Abstract
Beta-glucans are components of fungal cell walls and potent stimulants of innate immunity. The majority of research on biological activities of glucans has focused on β-(1,3)-glucans, which have been implicated in relation with fungal exposure-associated respiratory symptoms, and as important stimulatory agents in anti-fungal immune responses. Fungi - and bacteria and plants - produce a wide variety of glucans with vast differences in proportion and arrangement of their 1,3-, 1,4-, and 1,6-β-glycosidic linkages. Thus far the proinflammatory potential of different β-glucans has not been studied within the same experimental model. Therefore, we compared the potency of 13 different glucan preparations to induce in vitro production of IL1β, IL6, IL8 and TNF-α in human whole blood cultures. The strongest inducers of all cytokines were pustulan (β-(1,6)-glucan), lichenan (β-(1,3)-(1,4)-glucan), xyloglucan (β-(1,4)-glucan), and pullulan (α-(1,4)-(1,6)-glucan). Moderate to strong cytokine production was observed for curdlan (β-(1,3)-glucan), baker’s yeast glucan (β-(1,3)-(1,6)-glucan), and barley glucan (β-(1,3)-(1,4)-glucan), while all other glucan preparations induced only low or no detectable levels of cytokines. We therefore conclude that innate immunity reactions are not exclusively induced by β-(1,3)-glucans, but also by β-(1,6)- and β-(1,4)-structures. Thus, not only β-(1,3)-glucan, but also other β-glucans and particularly β-(1,6)-glucans should be considered in future research.
Keywords: Beta-glucans, inflammation, innate immunity, curdlan, pustulan, whole blood stimulation
Introduction
Beta-glucans are microbial associated molecular patterns (MAMPs) produced by bacteria and fungi with large variation in proportion and arrangement of their 1,3-, 1,4-, and 1,6-β-glycosidic linkages 1. Plants also produce β-glucans, which are by definition not MAMPs. These linear β-(1,4)-glucan chains with different amounts of β-(1,3)-linkages within the linear chain may nevertheless interact with the vertebrate immune system 2–4 as well. Fungal cell walls contain predominantly linear β-(1,3)-glucans with branches of shorter β-(1,6)-glucan chains 5–6, while in algae or bacteria the most common types are linear β-(1,3)-glucans. Another group of β-glucans with only β-(1,6)-linkages are produced by lichens 7–8 and some fungi 9–11. Recent reports suggest that these β-(1,6)-glucans may also induce both innate and adaptive immune responses 12–14, but the actual mechanisms by which they elicit inflammatory reactions have not yet been investigated.
Even though such vast differences between β-glucan structures are known, the majority of studies on glucans as MAMPs have focused on β-(1,3)-glucan 15. The principal innate immune receptor for β-(1,3)-glucan is dectin-1 16–17, which is primarily but not exclusively expressed on monocytic cell populations 18. Complement receptor 3 (CR3) 2, 19 has also been identified to recognize β-(1,3)-glucans, but its exact role in anti-microbial defense mechanisms has not yet been elucidated. The activation of cellular innate immunity reactions by receptor binding commonly includes the induction of cytokine production 20–22. The potential of β-(1,3)-glucans to stimulate cytokine production in human blood cells has been investigated in several experimental in vitro studies, with curdlan, a linear β-(1,3)-glucan from bacterial origin 23–25, schizophyllan, a fungal β-(1,3)-(1,6)-glucan, and a carboxymethylated glucan from baker’s yeast 26 as stimulating agents. While the various β-(1,3)-glucans used in each of these studies induced clearly elevated cytokine levels, their pro-inflammatory potencies have to our knowledge not been systematically compared within one study and in the same experimental test model. Differences in proinflammatory potency may be anticipated, since the pronounced structural differences most likely result in different affinities for innate immunity receptors, as has been shown for instance for binding to dectin-127.
The objective of this study was therefore, to test and compare the potential of 13 different glucans to induce cytokine production, using human whole blood cultures as the test system.
Material and Methods
Glucans
We used the β-(1,3)-glucans curdlan (Wako Chemicals GmbH, Neuss, Germany), laminarin (Sigma-Aldrich, Zwijndrecht, The Netherlands), pachyman (Megazyme, Wicklow, Ireland) and paramylon (Sigma-Aldrich, Zwijndrecht, The Netherlands), the β-(1,3)-(1,4)-glucans barley glucan, lichenan and oat glucan (all Megazyme), the β-(1,4)-glucan xyloglucan (Megazyme), the β-(1,3)-(1,6)-glucans baker’s yeast glucan (Sigma-Aldrich), schizophyllan and scleroglucan (gifts of Dr. U. Rau, Dep. Biotechnology, TU Braunschweig, Germany), and the β-(1,6)-glucan pustulan (EMD Chemicals, Gibbstown, NJ, USA). Additionally to the β-glucans we tested the α-(1,4)-(1,6)-glucan pullulan (Megazyme). All glucans were available as dry powder and were >90% pure according to the suppliers’ information. Stock solutions were made of one mg per ml, based on preliminary experiments in which solubilities were assessed. Glucans were considered soluble when the mixture consisted of one clear homogenous phase. Specifically, baker’s yeast glucan and curdlan were dissolved in 0.05M NaOH at room temperature, barley glucan, laminarin, lichenan and pustulan by autoclaving in ultrapure water, scleroglucan by autoclaving in 0.05M NaOH, and oat glucan, xyloglucan and pullulan were dissolved in ultrapure water at room temperature. Pachyman, paramylon and schizophyllan were dissolved by autoclaving in 0.05M NaOH followed by centrifugation at 1000g for 15 minutes to get rid of possible particulates and supernatants were collected. Pellets were dried and weighed and amounted to maximally 5% of the original weight.
Structures were confirmed by proton NMR spectra produced and evaluated by drs. D. W. Lowman, AppRidge International, LLC, Jonesborough, TN, USA, and J.P. Kamerling, Bio-Organic Chemistry of Carbohydrates at Utrecht University, respectively. Structural differences, biological sources and molecular weights are presented in table 1.
Table 1.
