Abstract
Clinical outcomes were compared between high-cylinder toric intraocular lens (IOL) implantation and the combined surgery of low-cylinder toric IOL implantation and limbal relaxing incision (LRI) for correcting preexisting high-amplitude corneal astigmatism. Fifty-seven eyes with preexisting corneal astigmatism of 2.5 diopter (D) or greater were divided into the following two groups: (1) eyes that underwent Alcon AcrySof® IQ Toric T6, T7, T8, or T9 IOL implantation (toric group); and (2) eyes that underwent the combined surgery of AcrySof® IQ Toric T5 IOL implantation and LRI (LRI group). Uncorrected visual acuity (UCVA), best-corrected visual acuity (BCVA), manifest, refractive and corneal cylinder (MC, RC, CC), were compared postoperatively. Corneal and ocular higher-order aberrations (HOA) were also compared. At 1 day postoperative, UCVA was significantly better and MC and RC were significantly less in the toric group, however, at 1 and 6 months postoperative, there was no significant difference in those parameters. Postoperative corneal and ocular HOA were significantly greater in the LRI Group. For correcting astigmatism in eyes with a high amount of preexisting astigmatism, high-cylinder toric IOL implantation achieves better clinical outcomes, especially in the early postoperative period, than the combined procedure of moderate-cylinder toric IOL implantation and LRI.
Keywords: toric intraocular lens, limbal relaxing incision, high-cylinder astigmatism, cataract surgery
Introduction
Until 2011 in the United States and Japan, the AcrySof® IQ Toric SN60 intraocular lens (IOL) (Alcon Laboratories, Inc., Fort Worth, TX, USA) model line-up consisted of models T3, T4, and T5, for which the maximum cylinder correction effect was 2.06 diopters (D) at the corneal plane. Hence, it was necessary for ophthalmologists to perform the combined surgery of toric IOL implantation and limbal relaxing incision (LRI) due to the shortcomings of the AcrySof® IQ Toric SN60AT5 (T5) for cases of astigmatism greater than 2.0D.1 However, in 2011, new higher-cylinder-power IOL models (AcrySof® IQ Toric SN60 T6, T7, T8, and T9; Alcon Laboratories, Inc.)2,3 became available in both the US and Japan, thus offering surgeons new options for treating eyes with high-amplitude astigmatism.
Several surgical options currently exist for the correction of astigmatism during cataract surgery, such as LRI or toric IOL implantation. There have been several studies comparing the various surgical methods for correcting astigmatism; eg, toric IOL and LRI,4 LRI and photoastigmatic keratectomy (PAK),5 and LRI and on-axis incision.6 However, no studies have compared the clinical outcomes of different surgical procedures for treating cataract patients with preexisting high-amplitude astigmatism.
The aim of this present study was to compare the clinical outcomes between the following two methods for correcting astigmatism in eyes with a high amount of preexisting corneal astigmatism: (1) AcrySof® IQ Toric T6 or higher IOL implantation alone, and (2) AcrySof® IQ Toric T5 IOL implantation combined with LRI.
Patients and methods
Patients
This retrospective, single center, nonrandomized, consecutive comparative, interventional clinical study involved 57 eyes of 47 patients with preexisting corneal astigmatism of 2.5D or greater. All eyes were operated on by a single surgeon (MO). Exclusion criteria were eyes with any pathologic features of the cornea, vitreous body, macular or optic nerves, or any anticipated difficulties with examination or analysis. The combined surgery of T5 toric IOL implantation and LRI was performed on 33 eyes (LRI group) between July 2009 and September 2011; and T6, T7, T8, or T9 implantation alone was performed on 24 eyes (toric group) between October 2011 and August 2012 to correct preexisting corneal astigmatism, because T6, T7, T8, and T9 first became commercially available in September 2011 in Japan. All procedures were approved by the Ethics Committee of Ouchi Eye Clinic, and informed consent was obtained from all subjects. The study was conducted in accordance with the tenets set forth in the Declaration of Helsinki.
