Abstract
Iodine is a micronutrient that is essential for the production of thyroid hormones. The primary source of iodine is the diet via consumption of foods that have been fortified with iodine, including salt, dairy products and bread, or that are naturally abundant in the micronutrient, such as seafood. Recommended daily iodine intake is 150 μg in adults who are not pregnant or lactating. Ingestion of iodine or exposure above this threshold is generally well-tolerated. However, in certain susceptible individuals, including those with pre-existing thyroid disease, the elderly, fetuses and neonates, or patients with other risk factors, the risk of developing iodine-induced thyroid dysfunction might be increased. Hypothyroidism or hyperthyroidism as a result of supraphysiologic iodine exposure might be either subclinical or overt, and the source of the excess iodine might not be readily apparent.
Introduction
Iodine (atomic weight 126.9 g per atom) is a micronutrient that is required for the synthesis of the thyroid hormones. It is a trace element in Earth’s upper crust and is found primarily in or near coastal areas. For adults who are not lactating or pregnant, the US Institute of Medicine, and jointly by the WHO, United Nations Children’s Fund (UNICEF) and the International Council for the Control of Iodine Deficiency Disorders (ICCIDD), recommend a daily iodine intake of 150 μg and state a tolerable upper level (the approximate threshold below which notable adverse effects are unlikely to occur in the healthy population) of 1,100 μg per day in adults.1,2
However, iodine is present in concentrations up to several thousand-fold higher than these amounts in medications, supplements and in the iodinated contrast agents used for radiologic studies (Box 1). In some susceptible individuals, the use of these iodine-containing substances can result in thyroid dysfunction as a result of the high iodine load. In certain circumstances, iodine excess can result in adverse thyroidal effects after only a single exposure to an iodine-rich substance.
Box 1. Sources of iodine exposure and potential excess.
Diet
Other sources
Vitamins (prenatal, labelled content per daily serving): 75–200 μg46
Amiodarone (per 200 mg): 75,000 μg
Iodinated contrast (free iodine content, per CT scan): 13,500 μg
Topical iodine (povidone iodine): variable, usually 1–5%
Expectorants, mouthwashes, vaginal douches: variable
Saturated solution of potassium iodide (per drop): 50,000 μg
Measures of iodine excess
Overall iodine levels cannot be reliably measured in individuals given the considerable day-to-day variation in iodine intake.3 Instead, median urinary iodine concentrations (UIC) have been widely used as a biomarker of population iodine intake, with levels >300 μg/l considered excessive in children and adults and levels >500 μg/l considered excessive in pregnant women.2
In dried blood spots from children, the concentration of thyroglobulin correlates with iodine exposure and could be a novel marker for monitoring population iodine status in this age group.4 In 2006, the international reference range of 4–40 μg/l for this assay was developed as a measure that indicates iodine sufficiency in children 5–14 years old.4 This standard has since been adopted and recommended by the WHO, UNICEF and the ICCIDD as a method of assessing iodine nutrition in school-aged children (≥6 years old).2 One study demonstrated that the mean concentration of thyroglobulin in dried blood spots was statistically significantly higher in healthy children aged 6–12 years in whom the median UIC was >300 μg/l than in those with lower UICs.5 These findings suggest that levels of thyroglobulin in dried blood spots could be developed as a sensitive marker of iodine excess in this population.5
Thyroidal adaptation to excess iodine
The acute Wolff–Chaikoff effect was described in 1948 by Drs Jan Wolff and Israel Lyon Chaikoff at the University of California Berkeley, USA.6 Wolff and Chaikoff observed a transient reduction (lasting ~24 h) in the synthesis of thyroid hormones in rats exposed to high amounts of iodide administered intraperitoneally. The mechanism for the acute Wolff–Chaikoff effect is not completely understood, but is thought to be at least partially explained by the generation of several inhibitory substances (such as intrathyroidal iodolactones, iodoaldehydes and/or iodolipids) on thyroid peroxidase activity.7 Reduced intrathyroidal deiodinase activity as a result of the increased iodine load might also contribute to decreased synthesis of thyroid hormones.