Beta-glucan types and structural differences
| Glucan | source | Linkages | MW (kDa) | Reference | |
|---|---|---|---|---|---|
| taxon | species | ||||
| baker’s yeast glucan | yeast | Saccharomyces cerevisiae | β-(1,3) β-(1,6) |
35–5000 | 54 |
| barley glucan | plant | Hordeum vulgare | β-(1,3) β-(1,6) |
23–137 | Producer’s Insert |
| curdlan | bacteria | Alcaligenes faecalis | β-(1,3) β-(1,6) |
53–2000 | 55 |
| laminarin | algae | Laminaria digitata | β-(1,3) β-(1,6) |
3.5–7.7 | 56–57 |
| lichenan | lichen | Cetraria islandica | β-(1,3) β-(1,6) |
20–35 | 58 |
| oat glucan | plant | Avena sativa | β-(1,3) β-(1,6) |
1–300 | 59–60 |
| pachyman | fungi | Poria cocos | β-(1,3) β-(1,6) |
21–100 | 55 |
| paramylon | algae | Euglena gracilis | β-(1,3) | 118 | 61 |
| pullulan | fungi | Aureobasidium pulllan | α-(1,4) α-(1,6) |
200 | 62 |
| pustulan | lichen | Umbilicaria sp. | β-(1,6) | 20 | 58 |
| schizophyllan | fungi | Schizophyllum commune | β-(1,3) β-(1,6) |
76.8–450 | 56 |
| scleroglucan | fungi | Sclerotium rolfsii/glucanicum | β-(1,3) β-(1,6) |
1000–5000 | 56, 63 |
| xyloglucan | plant | tamarind | β-(1,4) | 202 | Producer’s Insert |
Whole Blood Assay (WBA)
Approximately 15 ml peripheral blood of 7 healthy adult human donors was collected by venapuncture in sterile heparin tubes (Lithium Heparin, Venosafe; Terumo, Leuven, Belgium), and gently mixed for approximately one hour. Heparin was chosen based on previous pilot experiment showing that heparin gave more consistent and higher cytokine measurements (I.M. Wouters &L.A.M. Smit, not published). The whole blood stimulations were essentially performed as described earlier 28. Briefly, the glucan solutions were diluted with RPMI 1640 medium supplemented with 100 U/ml penicillin and 100 mg/ml streptomycin (Gibco, Breda, the Netherlands) to 500, 250 and 50 μg/ml.
As positive control LPS (E. Coli O55:B5, Fluka, Buchs, Switzerland) was used in the concentrations of 0.2, 0.02, 0.002 and 0.0002 ng/ml and the RPMI 1640 diluent was used as negative control. All solutions and controls were transferred in 100 μl aliquots to sterile flat-bottom 96-well cell culture plates (Corning Inc, New York, USA) and 100 μl heparinized blood samples were added.
After culturing for 24 h at 37°C, 5% CO2 and 96% relative humidity, the plates were centrifuged (15 min, 1000g) and supernatants were stored at −20°C. Enzyme immunoassays (EIAs) from Sanquin Reagents (Amsterdam, The Netherlands) were used to measure TNF-α (samples diluted 1/5 to 1/40), IL1β (1/40 to 1/320), IL6 (1/100 to 1/800) and IL8 (1/100 to 1/2700) in the supernatants, with the lower detection limits (LOD) being 17.7 pg/ml, 11.5 pg/ml, 6.04 pg/ml and 1.96 pg/ml respectively.
Polymyxin treatment and LPS measurement
To control for possible endotoxin contamination all glucan solutions were treated with polymyxin, a cationic agent that would remove endotoxin by binding to the negatively charged lipid A portion of the lipopolysaccharide 29–30. Polymyxin B-Agarose (Sigma-Aldrich) was washed three times with 0.1M ammonium bicarbonate buffer, followed by three washing steps with pyrogen-free water. One ml of 1 mg/ml polymyxin B-Agarose (binding capacity: 200–500 μg LPS per ml) was added to 1 ml of 1 mg/ml glucan and incubated on an end-over-end roller for 2 h at room temperature. The mixture was centrifuged 20 min at 1000g. The treated glucan solutions as well as the stock solutions without treatment were tested in the quantitative kinetic chromogenic Limulus Amoebocyte Lysate (LAL) assay (Lonza, LAL-Lysate lot GL155U) at a 1/4 dilution in pyrogen-free water.
LPS from E. coli O55:B5 (lot GL1157, 14 EU/ng) was used as the reference standard. The cut-off signal of the LAL-Assay was defined as the mean plus two standard deviations of the Vmax of the assay blanks. The corresponding sensitivity of the assay (LOD) for an undiluted sample was 0.012 EU/ml and thus for the (1/4) diluted glucan solutions the LOD was 0.048 EU/ml, which corresponds to approximately 0.003 ng pure LPS per ml. Since β-(1,3)-Glucans can produce false positive results in LAL assays, we used the β-G-Blocker (Lonza), to assess this possibility in some informative samples. The use of the β-G-Blocker did not affect the measured endotoxin content (data not shown). The results are presented in table 2. The polymyxin-treated glucan solutions were additionally tested in parallel with the non-treated glucans in the glucan inhibition assay31 to ensure that glucan concentrations were not affected by the treatment. Glucan levels measured before and after polymyxin treatment were similar, with an average absolute difference of 17% (1–31%).
Table 2.
Endotoxin concentration before and after polymyxin treatment of the LPS positive control (50 EU/ml=3.5 ng/ml) and glucan stock solutions (1mg/ml) as determined by the LAL assay; LOD=0.003 ng/ml.
| LPS concentration (ng/ml) | ||
|---|---|---|
| Before Polymyxin treatment | After Polymyxin treatment | |
| LPS control | 2.72 | 0.065 |
| baker’s yeast glucan | <LOD | <LOD |
| barley β-glucan | 0.079 | 0.012 |
| curdlan | 0.058 | 0.008 |
| laminarin | <LOD | <LOD |
| lichenan | 0.208 | 0.039 |
| oat β-glucan | 0.150 | 0.061 |
| pachyman | 0.033 | <LOD |
| paramylon | <LOD | <LOD |
| pullulan | 210 | 10 |
| pustulan | 0.024 | <LOD |
| schizophyllan | 0.024 | <LOD |
| scleroglucan | <LOD | <LOD |
| xyloglucan | 1071 | 362 |
Finally, polymyxin treated solutions were tested in the WBA as described before and results were compared with those of non-treated solutions. As a positive control for successful polymyxin treatment, a 3.5 ng/ml LPS standard preparation (E. coli O55:B5) was included parallel to the glucan solutions.