IOL power calculation
The toric IOLs used in this study and their respective cylinder powers (in D) at the corneal plane were as follows: T5 (2.06D), T6 (2.57D), T7 (3.08D), T8 (3.60D), and T9 (4.11D). The spherical power of the specific IOL used in each case was calculated using the SRK/T formula. The axial length was measured using the IOL master (Carl Zeiss Meditec AG, Jena, Germany), and in patients with a dense cataract, an ultrasonic applanation biometer (AL-2000; TOMEY Co., Ltd., Nagoya, Japan) was used. Keratometry readings were obtained by use an auto kerato-refractometer (ARK-560A; NIDEK Co., Ltd., Gamagori, Japan), with the keratometry readings being used to calculate the power and axis of the specific toric IOL. Calculation of the toric IOL power and axis placement was performed by use of the Alcon Laboratories Web Toric Calculator (Alcon Laboratories, Inc.). In the LRI group, estimated residual astigmatism after T5 implantation was recorded.
Outcome measures
In all eyes, uncorrected visual acuity (UCVA) and best corrected visual acuity (BCVA), manifest cylinder (MC), refractive cylinder (RC), and corneal cylinder (CC) were examined prior to surgery and at 1 day, 1 month, and 6 months postoperatively. MC was estimated with cylinder lens in BCVA, RC was estimated with auto refractometer readings, and CC was estimated with auto keratometer readings. Decimal visual acuity was converted to the Logarithm of Minimum Angle of Resolution (logMAR) scale for statistical analyses. The RC power, corneal astigmatism, and axis were examined by use of the above-described autokerato-refractometer used for the preoperative examination. The UCVA and BCVA were examined using decimal charts and converted to the logMAR scale for statistical analysis.
Corneal and ocular wavefront aberrations were measured and analyzed at 6 months postoperatively using wavefront aberrometry (OPD-Scan II ARK-10000; NIDEK Co., Ltd., Gamagori, Japan), after the pupil was pharmacologically dilated. Wavefront aberration from the anterior cornea and whole eye was calculated for the central 4 mm zone. The parameters analyzed included the root mean square (RMS) of higher-order aberrations (HOA) from the third to sixth orders; RMS of the spherical aberrations (square root of the sum of the squared coefficients of Z40 and Z60); and RMS of the coma aberrations (square root of the sum of the squared coefficients of Z3−1, Z31, Z5−1, and Z51).
The orientation axis of the toric IOL was also determined at the postoperative period using the vertical-arm reading of a slit-lamp biomicroscope (Slit Lamp BQ-900®; Haag-Streit AG, Koeniz, Switzerland) with the patient sitting in the upright position.
Surgical technique
Prior to surgery, 90° and 270° positions were marked on the corneal limbus with the patient seated in the upright position at the slit-lamp biomicroscope. At the beginning of the surgery, the 90° and 270° scale marks of the corneal gauge for toric IOL (Duckworth & Kent Ltd., Letchworth Garden, UK) were aligned with those marks, with the steepest meridian of the corneal limbus then being identified and marked using an Axis Marker for toric IOL (Duckworth & Kent Ltd.) in the toric group or using the Fukuyama LRI 60° marker (ASICO) in the LRI group. Two 0.9 mm corneal incisions were then made; one incision each at the 10- and 2-o’clock positions, respectively, using a 0.9 mm MVR knife (EdgeAhead Stiletto; Becton, Dickinson and Company, Franklin Lakes, NJ, USA). Bimanual phacoemulsification was performed using a 22-gauge (G) phacoemulsification needle and an Agarwal 22-G irrigating chopper (Microsurgical Technology, Inc., Redmond, WA, USA) through the 0.9 mm corneal incision. After the removal of the lens, the following group-specific procedures were performed: In the toric group, the AcrySof® IQ Toric IOL (T6, T7, T8, or T9) was then inserted via the newly created 2.2 mm incision located at the 90° position by use of an IOL injector (MONARCH® II; Alcon Laboratories, Inc.) using the wound-assisted technique.7 After the IOL insertion, the ophthalmic viscosurgical device was removed and the IOL was rotated into the final position under irrigation using the 22G irrigating chopper and a Sinskey hook. In the LRI group, the LRIs for the estimated residual astigmatism after T5 implantation were made at 1 mm inside the limbus at a preplanned depth and angle depending on the degree of astigmatism and our original patient-age-dictated nomogram (Table 1) for Japanese eyes, a modified version of the Nichamin’s nomogram.8 After making one pair of LRIs, a new, 2.2 mm, clear corneal incision was made at an orthogonalized position to the LRI, a position that was apart from, and did not affect, the pair of LRIs (Figure 1). The AcrySof® IQ T5 IOL was then inserted and rotated into the final position in the same fashion as was done in the toric group.
Table 1.