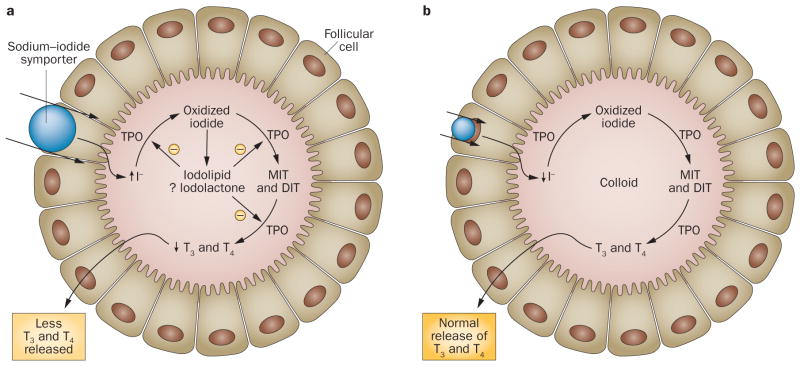
In most individuals, the decreased production of thyroid hormones is only transient and resumes after adaptation to the acute Wolff–Chaikoff effect.7 In rats, this adaptation is associated with a marked decrease in expression of the sodium–iodide symporter (NIS) that is present on the basolateral membrane of thyroid follicular cells.8 NIS is a 13-transmembrane glycoprotein that mediates the active transport of iodine from the circulation into the thyroid.9 The decrease in expression of the NIS occurs by 24 h after exposure to excess iodine and results in reduced intrathyroidal iodine concentrations. In turn, the reduced iodine levels lead to a decrease in levels of the iodinated substances that inhibit synthesis of thyroid hormones, which results in the resumption of normal production of thyroid hormone (Figure 1).
Figure 1.
The Wolff–Chaikoff effect. a | The proposed mechanism for the acute Wolff–Chaikoff effect. During initial iodine exposure, excess iodine is transported into the thyroid gland by the sodium–iodide symporter. This transport results in transient inhibition of TPO and a decrease in the synthesis of thyroid hormone. b | The mechanism by which adaptation to the acute Wolff–Chaikoff effect occurs. A decrease in the expression of the sodium–iodide symporter results in reduced iodine transport, which enables the synthesis of thyroid hormone to resume. Abbreviations: DIT, diiodotyrosine; I−, iodide; MIT, monoiodotyrosine; TPO, thyroid peroxidase. Permission obtained from Massachusetts Medical Society © Pramyothin, P. et al. N. Engl. J. Med. 365, 2123–2127 (2011).
In individuals with dysregulation of the thyroid follicular cell, excess iodine exposure can induce thyroid dysfunction, which might be transient or permanent.7
Iodine-induced hypothyroidism
Vulnerable patients with specific risk factors might have an increased risk of failing to adapt to the acute Wolff–Chaikoff effect.7 Susceptible patients include those with autoimmune thyroid disease; a previous history of surgery, 131I or antithyroid drug therapy for Graves disease; subacute thyroiditis; postpartum thyroiditis; type 2 amiodarone-induced thyrotoxicosis (AIT); hemithyroidectomy; IFNα therapy; and concomitant use of potential goitrogens, such as lithium. Failure to escape from the acute Wolff–Chaikoff effect might also be more likely during fetal development, a period when the hypothalamic–pituitary–thyroid axis is still immature, and during neonatal life.