Statistics
All data showed a log-normal distribution, and therefore Pearson correlation coefficients (r) were calculated based on ln-transformed values.
For three donors complete data were available - thus for all four cytokines after stimulation with each of the 13 glucans in three stimulatory concentrations. We used medians of the cytokine responses of those three donors in the analysis. Correlations between the cytokine responses of the three different donors were similar for all cytokines and all pair-wise comparisons between the seven donors (r>0.7) where applicable.
Figures were produced in Sigmaplot 10 (Systat Software, Inc., San Jose, USA).
Results
Comparability between individual responses
Since individual responses to β-glucan stimulation may differ qualitatively and quantitatively, a comparison between individual donors’ cytokine responses to the different glucans (figure 1) was made. The comparisons between IL6 responses in whole blood from three donors stimulated with 250 μg/ml of the glucans are presented in figure 1. The analogous correlations for IL6 induced by 125 and 25 μg/ml of the glucans, as well as the between-donor comparisons of IL 1β, IL 8 and TNF-α production showed essentially the same pattern (all r>0.8).
Figure 1. Comparison between IL 6 concentrations in whole blood of three donors after stimulation with 250μg/ml glucan.
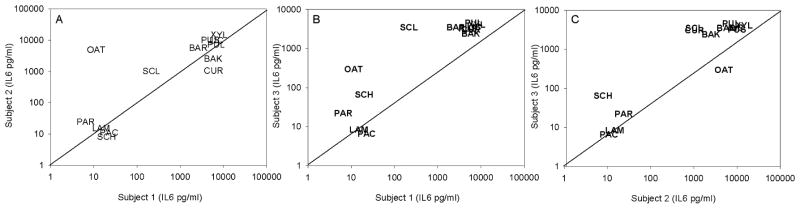
A: donor 1 (x-axis) vs donor 2 (y-axis); B: donor 1 (x-axis) vs donor 3 (y-axis); C: donor 2 (x-axis) vs donor 3 (y-axis).
BAK: baker’s yeast glucan; BAR: barley β-glucan; CUR: curdlan; LAM: laminarin; LIC: lichenan; oat: oat β-glucan; PAC: pachyman; PAR: paramylon; PUL: pullulan; PUS: pustulan; SCH: schizophyllan; SCL: scleroglucan; XYL: xyloglucan
Some quantitative differences in cytokine responses were observed for different donors (figure 1), but the ranking of the various glucans into weak, moderate, strong and very strong cytokine inducers was practically the same for all donors. An exception was oat β-glucan which did not induce significant production of any cytokine in whole blood from donor 1, while high IL-6 production – and also high production of IL 1β, IL 8 and TNF-α (not shown) - was observed for both donor 2 and 3 (figure 1A, B).
Cytokine induction potency of different glucans
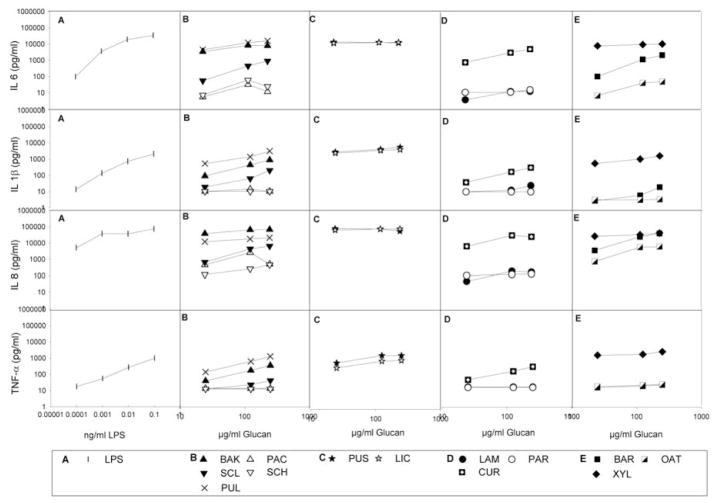
Figure 2 presents an overview of the median cytokine levels after stimulation with LPS and the various glucan preparations.
Figure 2.
Median cytokine levels in whole blood of three donors after 24h stimulation with LPS and various glucan preparations. The different panels represent in vertical direction the measured median levels of IL6, IL1β, IL8 and TNFα and in horizontal direction the results for LPS (A) and glucans derived from fungi (B), lichen (C), algae and bacteria (D) and plants (E);
Generally, the two lichen glucans - the β-(1,6)-glucan pustulan and the β-(1,3)-(1,4)-glucan lichenan - and the plant xyloglucan (β-(1,4)-glucan) as well as pullulan – a fungal α-(1,4)-(1,6)-glucan - were the strongest cytokine inducers. Baker’s yeast glucan, (fungal β-(1,3)-(1,6)-glucan), barley β-glucan (plant β-(1,3)-(1,4)-glucan) and curdlan (bacterial β-(1,3)-glucan) also induced strong production of most cytokines. Scleroglucan (fungal β-(1,3)-(1,6)-glucan) and oat β-glucan (plant β-(1,3)-(1,4)-glucan) induced modest cytokine production, and all other glucans only weak or no cytokine production at all.