Nomogram for limbal relaxing incision (LRI) to correct estimated residual astigmatism after AcrySof® IQ Toric T5 intraocular lens (IOL) implantation
| Degree of arc to be incised
| ||
|---|---|---|
| Cylinder (in diopters) | Against-the-rule and oblique | With-the-rule |
| 0.75 | 30 | 50 |
| 1.00 | 40 | 60 |
| 1.50 | 50 | 70 |
| 2.00 | 60 | 80 |
| ≥3.00 | 90 | 100 |
| Patient age | Depth to be incised | |
| Under 70 years | 90% of the central corneal thickness | |
| 70 to 80 years | 85% of the central corneal thickness | |
| Over 80 years | 80% of the central corneal thickness | |
Note: AcrySof® IQ Toric T5 intraocular lens (Alcon Laboratories, Inc., Fort Worth, TX, USA).
Figure 1.
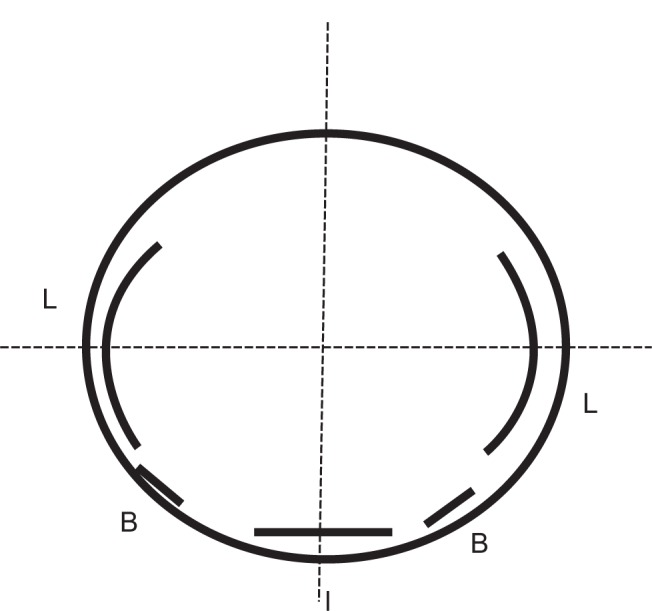
Diagram of combined surgery of limbal relaxing incision (LRI) and toric intraocular lens (IOL) implantation.
Notes: Bimanual phacoemulsification was performed through the two 0.9 mm corneal incisions (B). The LRIs for the estimated residual astigmatism after T5 implantation were made (L). After making one pair of LRIs, a new, 2.2 mm, clear corneal incision was made at an orthogonalized position to the LRI (I).
Selection of the toric IOL style (T6 to T9) in the toric group, estimated residual astigmatism after T5 implantation in the LRI group, and axis placement in both groups were calculated by Alcon web toric calculator following calculation of surgically induced astigmatism using the online “doctor-hill.com” Surgically Induced Astigmatism Calculator.9
Statistical analysis
The Welch’s t-test was used to compare patient age, visual acuity, subjective and objective cylinder, or other continuous variables. Discrete variables between groups were compared using the Fisher’s exact probability test. A P-value of <0.05 was considered statistically significant.
Results
All 57 eyes completed the scheduled examinations. The baseline clinical data of the toric group and LRI group is shown in Table 2. No significant differences in age, sex, preoperative UCVA, BCVA, MC, RC, or CC were observed between the two groups.
Table 2.
Baseline data of the LRI group and toric group patients
| LRI group | Toric group | P-value | |
|---|---|---|---|
| Patient age | 72.4±11.9 | 77.4±11.3 | P=0.18 |
| Patient sex (M/F) | 15:18 | 11:13 | P=0.62 |
| UCVA (logMAR) | 0.18 (0.74±0.41) | 0.09 (1.05±0.54) | P=0.32 |
| BCVA (logMAR) | 0.42 (0.37±0.41) | 0.28 (0.55±0.54) | P=0.72 |
| Manifest spherical (D) | −0.52±3.45 | −2.09±4.81 | P=0.50 |
| Equivalent (range) | (−7.0∼5.0) | (−12.75∼4.75) | |
| Manifest cylinder (D) | 3.53±1.92 | 3.33±1.30 | P=0.71 |
| Refractive cylinder (D) | 3.98±1.92 | 3.80±1.42 | P=0.45 |
| Corneal cylinder (D) | 3.07±1.28 | 3.16±1.21 | P=0.47 |
Abbreviations: LRI, limbal relaxing incision; UCVA, uncorrected visual acuity; BCVA, best-corrected visual acuity; D, diopters; logMAR, Logarithm of Minimum Angle of Resolution; M/F, male/female.