The underlying mechanism of iodine-induced hypothyroidism remains unclear, but could be attributable to failure to adapt to the acute Wolff–Chaikoff effect, probably because of a damaged thyroid as a result of previous pathological insults. Exposure to high concentrations of iodine might also decrease the release of thyroid hormone, as reported in several small studies that show mild decreases in serum levels of thyroid hormone and increases in the serum level of TSH to the upper limit of the normal range.10–12 Administration of iodine to patients with severe hyperthyroidism or thyroid storm is efficacious, as it results in an acute decrease in the release of thyroid hormones.13
Iodine-induced hyperthyroidism
In some susceptible patients, an excess iodine load provides a rich substrate for increased production of thyroid hormones. Iodine-induced hyperthyroidism (the Jod–Basedow phenomenon) was first described in the early 1800s, when thyrotoxicosis was observed to be more common among patients with endemic goiter treated with iodine supplementation than in individuals without goiter.14 Iodine-induced hyperthyroidism might be transient or permanent, and risk factors include nontoxic or diffuse nodular goiter, latent Graves disease and long standing iodine deficiency.7 In addition, iodine-induced hyperthyroidism in euthyroid patients with nodular goiter in iodine-sufficient areas has also been reported when iodine supplementation is excessive.15
Sources of iodine excess
Iodine supplementation
Globally, iodine supplementation has been the primary method over the past century to decrease iodine deficiency, which is the leading cause of preventable mental retardation.16 Iodine has been administrated as iodized oil orally and intramuscularly, introduced into the water supply, used in crop irrigation, incorporated into animal fodder and introduced into food through salt iodization, bread iodophors and other products.17 Fortified micronutrient biscuits have also been successfully used to raise the median UICs of schoolgirls (aged 10–15 years) in India.18
Although iodine supplementation has decreased the number of people at risk of iodine deficiency and its associated sequelae, particularly in the past few decades, the use of iodine has also led to concerns of excessive iodine exposure in some individuals. A study published in 2012 reported that the median UIC (730 μg/l) of the populations of two Somali refugee camps in Kenya who were receiving iodine supplementation were in the range consistent with excessive iodine intake.19 In a study of >200 Chinese adults, subclinical hypothyroidism was more common in those supplemented with a 400 μg iodine tablet than in those given placebo.20 These findings are similar to the results of other studies in Denmark21 and New Zealand,22 which also showed an increased prevalence of transient hyperthyroidism. The incidence of thyrotoxicosis was increased following periods of mandatory salt iodization, compared with when supplementation was not required, in both Spain23 and Zimbabwe.24 Another iodine supplementation programme in Bangladesh has shown no increased risk of thyroid dysfunction.25
Iodine supplementation also affects other aspects of thyroid health. For example, high iodine intake seems to increase the prevalence of autoimmune thyroiditis in the Bio Breeding/Worcester rat model and in humans.26,27 The number of reported cases of thyroid cancer, particularly papillary thyroid cancer, has also increased following iodine supplementation in some studies, including a nearly 20-year study in northeastern China28 and a >50-year study in Denmark.29
Diet
The diet is the main way of achieving adequate iodine nutrition. Dairy products (due to the use of iodophor cleaners for milk cans and teats), some breads (due to the use of iodate bread conditioners), seaweed and other seafood and iodized salt are the most common iodine-containing foods.30 In children from the USA aged 6–12 years, dairy intake is a particularly good source of adequate iodine, probably due to the abundance of dairy content in the diet of children in this age range.31 Australia introduced iodization of bread in 2009, which resulted in a modest increase in the median UIC of a small group of pregnant women.32 The amount of iodine that can be obtained from plant-based food is minimal and is dependent on the local environment (that is, iodine levels in the soil, groundwater used for irrigation, crop fertilizers and livestock feed).33
The many varieties of seaweed are a unique potential source of excess ingestion of iodine. Seaweed is a popular food item in many parts of the world, particularly in Japan34 and other Asian countries, where ingestion of seaweed soup is common in the everyday diet and is a frequent practice during the postpartum period.35 However, the iodine content of seaweed can vary widely.36 Cases of kelp-induced thyrotoxicosis have been widely reported, including one woman who drank kelp-containing tea for 4 weeks37 and another patient who had a long-standing history of using kelp-containing dietary supplements.38 In other reports, chronic seaweed ingestion has also been reported to be associated with a modest increase in serum levels of TSH without overt thyroid dysfunction.39,40
The iodine-rich content of the Japanese diet is unique and demonstrates how chronic exposure to excess iodine could result in several adaptive mechanisms. A 2013 paper reported a case of delayed onset congenital hypothyroidism in an infant with a mutation in the gene that encodes dual oxidase (DUOX2), an enzyme known to be associated with transient congenital hypothyroidism, that was exacerbated by the infant’s mother ingesting large amounts of seaweed during pregnancy.41 Among Japanese schoolchildren (aged 6–12 years old), high UIC are associated with smaller thyroid glands than are found in children living in other iodine-sufficient areas.42 A comparison of individuals with negative thyroid antibody titres living in coastal regions versus noncoastal areas of Japan showed that people from areas in which iodine-rich seaweed is abundant have an increased prevalence of hypothyroidism (12.1% versus 2.3%).43 Finally, a small study in Japan found that consumption of seaweed was positively associated with an increased risk of papillary thyroid cancers in postmenopausal women (HR 1.71, 95% CI 1.01–2.90).44 The high iodine intake in Japan has probably reduced the adverse effects of the 2011 Fukushima reactor accident on thyroid function and radiation-induced thyroid cancer, as the uptake of radioactive iodine is inversely proportional to the ambient iodine intake.