Comparison of different cytokine responses
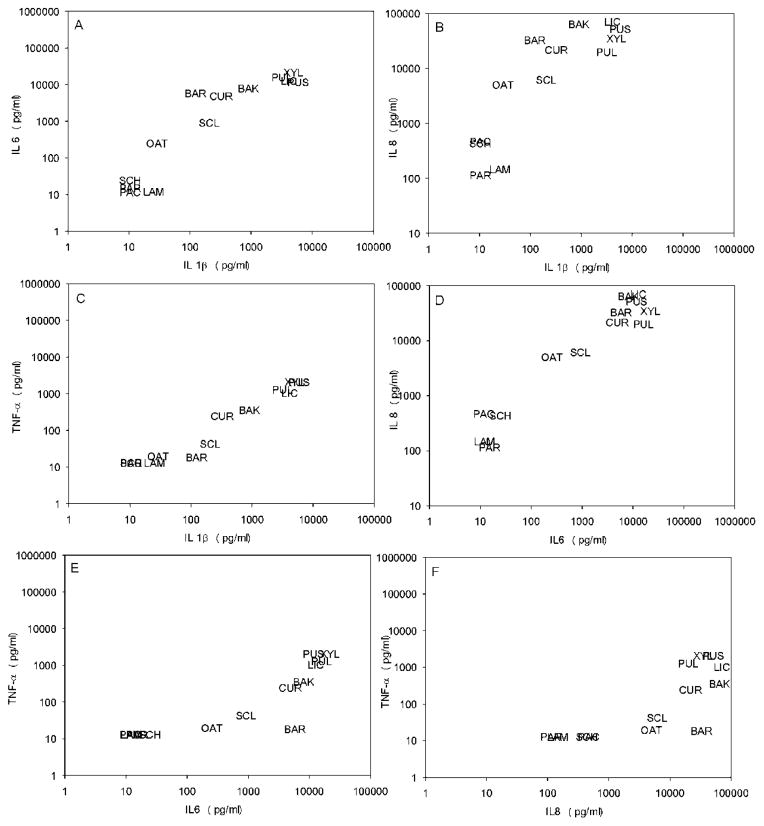
To investigate whether the profile of proinflammatory cytokines – i.e. their relative abundance or absence compared to other cytokines - induced by different glucans may show some qualitative variation, we also compared the induced levels of the four cytokines (fig. 3).
Figure 3. Comparison of productions of different cytokines in response to the various glucans.
A: IL 1β vs. IL 6; B: IL 1β vs. IL 8; C: IL 1β vs. TNF α; D: IL 6 vs IL8; E: IL 6 vs. TNF α; F: IL 8 vs. TNF α; Abbreviations as in figure 1.
In general the levels of different cytokines produced in response to a certain glucan were highly correlated. The strongest correlations (r>0.9) were found between IL8 and IL6, between TNF-α and IL1β, and between IL 6 and IL1β. IL8 correlated only moderately with TNF-α and IL1β (0.6<r<0.8), while IL6 correlated only moderately with TNF-α (r=0.7).
Thus, we confirmed that the glucans could be grouped - irrespective of the blood donor or cytokine measured - into four categories of proinflammatory potential, ranging from very strong to practically none.
Endotoxin activity in the glucan solutions
Endotoxin is a very potent proinflammatory agent, as confirmed in figure 2, where even the lowest concentrations of endotoxin - which are barely measurable in the LAL-test – could induce moderate to strong cytokine responses.
Polymyxin treatment of LPS removed more than 97% of the endotoxin reactivity (table 2). Polymyxin treatment of the glucan preparations also removed most, but not all (60–95%) of the endotoxin reactivity as measured in the LAL assay, and the effectiveness of the treatment varied depending on the glucan preparation (table 2). Figure 4 shows the IL6 concentrations after whole blood stimulation with polymyxin- treated and non-treated LPS and various glucans with strong cytokine inducing potency. As expected from the LAL measurements, the treatment of the standard E. coli LPS solution caused a >95% loss of its IL-6 inducing potency. However, the cytokine stimulatory potency of glucans in the WBA was not or much less affected, and definitely not completely abolished by the polymyxin treatment.
Figure 4.
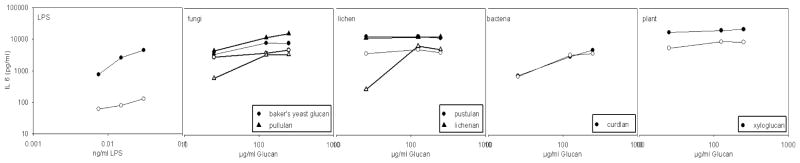
Comparison of IL6 concentrations induced in the WBA by six glucans (baker’s yeast glucan, pullulan, pustulan, lichenan, curdlan, xyloglucan) and the LPS positive control solution before (closed symbols) and after polymyxin treatment (open symbols).
Overall, the results of the polymyxin treatment confirmed that the cytokine inducing potential of most of the glucan preparations was not due to endotoxin contamination. Moreover, none of the glucan solutions induced IL6 responses corresponding to its apparent endotoxin content.
Discussion
A variety of differently structured glucans showed strong proinflammatory potential in the in vitro whole blood assay. Compared to endotoxin, there was no dose-response seen for many of the glucans which clearly induced a cytokine response. This is most likely the result from the relatively high stimulating concentrations applied. The high concentrations were selected in order to have the highest chance of detecting also low cytokine induction potential of the various glucans, as established in a pilot experiment (data not shown). It thus is likely that the strongest inflammatory response inducing glucans will also lead to significant cytokine production at much lower concentrations in the range of 1 μg/ml or lower.
The levels of different cytokines produced in response to a certain glucan were highly correlated, suggesting that all glucan types elicit a similar, common type of inflammatory response. Interestingly the glucans with the highest cytokine production potency had very different structures and molecular weights (table 1): the pure β-(1,6)-glucan pustulan (20 kDa), the β-(1,3)-(1,4)-glucan lichenan (20–35 kDa), the β-(1,4)-glucan xyloglucan(202 kDa) and the α-glucan pullulan (118 kDa). The β-(1,3)-(1,6)-glucan from baker’s yeast (35–5000 kDa), β-(1,3)-glucan curdlan (53–2000 kDa) and β-(1,3)-(1,4)-glucan barley β-glucan (391 kDa) induced strong to moderate cytokine production, while scleroglucan (β-(1,3)-(1,6)-glucan, 1000–5000 kDa) and oat β-glucan (β-(1,3)-(1,4)-glucan, 1–300 kDa) induced moderate IL6 and IL8 production and only low amounts of TNF-α and IL1β. All other glucan preparations did not stimulate significant cytokine productions.