The UCVA and BCVA of each examination period are shown in Figure 2. As is shown in the figure, UCVA was significantly better in the toric group at 1 day postoperative, and a similar tendency was observed up until 1 month postoperatively although no difference was seen (P=0.056). However, no difference was observed at 6 months postoperatively (Figure 2A). BCVA was also better in the toric group at 1 day postoperatively, although no statistical significance was observed (P=0.07) (Figure 2B).
Figure 2.

Pre- and post-operative uncorrected visual acuity (UCVA) and best-corrected visual acuity (BCVA).
Notes: (A) UCVA is significantly better in the toric group than in the LRI group at 1 day postoperative (*P=0.04). (B) BCVA is better in the toric group than in the LRI group at 1 day postoperative, although no statistical significance was observed (*P=0.04).
Abbreviations: LRI, limbal relaxing incision; pre, preoperative; 1D, 1 day postoperatively; 1M, 1 month postoperatively; 6M, 6 months postoperatively; logMAR, Logarithm of Minimum Angle of Resolution.
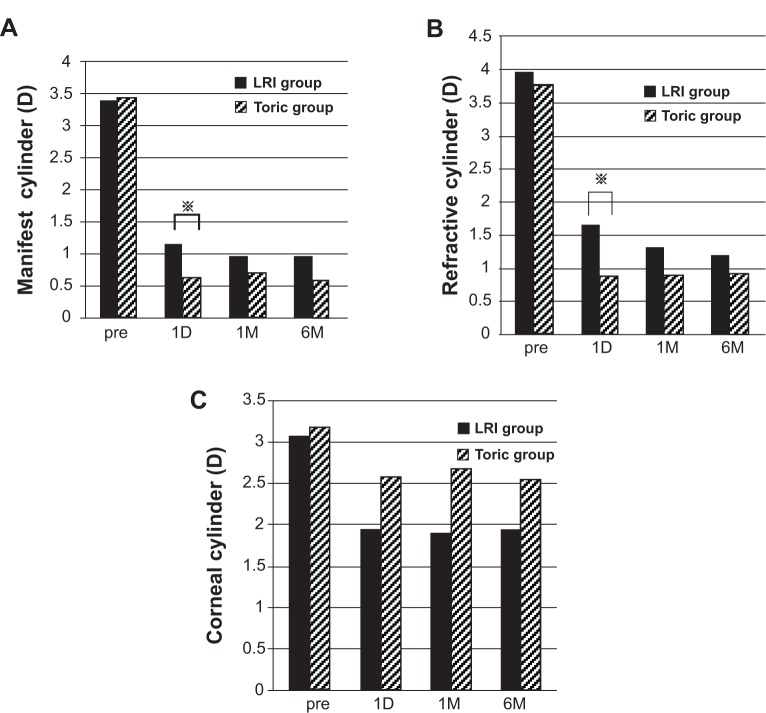
MC was less in the toric group than in the LRI group at all postoperative periods, and statistical significance was found at 1 day postoperatively (P=0.05) (Figure 3A). RC was less in the toric group than in the LRI group at all postoperative periods, and a statistical significance was found at 1 day postoperatively (P=0.005) (Figure 3B). The mean corneal astigmatism in the LRI group decreased to approximately 2D postoperatively, while the corneal astigmatism in the toric group did not change significantly after surgery (Figure 3C).
Figure 3.
Pre- and post-operative cylinder.
Notes: (A) Pre- and post-operative manifest cylinder (MC). Postoperative MC was found to be significantly larger in the LRI group than in the toric group at 1 day postoperatively (*P=0.05). (B) Pre- and post-operative refractive cylinder (RC). Postoperative RC was found to be significantly larger in the LRI group than in the toric group at 1 day postoperatively (*P=0.02). (C) Pre- and post-operative corneal cylinder (CC). The mean CC in the LRI group decreased to almost 2 diopters (D) at 1 day postoperatively up through 6 months postoperatively, although the mean CC in the toric group was of similar value at the preoperative period and throughout all postoperative periods.
Abbreviations: LRI, limbal relaxing incision; pre, preoperative; 1D, 1 day postoperatively; 1M, 1 month postoperatively; 6M, 6 months postoperatively.