Salt iodization is viewed as one of the safest and most effective methods of achieving iodine sufficiency across a population.16 Iodine fortification of all food-grade salt is mandated in ~120 countries, although the enforcement and degree of implementation of these efforts in individual countries are unknown.45 Although salt iodization is not obligatory in the USA and the FDA does not require that iodine content is listed on food labels, it is reassuring that >90% of households in the USA have access to iodized salt.16 Salt is not generally considered to be a source of excess iodine, although it is one of the most widely available iodine-containing foods.
Vitamins and supplements
The iodine content of multivitamins is not uniform.46 Supplements in the USA are not regulated by the FDA to the same rigorous standards as FDA-approved medications; therefore, their actual contents might not match the labelled content. In a US study published in 2009, iodine (as potassium iodide or kelp) was a labelled ingredient in only 51% of 223 prenatal nonprescription and prescription multivitamins.46 However, among the 25 brands containing iodine derived from kelp, measured values (33–610 μg per daily dose) were frequently discordant with the labelled values (75–300 μg per daily dose), including 13 brands with a >50% discrepancy between the measured and labelled values. Thus, potassium iodide, and not kelp, should be used in vitamin preparations.
Several cases of congenital hypothyroidism caused by ingestion of excess maternal iodine tablets during pregnancy have been reported.47 Similarly, hypothyroidism in neonates born to mothers who ingested excessive amounts of seaweed or seaweed soup during both pregnancy48 and lactation have been reported.49,50 Given the risks of potential iodine-induced thyroid dysfunction, the American Thyroid Association recommends against ingestion of an iodine or kelp daily supplement containing >500 μg iodine for all individuals, except for certain medical indications.51
Medications
Amiodarone, an iodine-rich medication used in the management of ventricular and supraventricular tachyarrhythmias, is probably the most important and common source of medication-induced thyroid dysfunction. Amiodarone is 37% iodine by weight and has some structural resemblance to the thyroid hormones T3 and T4. Thus, one 200 mg tablet of amiodarone contains 75 mg iodine, which is several hundred-fold higher than the recommended daily intake of 150 μg in adults. The drug has a long half-life of ~100 days, is very lipophilic and accumulates in various tissues, including adipose tissue, liver and the lungs. As a result of its high iodine content, use of amiodarone is associated with thyrotoxicosis in 9.6% of patients with low ambient iodine intake, and hypothyroidism in 22.0% of patients with normal ambient iodine intake.52 Overall, amiodarone-induced hyperthyroidism is more common in iodine-deficient areas, whilst amiodarone-induced hypothyroidism is more common in iodine-sufficient areas.