Low amounts of LPS, a component of the cell wall of gram negative bacteria, can induce strong cytokine responses and thus easily cause false positives. Liebers et al. reported for 5 pg/ml LPS already substantial cytokine induction32, and we can report that already the low concentrations of 2 pg/ml LPS induces moderate levels of IL1β and TNF-α and high levels of IL6 and IL8. We found relatively low to high endotoxin levels in the LAL measurements of some glucan preparations, and although even here the measured LPS concentration (maximally 1071 ng/ml) relates to only 0.1% of the total dry weight of the glucan preparations. This low amount might indeed have accounted for part of the strong cytokine inducing potency of some glucans. Therefore we treated all glucan solutions with polymyxin to remove possible LPS contaminations. According to the LAL measurements the endotoxin activity of most glucan preparations was reduced by >90% and of the LPS control by 97 % after polymyxin treatment and while the cytokine inducing potential of all polymyxin treated glucans was retained, the treated LPS control solution lost >95% of its cytokine inducing capacity. Therefore, the proinflammatory potential of the glucans with an apparent endotoxin contamination was most likely not due to LPS contamination.
Peptidoglycan (PGN) is a component of the bacterial cell wall especially of Gram positive bacteria and is a MAMP that induces signaling through the Toll-like and NOD-like receptor pathways. Since PGN could be another possible contaminant, all glucan solutions used in this study were also tested for PGN at the University of Iowa, Pulmonary Toxicology Facility using an enzyme immunoassay. Pustulan showed the highest concentration of PGN in the analysis - namely 1.75 μg/ml in a 1 mg/ml preparation - thus slightly less than 0.2% of the dissolved glucan in the solution. This would mean that the highest dilution of pustulan in the WBA (25μg/ml) - which still gave strong cytokine responses - contained maximally 45 ng/ml PGN. Rockel et al.33 reported that PGN alone does not stimulate a cytokine response in human whole blood at 10 μg/ml, and van der Meer et al.34 found the same result for muramyldipeptide in human whole blood. Thus the concentration of PGN measured in our glucan preparations was clearly below the minimum level required for cytokine induction. We therefore conclude that the proinflammatory potential of the glucans used in this study was most likely not due to PGN contamination.
Despite the fact that we can conclude that the measured cytokine productions in this study were induced by the various investigated glucans rather than by LPS or PGN, there still may be the question of a possible interaction with another substance that may have been present in our glucan solutions. The NMR analysis as well as the producer’s information does not exclude the presence of minor contamination at <5%, but it is highly unlikely that such contaminants might account for the high cytokine production seen with some of the glucans.
Our results for curdlan are similar to those of previous studies reporting high levels of IL 1β, TNF-α, IL 6 and IL 8 24–25 after whole blood stimulations with curdlan. In vivo inhalation exposure studies in humans reported that IL 8 concentrations in nasal lavage samples were significantly higher after inhalation of dust spiked with curdlan than after exposure to non-spiked airborne dust alone35. We are aware that it is challenging to extrapolate results of in vitro work back to the biology of the intact organism and the glucan concentrations used in the present and other studies to examine proinflammatory potential in vitro are much higher than the in vivo exposure dose of glucans that would be expected after inhalation of airborne glucans the home or occupational environment36–38 - which would only amount to ng glucan per kg bodyweight. Despite the pro-inflammatory nature of β-(1,3)-glucans in the previously reported in vitro studies23–26, a series of studies investigating inhalation exposures of pure β-(1,3)-glucans in guinea pigs, mice or humans showed conflicting results - reporting no effects or either a neutrophilic or eosinophilic response (reviewed by Douwes39 and Rylander 40). The same is observed in epidemiological studies reporting associations between indoor β-(1,3)-glucan exposure and inflammatory reactions of the respiratory system35, 41–45, as well as protective effects of glucan exposure in early childhood against the development of asthma and allergy46–49. Apart from the chemical structure, the solubility and the dose level have been suggested to play a role, based on comparisons between studies and a limited number of glucans within each study. In our current study – including a broad range of glucans, with confirmed structures within the same experimental model - no clear relation between chemical characteristics and cytokine inducing potency could be recognized. Thus, one may conclude, that while in vitro studies may not be easily extrapolated to the biology of the intact organism, such studies are necessary to uncover the mechanisms behind the effects found in intact organisms.
For β-(1,3)-glucan, the pattern recognition receptor dectin-1 has been described as a primary receptor 16, and binding to it will likely lead to the induction of cytokine responses. Since dectin-1 only binds structures containing a β-(1,3)-linked backbone, but not pure β-(1,6)-glucan or the plant type linear β-(1,3)-(1,4)-glucan like lichenan, barley or oat β-glucan27, 50, it is unlikely that the cytokine production induced by the tested glucans in this study was in all cases dectin-1 mediated. Thus, other dectin-1 independent mechanisms have to be considered. Complement receptor 3 (CR3) has been reported to bind β-glucans. CR3 binds barley and yeast glucans, as well as other polysaccharides via its lectin site located C-terminal to the I-domain 51. Further CR3-mediated interaction with neutrophils may induce phagocytosis and thus lead to the induction of cytokine production by glucans, such as pustulan, lichenan, barley and oat β-glucan, which are not binding to dectin-1. A third possible mechanism could be anti-β-glucan antibody coating the glucan particles, leading to opsonization of the according glucans, followed by dectin-1 or CR-3 independent monocyte activation via binding to the cell’s membrane Fc-receptors. In a previous study we confirmed recent findings of Chiani et al.12 that high anti-β-glucan antibody levels can be found in many normal human sera. The predominant antigenic structure appeared in both studies to be associated with β-(1,6)-linkages 12–13, which may explain induction of cytokine production by glucans containing β-(1,6)-linkages not binding to Dectin-1 or CR3. Interestingly both β-(1,3)-(1,6)-glucans - scleroglucan and baker’s yeast glucan - were capable of dectin-1 binding 27 and in our serologic studies we found high anti-scleroglucan as well as anti-baker’s-yeast-glucan IgG levels 13, but only baker’s yeast glucan induced moderate cytokine production. Furthermore, barley β-glucan - not binding to dectin-1 27, 50 and in our serologic study an apparently poor IgG binding antigen 13 – induced considerable cytokine production in the whole blood assay. Thus it seems reasonable to assume that the induction of cytokine production by several glucans may involve different and more complex mechanisms than we are yet aware of.