Postoperative corneal HOA and coma aberration registered significantly higher in the LRI group than in the toric group, although no significant difference was seen preoperatively between the two groups (Figure 4A and B). Moreover, postoperative ocular HOA and coma aberration were significantly higher in the LRI group than in the toric group (Figure 4C). Change in corneal astigmatism between 1 day and 1 month postoperatively as determined by using Jaffe’s vector analysis11 was significantly larger in the LRI group than in the toric group (LRI group: 0.84±0.61, toric group: 0.45±0.23D, P=0.01). No significant difference was seen in the error of the orientation axis of the toric IOL between the groups (LRI group: 6.27±4.48 degrees, toric group: 4.46±7.04 degrees, P=0.41).
Figure 4.
Pre- and post-operative HOA.
Notes: (A) Preoperative corneal aberrations. (B) Postoperative corneal aberrations. (C) Postoperative ocular aberrations. HOA: RMS of HOA from the third to sixth orders. SA: RMS of the spherical aberration (square root of the sum of the squared coefficients of Z40 and Z60). Coma: RMS of the coma aberrations (square root of the sum of the squared coefficients of Z3−1, Z31 Z5−1, and Z51).
Abbreviations: HOA, higher-order aberrations; LRI, limbal relaxing incision; RMS, root mean square; SA, spherical aberration.
Discussion
The findings of the present study demonstrated that although both groups achieved the same good results in eyes with high-amplitude astigmatism at 6 months postoperatively, the toric group achieved better UCVA and lower residual RC and MC errors than did the LRI group in the early postoperative period. Moreover, ocular total HOA and ocular coma aberration were greater in the LRI group, even at 6 months postoperatively.
In addition, a relatively large change in corneal astigmatism was observed through the early postoperative period in the LRI group, although stable results were seen in the toric group throughout the postoperative period. Thus, prompt stabilization of corneal curvature might have contributed to the better visual acuity and lesser residual astigmatism of the toric group in the early postoperative period.
Fouda et al reported that subsequent PAK achieved less residual astigmatism than LRI during cataract surgery, but caused greater HOA than LRI, and prompted the necessity of a secondary operation in PAK after IOL implantation.5 According to Kaufmann et al greater astigmatism-correcting effect was achieved by LRI than by on-axis incision, but in that report, the mean postoperative RC remained at 1.5D, whereas the mean preoperative cylinder was 2.0D in the LRI group.6 Mingo-Botín et al described the comparison of LRI and toric IOL and reported that cases of toric IOL implantation had better refractive outcomes with favorable predictability and less spectacle dependence.4 However, their study was conducted before release of high-cylinder models, and the mean preoperative cylinder in their study was 1.89D. In regard to high-power toric IOLs, Hoffmann et al reported the clinical outcomes of AcrySof IQ Toric T6 to T9, and the findings in that study were similar to those of the toric group in our study.12 In that report, they stated that high-power toric IOLs can reduce preexisting high-amplitude corneal astigmatism with good predictability. However, to the best of our knowledge, no previous study has compared the clinical results of different surgical methods for treating cataract patients with preexisting high-amplitude corneal astigmatism.
Numerous human-related factors such as the alignment of eye rotation, limbal marking, and alignment of the toric axis affect the clinical results of astigmatism correction during cataract surgery, thus possibly explaining why the simpler surgical procedure of performing the high-cylinder-style toric IOL implantation alone achieved better and more stable clinical results. On the other hand, misalignment of the high-cylinder toric IOL itself might be a critical error, for in the LRI group, even if there was misalignment of the IOL, a correctly performed LRI still provided correction; thus, the risk of a major critical error is decreased. However, it should be noted that there are certain negative aspects associated with LRI, such as that it involves a degree of uncertainty due to the difficulty of performing an equally relaxing incision, or that a relatively long amount of time is needed until stabilization of the correcting effect is acquired. In fact, there was a large increase in VA in the LRI group at 1 day postoperative, yet the VA continued to increase throughout the 6-month postoperative period, thus indicating that more than 1 month is required for stabilization of the surgical effect of LRI. However, the advantage of the procedure used in the toric group is that final refraction can be achieved immediately at 1 day postoperatively.
The significant difference in HOA between the two groups, which was seen even at the 6 month follow-up, is also notable. Greater HOA has a significant adverse effect on visual function; however, this adverse effect is not apparent in terms of visual acuity.13 It is important to note that this present study did have some limitations, as the LRI group operations were performed in the first half of the study period and the toric group operations were performed in the latter half, so it is possible that a learning curve on the part of the surgeon may have affected the results. However, no significant differences were observed in relation to error of the orientation axis of the toric IOL in the postoperative examinations.