Two types of AIT have been described: type 1 AIT is associated with increased synthesis of thyroid hormones, whereas type 2 AIT is characterized by destructive thyroiditis. Both occur approximately three times more frequently in men than in women, which is in contrast to amiodarone-induced hypothyroidism.53 A comprehensive update on this topic was published in 2012.54 Type 1 AIT is a form of the Jod–Basedow phenomenon, in which excess iodine exposure results in thyroid autonomy as a result of altered thyroid function regulation. Type 1 AIT is treated with thionamides, β-blockers, and if available, perchlorate; corticosteroids could also be used if the initial treatment is not rapidly effective. By contrast, type 2 AIT is characterized by parts of the thyroid gland being destroyed, which results in thyroid hormones leaking into the circulation. Type 2 AIT is usually managed with corticosteroids; one trial has demonstrated that treatment with perchlorate did not improve the likelihood of restoring euthyroidism.55 In severe cases of AIT, thyroidectomy might be required,56 with the use of iopanoic acid, if available, for the rapid control of hyperthyroidism before the surgery.57 The discontinuation of amiodarone might be considered, if this strategy is tolerated by the patient and in collaborative discussion with the patient’s cardiologist.
Some patients present with a mixed form of AIT, displaying features of both type 1 AIT and type 2 AIT.58 Differentiating between type 1 AIT and type 2 AIT can be difficult, but the use of radioiodine uptake and colour flow Doppler ultrasonography has been proposed.58 Finally, the risk of AIT might be further increased in some patients who are concomitantly treated with warfarin, due to a variety of mechanisms that can occur in patients who receive amiodarone that result in the potentiation of the effects of warfarin.59
Radiologic studies
Use of iodinated contrast agents in diagnostic radiologic studies is a common source of excess iodine exposure in many patients.60 A single dose of iodinated contrast can contain up to 13,500 μg of free iodine and 15–60 g of bound iodine (which is more than several thousand times above the recommended daily intake).60 Following exposure to an iodinated contrast agent, iodine stores in the body remain raised and provide a continuous pool that can potentially induce thyroid dysfunction. In euthyroid, healthy adults from the USA without previous thyroid or renal disease, UIC did not return to baseline in a study with a small number of participants until a median of 43 days following exposure to the contrast agent.61 In another study of patients with athyreosis in Brazil, UICs did not normalize until 1 month after receiving a single intravenous contrast dose given for a CT scan.62
Several case reports have demonstrated the effects of thyroid dysfunction arising after use of an iodinated contrast agent. For example, a 53-year-old woman in the USA developed thyroid storm and cardiopulmonary arrest immediately after undergoing an iodinated radiologic study.63 Two studies in Germany and the USA showed that a small proportion of patients who received either coronary angiography or an iodinated CT scan developed subclinical hypothyroidism ~1 week after the exam.64,65 A Turkish study of 101 patients who underwent coronary angiography found a small increased risk of subclinical hyperthyroidism at up to 8 weeks after the iodine exposure.66 However, one small study showed that intravenous administration of an iodine contrast agent during pregnancy did not result in a notably increased incidence of fetal thyroid dysfunction.67 Finally, we have reported the occurrence of iodine-induced hypothyroidism in three neonates who received intravenous contrast agents to evaluate congenital cardiac defects.68
One of the most rigorous studies that has examined the association between iodinated contrast use and thyroid dysfunction was a large case–control study that used medical records from two hospitals in Boston, USA, over a 20-year period.60 In this study, patients without pre-existing hypothyroidism or hyperthyroidism who received a single iodinated contrast dose had a 2–3-fold increased risk of developing either incident hyperthyroidism (including overt hyperthyroidism) or overt hypothyroidism at a median of 9 months following exposure, compared with patients who did not receive the high iodine load.
Iodine-induced thyroid dysfunction is a potential consequence in patients with nodular goiters and/or elderly patients.69,70 Guidelines from the Contrast Media Safety Committee of the European Society of Urogenital Radiology advocate that high-risk patients are monitored for thyroid dysfunction following iodinated contrast use.71 No guidelines are currently available for screening or follow-up of at-risk patients receiving iodinated contrast in the USA.