Our study is reporting a comparison of the proinflammatory potential of various glucan types. Our results clearly show that not only the β-(1,3)-glucans stimulate innate immunity but that also β-(1,6)- and β-(1,4)-structures can elicit strong cytokine responses. The β-(1,6)-glucans even seem to have a much greater potential to elicit immune responses compared to the much investigated β-(1,3)-glucans. This is a new observation and in line with previously published studies12, 14, 52–53.
Since it is generally accepted that the - in this study less reactive - β-(1,3)-glucans may have an effect on the immune system after inhalation of considerably lower concentrations, similar in vivo responses to inhaled low concentrations of the - in this study highly reactive -β-(1,6)-glucans are plausible. Therefore, we feel that it is reasonable to suggest that the biology of β-(1,6)-glucans may be an interesting new addition to future research, both in epidemiological exposure studies and in studies investigating immunological mechanisms for anti-fungal responses and general immune system skewing.
Acknowledgments
We would like to thank D.W. Lowman, for the NMR measurements and J.P. Kamerling, for his help with the interpretation of the resulting spectra. We thank N. Metwali for peptidoglycan measurements.
This study was financially supported by the European Commission as part of GABRIEL, contract number 018996 under the Integrated Program LSH-2004-1.2.5-1. I. N is financially supported by the German Research Foundation (Deutsche Forschungsgemeinschaft) Grant NO 928/1-1. PST is supported by U.S. NIH P30 ES005605.
References
- 1.Stone BA, Clarke AE. Chemistry and Biology of (1->3)-β-Glucans. La Trobe University Press; 1992. p. 803. [Google Scholar]
- 2.Xia Y, Vetvicka V, Yan J, Hanikyrova M, Mayadas T, Ross GD. The beta-glucan-binding lectin site of mouse CR3 (CD11b/CD18) and its function in generating a primed state of the receptor that mediates cytotoxic activation in response to iC3b-opsonized target cells. Journal of Immunology. 1999;162:2281–90. [PubMed] [Google Scholar]
- 3.Hong F, Yan J, Baran JT, et al. Mechanism by which orally administered beta-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. Journal of Immunology. 2004;173:797–806. doi: 10.4049/jimmunol.173.2.797. [DOI] [PubMed] [Google Scholar]
- 4.Tada R, Ikeda F, Aoki K, et al. Barley-derived beta-D-glucan induces immunostimulation via a dectin-1-mediated pathway. Immunology Letters. 2009;123:144–8. doi: 10.1016/j.imlet.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Bowman SM, Free SJ. The structure and synthesis of the fungal cell wall. Bioessays. 2006;28:799–808. doi: 10.1002/bies.20441. [DOI] [PubMed] [Google Scholar]
- 6.Kapteyn JC, Van Den Ende H, Klis FM. The contribution of cell wall proteins to the organization of the yeast cell wall. Biochim Biophys Acta. 1999;1426:373–83. doi: 10.1016/s0304-4165(98)00137-8. [DOI] [PubMed] [Google Scholar]
- 7.Nishikawa Y, Tanaka M, Shibata S, Fukuoka F. Polysaccharides of lichens and fungi. IV. Antitumour active O-acetylated pustulan-type glucans from the lichens of Umbilicaria species. Chem Pharm Bull (Tokyo) 1970;18:1431–4. doi: 10.1248/cpb.18.1431. [DOI] [PubMed] [Google Scholar]
- 8.Carbonero ER, Smiderle FR, Gracher AHP, et al. Structure of two glucans and a galactofuranomannan from the lichen Umbilicaria mammulata. Carbohydrate Polymers. 2006;63:13–8. [Google Scholar]
- 9.Aimanianda V, Clavaud C, Simenel C, Fontaine T, Delepierre M, Latge JP. Cell wall beta-(1,6)-glucan of Saccharomyces cerevisiae: structural characterization and in situ synthesis. J Biol Chem. 2009;284:13401–12. doi: 10.1074/jbc.M807667200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sassaki GL, Ferreira JC, Glienke-Blanco C, et al. Pustulan and branched beta-galactofuranan from the phytopathogenic fungus Guignardia citricarpa, excreted from media containing glucose and sucrose. Carbohydrate Polymers. 2002;48:385–9. [Google Scholar]
- 11.Kruppa MD, Lowman DW, Chen YH, et al. Identification of (1 -> 6)-beta-D-glucan as the major carbohydrate component of the Malassezia sympodialis cell wall. Carbohydrate Research. 2009;344:2474–9. doi: 10.1016/j.carres.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiani P, Bromuro C, Cassone A, Torosantucci A. Anti-beta-glucan antibodies in healthy human subjects. Vaccine. 2009;27:513–9. doi: 10.1016/j.vaccine.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 13.Noss I, Wouters I, Heederik D, Doekes G. IgG to β-glucans in the general population. 2010 submitted. [Google Scholar]
- 14.Rubin-Bejerano I, Abeijon C, Magnelli P, Grisafi P, Fink GR. Phagocytosis by human neutrophils is stimulated by a unique fungal cell wall component. Cell Host & Microbe. 2007;2:55–67. doi: 10.1016/j.chom.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herre J, Gordon S, Brown GD. Dectin-1 and its role in the recognition of beta-glucans by macrophages. Mol Immunol. 2004;40:869–76. doi: 10.1016/j.molimm.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Brown GD, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature. 2001;413:36–7. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 17.Brown GD, Herre J, Williams DL, Willment JA, Marshall AS, Gordon S. Dectin-1 mediates the biological effects of beta-glucans. J Exp Med. 2003;197:1119–24. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willment JA, Marshall ASJ, Reid DM, et al. The human beta-glucan receptor is widely expressed and functionally equivalent to murine Dectin-1 on primary cells. European Journal of Immunology. 2005;35:1539–47. doi: 10.1002/eji.200425725. [DOI] [PubMed] [Google Scholar]