In conclusion, the findings of this study show that for the treatment of cataract patients with a high amount of astigmatism greater than 2.5D, AcrySof® IQ Toric T6, T7, T8, and T9 implantation can achieve better clinical outcomes, especially in the early postoperative period, than the combined procedure of AcrySof® IQ Toric T5 implantation and LRI.
Footnotes
Disclosure
The author declares no conflicts of interest in this work.
References
- 1.Ouchi M, Kinoshita S. AcrySof IQ Toric IOL implantation combined with limbal relaxing incision during cataract surgery for eyes with astigmatism >2.5D. J Refractive Surg. 2011;27(9):643–647. doi: 10.3928/1081597X-20110317-03. [DOI] [PubMed] [Google Scholar]
- 2.Cervantes-Coste G, Garcia-Ramirez L, Mendoza-Schuster E, Velasco-Barona C. High-cylinder acrylic toric intraocular lenses: a case series of eyes with cataracts and large amounts of corneal astigmatism. J Refract Surg. 2012;28(4):302–304. doi: 10.3928/1081597X-20120208-06. [DOI] [PubMed] [Google Scholar]
- 3.Visser N, Ruíz-Mesa R, Pastor F, Bauer NJ, Nuijts RM, Montés-Micó R. Cataract surgery with toric intraocular lens implantation in patients with high corneal astigmatism. J Cataract Refract Surg. 2011;37:1403–1410. doi: 10.1016/j.jcrs.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 4.Mingo-Botín D, Muñoz-Negrete FJ, Won Kim HR, Morcillo-Laiz R, Rebolleda G, Oblanca N. Comparison of toric intraocular lenses and peripheral corneal relaxing incisions to treat astigmatism during cataract surgery. J Cataract Refract Surg. 2010;36(10):1700–1708. doi: 10.1016/j.jcrs.2010.04.043. [DOI] [PubMed] [Google Scholar]
- 5.Fouda S, Kamiya K, Aizawa D, Shimizu K. Limbal relaxing incision during cataract extraction versus photoastigmatic keratectomy after cataract extraction in controlling pre-existing corneal astigmatism. Graefes Arch Clin Exp Ophthalmol. 2010;248(7):1029–1035. doi: 10.1007/s00417-009-1272-6. [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann C, Peter J, Ooi K, Phipps S, Cooper P, Goggin M. Queen Elizabeth Astigmatism Study Group: limbal relaxing incisions versus on-axis incisions to reduce corneal astigmatism at the time of cataract surgery. J Cataract Refract Surg. 2005;31:2261–2265. doi: 10.1016/j.jcrs.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 7.Tsuneoka H, Hayama A, Takahama M. Ultrasmall-incision bimanual phacoemulsification and AcrySof SA30AL implantation through a 2.2 mm incision. J Cataract Refract Surg. 2003;29:1070–1076. doi: 10.1016/s0886-3350(03)00076-2. [DOI] [PubMed] [Google Scholar]
- 8.Nichamin LD. Correction of keratometric astigmatism: incisional surgery. In: Fine IH, Packer M, Hoffman RS, editors. Refractive Lens Surgery. Springer-Verlag; New York: 2005. pp. 49–57. [Google Scholar]
- 9.AcrySof Toric Web Based Calculators. [Accessed March 21, 2014]. Available at: http://www.acrysoftoriccalculator.com.
- 10.doctor-hill.com surgically induced astigmatism calculator. [Accessed January 25, 2014]. Available at: www.sia-calculator.com.
- 11.Jaffe NS, Clayman HM. The pathophysiology of corneal astigmatism after cataract extraction. Trans Am Ophthalmol Soc. 1975;79:615–630. [Google Scholar]
- 12.Hoffmann PC, Auel S, Hütz WW. Results of higher power toric intraocular lens implantation. J Cataract Refract Surg. 2011;37:1411–1418. doi: 10.1016/j.jcrs.2011.02.028. [DOI] [PubMed] [Google Scholar]
- 13.Fernández-Sánchez V, Ponce ME, Lara F, Montés-Micó R, Castejón-Mochón JF, López-Gil N. Effect of 3rd-order aberrations on human vision. J Cataract Refract Surg. 2008;34:1339–1344. doi: 10.1016/j.jcrs.2008.04.017. [DOI] [PubMed] [Google Scholar]