Topical iodine
The use of transdermal iodine and thyroid dysfunction associated with this practice is often seen in hospitalized neonates.72 A study in Israel reported significantly higher serum levels of TSH in preterm neonates on whom topical iodinated antiseptic cleansers had been used than in preterm neonates on whom alcohol-based topical cleansers had been used (15.4 mIU/l versus 7.8 mIU/l, P<0.01).73 Iodine is also frequently used as a topical antiseptic in many surgical settings and for burn victims, whose ability to absorb topical iodine might be increased.74 Iodine-induced thyrotoxicosis has been described in a paraplegic woman in the USA who had applied topical povidone-iodine prior to urinary self-catheterization several times daily for many years.7
Other sources of excess iodine exposure
Other sources of potential excess iodine exposure include various expectorants, food preservatives, prescribed medications, parenteral nutrition preparations, mouthwashes75 and vaginal douches.11 Reversible increases in serum levels of TSH have been observed among US astronauts drinking iodinated water as purified drinking water76 and individuals ingesting water purified with iodinated tablets.77 In the 1990s, due to the use of a faulty iodine-based water filtration system, small increases in serum levels of TSH were detected in American Peace Corp workers in Niger; these changes resolved when the iodinated water source was no longer used.78
Indications for supraphysiologic iodine
Administration of supraphysiologic levels of iodine is medically appropriate in some very specific settings. A saturated solution of potassium iodide, of which the US generic formulation contains 1,000 mg potassium iodide per ml, can be used for the rapid treatment of hyperthyroidism, usually in patients with thyroid storm or in the preoperative period in patients with Graves disease, in conjunction with antithyroid drugs and other therapies.13 In such patients, pharmacologic doses of iodine should be administered after a thionamide has been given to block excess synthesis of thyroid hormones. Finally, individuals in the vicinity of a nuclear power plant can be directed to take potassium iodide in the event of a nuclear accident to prevent uptake of radioactive iodine into the thyroid, as was carried out in Poland following the Chernobyl nuclear accident.79
Conclusions
Iodine is required for the production of thyroid hormones. Iodine is primarily taken in through the diet, with the recommended amount at 150 μg per day in adults who are not pregnant or lactating. Although excess iodine exposure generally does not result in any apparent clinical consequences, thyroid dysfunction can occur in vulnerable patients with specific risk factors, including those with pre-existing thyroid disease, the elderly, fetuses and neonates. As iodine-induced hypothyroidism or hyperthyroidism might be either subclinical or overt, excess iodine exposure should be suspected if the aetiology of thyroid dysfunction is not discernible.
Key points.
Recommendations for iodine intake in adults who are not pregnant or lactating are 150 μg of iodine a day
Excess iodine exposure or ingestion can result in thyroid dysfunction in certain susceptible individuals, but is generally well-tolerated in most people
lodine-induced thyroid dysfunction might be subclinical or overt and either transient or permanent
Sources of iodine excess include iodine supplementation to prevent iodine deficiency at a population level, the diet, vitamins and supplements, medications, contrast media and topical iodine
Supraphysiologic doses of iodine are appropriate in certain specific medical indications, including its use in the treatment of severe hyperthyroidism before thyroid surgery and as potassium iodide following a nuclear accident
Review criteria.
PubMed was searched using the terms “iodine”, “excess”, “thyroid”, “hypothyroidism”, “hyperthyroidism”, “dysfunction”. No limits were set regarding year of publication, language, or type of paper.
Acknowledgments
A.M. Leung would like to acknowledge the support of NIH grant 7K23HD06855204.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
Both authors contributed equally to all aspects of this manuscript.
Contributor Information
Angela M. Leung, Division of Endocrinology, Department of Medicine, David Geffen School of Medicine, University of California Los Angeles, VA Greater Los Angeles Healthcare System, 11301 Wilshire Boulevard (111D), Los Angeles, CA 90073, USA
Lewis E. Braverman, Section of Endocrinology, Diabetes, and Nutrition, Department of Medicine, Boston University School of Medicine, 88 East Newton Street, Evans 201, Boston, MA 02118, USA
References
- 1.Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes. National Academy Press; Washington, D.C: 2006. pp. 320–327. [Google Scholar]
- 2.WHO, UNICEF and ICCIDD. Assessment of the iodine deficiency disorders and monitoring their elimination. 2007 WHO/NHD/01.1 [online], http://whqlibdoc.who.int/publications/2007/9789241595827eng.pdf.