- 19.van Bruggen R, Drewniak A, Jansen M, et al. Complement receptor 3, not Dectin-1, is the major receptor on human neutrophils for beta-glucan-bearing particles. Mol Immunol. 2009;47:575–81. doi: 10.1016/j.molimm.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunology Letters. 2003;85:85–95. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 21.Schroder K, Tschopp J. The inflammasomes. Cell. 140:821–32. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 22.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–20. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 23.Krüger T, Sigsgaard T, Bonefeld-Jørgensen EC. Ex vivo induction of cytokines by mould components in whole blood of atopic and non-atopic volunteers. Cytokine. 2004;25:73–84. doi: 10.1016/j.cyto.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Sigsgaard T, Bonefeld-Jørgensen EC, Kjaergaard SK, Mamas S, Pedersen OF. Cytokine release from the nasal mucosa and whole blood after experimental exposures to organic dusts. Eur Respir J. 2000;16:140–5. doi: 10.1034/j.1399-3003.2000.16a25.x. [DOI] [PubMed] [Google Scholar]
- 25.Wouters IM, Douwes J, Thorne PS, Heederik D, Doekes G. Inter- and intraindividual variation of endotoxin- and beta(1 --> 3)-glucan-induced cytokine responses in a whole blood assay. Toxicol Ind Health. 2002;18:15–27. doi: 10.1191/0748233702th126oa. [DOI] [PubMed] [Google Scholar]
- 26.Kubala L, Ruzickova J, Nickova K, Sandula J, Ciz M, Lojek A. The effect of (1-->3)-beta-D-glucans, carboxymethylglucan and schizophyllan on human leukocytes in vitro. Carbohydr Res. 2003;338:2835–40. doi: 10.1016/j.carres.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Adams EL, Rice PJ, Graves B, et al. Differential high affinity interaction of Dectin-1 with natural or synthetic glucans is dependent upon primary structure and is influenced by polymer chain length and side chain branching. J Pharmacol Exp Ther. 2008 doi: 10.1124/jpet.107.133124. [DOI] [PubMed] [Google Scholar]
- 28.Smit LA, Heederik D, Doekes G, Krop EJ, Rijkers GT, Wouters IM. Ex vivo cytokine release reflects sensitivity to occupational endotoxin exposure. Eur Respir J. 2009;34:795–802. doi: 10.1183/09031936.00161908. [DOI] [PubMed] [Google Scholar]
- 29.Srimal S, Surolia N, Balasubramanian S, Surolia A. Titration calorimetric studies to elucidate the specificity of the interactions of polymyxin B with lipopolysaccharides and lipid A. Biochemical Journal. 1996;315:679–86. doi: 10.1042/bj3150679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Storm DR, Rosenthal KS, Swanson PE. Polymyxin and Related Peptide Antibiotics. Annual Review of Biochemistry. 1977;46:723–63. doi: 10.1146/annurev.bi.46.070177.003451. [DOI] [PubMed] [Google Scholar]
- 31.Douwes J, Doekes G, Montijn R, Heederik D, Brunekreef B. Measurement of beta(1-->3)-glucans in occupational and home environments with an inhibition enzyme immunoassay. Appl Environ Microbiol. 1996;62:3176–82. doi: 10.1128/aem.62.9.3176-3182.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liebers V, Stubel H, Duser M, Bruning T, Raulf-Heimsoth M. Standardization of whole blood assay for determination of pyrogenic activity in organic dust samples. Int J Hyg Environ Health. 2009 doi: 10.1016/j.ijheh.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Rockel C, Hartung T, Hermann C. Different Staphylococcus aureus whole bacteria mutated in putative pro-inflammatory membrane components have similar cytokine inducing activity. Immunobiology. 2011;216:316–21. doi: 10.1016/j.imbio.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 34.van der Meer JH, Netea MG, Dinarello CA. Modulation of muramyl dipeptide stimulation of cytokine production by blood components. Clin Exp Immunol. 2009;156:428–33. doi: 10.1111/j.1365-2249.2009.03926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonlokke JH, Stridh G, Sigsgaard T, et al. Upper-airway inflammation in relation to dust spiked with aldehydes or glucan. Scand J Work Environ Health. 2006;32:374–82. doi: 10.5271/sjweh.1033. [DOI] [PubMed] [Google Scholar]
- 36.Rao CY, Riggs MA, Chew GL, et al. Characterization of airborne molds, endotoxins, and glucans in homes in New Orleans after Hurricanes Katrina and Rita. Appl Environ Microbiol. 2007;73:1630–4. doi: 10.1128/AEM.01973-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stuurman B, Meijster T, Heederik D, Doekes G. Inhalable beta(1->3)glucans as a non-allergenic exposure factor in Dutch bakeries. Occup Environ Med. 2008;65:68–70. doi: 10.1136/oem.2007.032623. [DOI] [PubMed] [Google Scholar]
- 38.Noss I, Wouters IM, Bezemer G, et al. beta-(1,3)-Glucan exposure assessment by passive airborne dust sampling and new sensitive immunoassays. Appl Environ Microbiol. 2010;76:1158–67. doi: 10.1128/AEM.01486-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Douwes J. (1-->3)-Beta-D-glucans and respiratory health: a review of the scientific evidence. Indoor Air. 2005;15:160–9. doi: 10.1111/j.1600-0668.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- 40.Rylander R. Organic Dust Induced Pulmonary Disease - the Role of Mould Derived B-Glucan. Annals of Agricultural and Environmental Medicine. 2010;17:9–13. [PubMed] [Google Scholar]
- 41.Douwes J, Zuidhof A, Doekes G, et al. (1-->3)-beta-D-glucan and endotoxin in house dust and peak flow variability in children. Am J Respir Crit Care Med. 2000;162:1348–54. doi: 10.1164/ajrccm.162.4.9909118. [DOI] [PubMed] [Google Scholar]
