholar]
- Russell D. R., Bennett G. N. Construction and analysis of in vivo activity of E. coli promoter hybrids and promoter mutants that alter the -35 to -10 spacing. Gene. 1982 Dec;20(2):231–243. doi: 10.1016/0378-1119(82)90042-7. [DOI] [PubMed] [Google Scholar]
- Schottel J. L., Sninsky J. J., Cohen S. N. Effects of alterations in the translation control region on bacterial gene expression: use of cat gene constructs transcribed from the lac promoter as a model system. Gene. 1984 May;28(2):177–193. doi: 10.1016/0378-1119(84)90255-5. [DOI] [PubMed] [Google Scholar]
- Stillman B. W., Tamanoi F., Mathews M. B. Purification of an adenovirus-coded DNA polymerase that is required for initiation of DNA replication. Cell. 1982 Dec;31(3 Pt 2):613–623. doi: 10.1016/0092-8674(82)90317-8. [DOI] [PubMed] [Google Scholar]
- Vaessen R. T., Kreike J., Groot G. S. Protein transfer to nitrocellulose filters. A simple method for quantitation of single proteins in complex mixtures. FEBS Lett. 1981 Feb 23;124(2):193–196. doi: 10.1016/0014-5793(81)80134-2. [DOI] [PubMed] [Google Scholar]
- Zaidenzaig Y., Shaw W. V. Affinity and hydrophobic chromatography of three variants of chloramphenicol acetyltransferases specified by R factors in Escherichia coli. FEBS Lett. 1976 Mar 1;62(3):266–271. doi: 10.1016/0014-5793(76)80072-5. [DOI] [PubMed] [Google Scholar]
- de Boer H. A., Comstock L. J., Vasser M. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci U S A. 1983 Jan;80(1):21–25. doi: 10.1073/pnas.80.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]