- 3.Rasmussen LB, Ovesen L, Christiansen E. Day-to-day and within-day variation in urinary iodine excretion. Eur J Clin Nutr. 1999;53:401–407. doi: 10.1038/sj.ejcn.1600762. [DOI] [PubMed] [Google Scholar]
- 4.Zimmermann MB, et al. Assessment of iodine status using dried blood spot thyroglobulin: development of reference material and establishment of an international reference range in iodine-sufficient children. J Clin Endocrinol Metab. 2006;91:4881–4887. doi: 10.1210/jc.2006-1370. [DOI] [PubMed] [Google Scholar]
- 5.Zimmermann MB, et al. Thyroglobulin is a sensitive measure of both deficient and excess iodine intakes in children and indicates no adverse effects on thyroid function in the UIC range of 100–299 μg/l: a UNICEF/ICCIDD study group report. J Clin Endocrinol Metab. 2013;98:1271–1280. doi: 10.1210/jc.2012-3952. [DOI] [PubMed] [Google Scholar]
- 6.Wolff J, Chaikoff IL. Plasma inorganic iodide as a homeostatic regulator of thyroid function. J Biol Chem. 1948;174:555–564. [PubMed] [Google Scholar]
- 7.Pramyothin P, Leung AM, Pearce EN, Malabanan AO, Braverman LE. Clinical problem-solving. A hidden solution. N Engl J Med. 2011;365:2123–2127. doi: 10.1056/NEJMcps1008908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eng PH, et al. Escape from the acute Wolff–Chaikoff effect is associated with a decrease in thyroid sodium/iodide symporter messenger ribonucleic acid and protein. Endocrinology. 1999;140:3404–3410. doi: 10.1210/endo.140.8.6893. [DOI] [PubMed] [Google Scholar]
- 9.Dai G, Levy O, Carrasco N. Cloning and characterization of the thyroid iodide transporter. Nature. 1996;379:458–460. doi: 10.1038/379458a0. [DOI] [PubMed] [Google Scholar]
- 10.Saberi M, Utiger RD. Augmentation of thyrotropin responses to thyrotropin-releasing hormone following small decreases in serum thyroid hormone concentrations. J Clin Endocrinol Metab. 1975;40:435–441. doi: 10.1210/jcem-40-3-435. [DOI] [PubMed] [Google Scholar]
- 11.Safran M, Braverman LE. Effect of chronic douching with polyvinylpyrrolidone-iodine on iodine absorption and thyroid function. Obstet Gynecol. 1982;60:35–40. [PubMed] [Google Scholar]
- 12.Paul T, et al. The effect of small increases in dietary iodine on thyroid function in euthyroid subjects. Metabolism. 1988;37:121–124. doi: 10.1016/s0026-0495(98)90004-x. [DOI] [PubMed] [Google Scholar]
- 13.Bahn RS, et al. Hyperthyroidism and other causes of thyrotoxicosis: Management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid. 2011;21:593–646. doi: 10.1089/thy.2010.0417. [DOI] [PubMed] [Google Scholar]
- 14.Coindet JF. Nouvelles recherches sur les effets de l’iode, et sur les precautions a suivre dans le traitement de goitre par le nouveau remede [French] Ann Chim Phys. 1821;16:252–256. [Google Scholar]
- 15.Vagenakis AG, et al. Iodide-induced thyrotoxicosis in Boston. N Engl J Med. 1972;287:523–527. doi: 10.1056/NEJM197209142871101. [DOI] [PubMed] [Google Scholar]
- 16.International Council for the Control of Iodine Deficiency Disorders. 2013 [online], http://www.iccidd.org.