- 42.Rylander R. Indoor air-related effects and airborne (1 --> 3)-beta-D-glucan. Environ Health Perspect. 1999;107 (Suppl 3):501–3. doi: 10.1289/ehp.99107s3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thorn J, Beijer L, Rylander R. Airways inflammation and glucan exposure among household waste collectors. Am J Ind Med. 1998;33:463–70. doi: 10.1002/(sici)1097-0274(199805)33:5<463::aid-ajim5>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 44.Thorn J, Rylander R. Airways inflammation and glucan in a rowhouse area. Am J Respir Crit Care Med. 1998;157:1798–803. doi: 10.1164/ajrccm.157.6.9706081. [DOI] [PubMed] [Google Scholar]
- 45.Young SH, Ostroff GR, Zeidler-Erdely PC, Roberts JR, Antonini JM, Castranova V. A comparison of the pulmonary inflammatory potential of different components of yeast cell wall. Journal of Toxicology and Environmental Health-Part a-Current Issues. 2007;70:1116–24. doi: 10.1080/15287390701212224. [DOI] [PubMed] [Google Scholar]
- 46.Douwes J, van Strien R, Doekes G, et al. Does early indoor microbial exposure reduce the risk of asthma? The Prevention and Incidence of Asthma and Mite Allergy birth cohort study. J Allergy Clin Immunol. 2006;117:1067–73. doi: 10.1016/j.jaci.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Gehring U, Heinrich J, Hoek G, et al. Bacteria and mould components in house dust and children’s allergic sensitisation. Eur Respir J. 2007;29:1144–53. doi: 10.1183/09031936.00118806. [DOI] [PubMed] [Google Scholar]
- 48.Iossifova YY, Reponen T, Bernstein DI, et al. House dust (1–3)-beta-D-glucan and wheezing in infants. Allergy. 2007;62:504–13. doi: 10.1111/j.1398-9995.2007.01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schram-Bijkerk D, Doekes G, Douwes J, et al. Bacterial and fungal agents in house dust and wheeze in children: the PARSIFAL study. Clin Exp Allergy. 2005;35:1272–8. doi: 10.1111/j.1365-2222.2005.02339.x. [DOI] [PubMed] [Google Scholar]
- 50.Palma AS, Feizi T, Zhang Y, et al. Ligands for the beta-glucan receptor, Dectin-1, assigned using “designer” microarrays of oligosaccharide probes (neoglycolipids) generated from glucan polysaccharides. J Biol Chem. 2006;281:5771–9. doi: 10.1074/jbc.M511461200. [DOI] [PubMed] [Google Scholar]
- 51.Thornton BP, Vetvicka V, Pitman M, Goldman RC, Ross GD. Analysis of the sugar specificity and molecular location of the beta-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18) Journal of Immunology. 1996;156:1235–46. [PubMed] [Google Scholar]
- 52.Agarwal S, Specht CA, Haibin H, et al. Linkage specificity and role of properdin in activation of the alternative complement pathway by fungal glycans. MBio. 2011:2. doi: 10.1128/mBio.00178-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Noss I, Wouters IM, Smit LA, et al. IgG to Various Beta-Glucans in a Human Adult Population. Int Arch Allergy Immunol. 2011;157:98–108. doi: 10.1159/000324674. [DOI] [PubMed] [Google Scholar]
- 54.Bacon JS, Farmer VC, Jones D, Taylor IF. The glucan components of the cell wall of baker’s yeast (Saccharomyces cerevisiae) considered in relation to its ultrastructure. Biochem J. 1969;114:557–67. doi: 10.1042/bj1140557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saito H, Misaki A, Harada T. A Comparison of the Structure of Curdlan and Pachyman. Agr Biol Chem. 1968;32:1261–9. [Google Scholar]
- 56.Mueller A, Raptis J, Rice PJ, et al. The influence of glucan polymer structure and solution conformation on binding to (1-->3)-beta-D-glucan receptors in a human monocyte-like cell line. Glycobiology. 2000;10:339–46. doi: 10.1093/glycob/10.4.339. [DOI] [PubMed] [Google Scholar]
- 57.Beattie A, Percival E, Hirst EL. Studies on Metabolism of Chrysophyceae - Comparative Structural Investigations on Leucosin (Chrysolaminarin) Separated from Diatoms and Laminarin from Brown Algae. Biochemical Journal. 1961;79:531. doi: 10.1042/bj0790531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olafsdottir ES, Ingolfsdottir K. Polysaccharides from lichens: structural characteristics and biological activity. Planta Med. 2001;67:199–208. doi: 10.1055/s-2001-12012. [DOI] [PubMed] [Google Scholar]
- 59.Wood PJ. Cereal beta-glucans in diet and health. Journal of Cereal Science. 2007;46:230–8. [Google Scholar]
- 60.Wu J, Zhang Y, Wang L, Xie B, Wang H, Deng S. Visualization of single and aggregated hulless oat (Avena nuda L.) (1-->3),(1-->4)-beta-D-glucan molecules by atomic force microscopy and confocal scanning laser microscopy. J Agric Food Chem. 2006;54:925–34. doi: 10.1021/jf0523059. [DOI] [PubMed] [Google Scholar]
- 61.Aketagawa J, Tanaka S, Tamura H, Shibata Y, Saito H. Activation of limulus coagulation factor G by several (1-->3)-beta-D-glucans: comparison of the potency of glucans with identical degree of polymerization but different conformations. J Biochem. 1993;113:683–6. doi: 10.1093/oxfordjournals.jbchem.a124103. [DOI] [PubMed] [Google Scholar]
- 62.Muller A, Rice PJ, Ensley HE, et al. Receptor binding and internalization of a water-soluble (1->3)-beta-D-glucan biologic response modifier in two monocyte macrophage cell lines. Journal of Immunology. 1996;156:3418–25. [PubMed] [Google Scholar]
- 63.Suzuki T, Ohno N, Saito K, Yadomae T. Activation of the complement system by (1----3)-beta-D-glucans having different degrees of branching and different ultrastructures. J Pharmacobiodyn. 1992;15:277–85. doi: 10.1248/bpb1978.15.277. [DOI] [PubMed] [Google Scholar]