- 17.Zimmermann MB, Jooste PL, Pandav CS. Iodine-deficiency disorders. Lancet. 2008;372:1251–1262. doi: 10.1016/S0140-6736(08)61005-3. [DOI] [PubMed] [Google Scholar]
- 18.Goyle A, Prakash S. Efficacy of multi-micronutrient fortified biscuits on urinary iodine levels of adolescent girls from Jaipur, India. Malays J Nutr. 2011;17:143–150. [PubMed] [Google Scholar]
- 19.Kassim IA, et al. Excessive iodine intake during pregnancy in Somali refugees. Matern Child Nutr. 2012;8:49–56. doi: 10.1111/j.1740-8709.2010.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sang Z, et al. Exploration of the safe upper level of iodine intake in euthyroid Chinese adults: a randomized double-blind trial. Am J Clin Nutr. 2012;95:367–373. doi: 10.3945/ajcn.111.028001. [DOI] [PubMed] [Google Scholar]
- 21.Laurberg P, et al. The Danish investigation on iodine intake and thyroid disease, DanThyr: status and perspectives. Eur J Endocrinol. 2006;155:219–228. doi: 10.1530/eje.1.02210. [DOI] [PubMed] [Google Scholar]
- 22.Thomson CD, Campbell JM, Miller J, Skeaff SA. Minimal impact of excess iodate intake on thyroid hormones and selenium status in older New Zealanders. Eur J Endocrinol. 2011;165:745–752. doi: 10.1530/EJE-11-0575. [DOI] [PubMed] [Google Scholar]
- 23.Galofre JC, Fernandez-Calvet L, Rios M, Garcia-Mayor RV. Increased incidence of thyrotoxicosis after iodine supplementation in an iodine sufficient area. J Endocrinol Invest. 1994;17:23–27. doi: 10.1007/BF03344958. [DOI] [PubMed] [Google Scholar]
- 24.Todd CH, et al. Increase in thyrotoxicosis associated with iodine supplements in Zimbabwe. Lancet. 1995;346:1563–1564. doi: 10.1016/s0140-6736(95)92095-1. [DOI] [PubMed] [Google Scholar]
- 25.Parveen S, Latif SA, Kamal MM, Uddin MM. Effects of long term iodized salt consumption on serum T3, T4 and TSH in an iodine deficient area of Bangladesh. Mymensingh Med J. 2007;16:57–60. doi: 10.3329/mmj.v16i1.249. [DOI] [PubMed] [Google Scholar]
- 26.Allen EM, Appel MC, Braverman LE. The effect of iodine ingestion on the development of spontaneous lymphocytic thyroiditis in the diabetes-prone BB/W rat. Endocrinology. 1986;118:1977–1998. doi: 10.1210/endo-118-5-1977. [DOI] [PubMed] [Google Scholar]
- 27.Kahaly GJ, Dienes HP, Beyer J, Hommel G. Iodide induces thyroid autoimmunity in patients with endemic goiter: a randomized, double-blind, placebo-controlled trial. Eur J Endocrinol. 1998;139:290–297. doi: 10.1530/eje.0.1390290. [DOI] [PubMed] [Google Scholar]
- 28.Dong W, et al. The changing incidence of thyroid carcinoma in Shenyang, China before and after universal salt iodization. Med Sci Monit. 2013;19:49–53. doi: 10.12659/MSM.883736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blomberg M, Feldt-Rasmussen U, Andersen KK, Kjaer SK. Thyroid cancer in Denmark 1943–2008, before and after iodine supplementation. Int J Cancer. 2012;131:2360–2366. doi: 10.1002/ijc.27497. [DOI] [PubMed] [Google Scholar]
- 30.Murray CW, Egan SK, Kim H, Beru N, Bolger PM. US Food and Drug Administration’s Total Diet Study: dietary intake of perchlorate and iodine. J Expo Sci Environ Epidemiol. 2008;18:571–580. doi: 10.1038/sj.jes.7500648. [DOI] [PubMed] [Google Scholar]
- 31.Perrine CG, Sullivan KM, Flores R, Caldwell KL, Grummer-Strawn LM. Intakes of dairy products and dietary supplements are positively associated with iodine status among U.S. children. J Nutr. 2013;143:1155–1160. doi: 10.3945/jn.113.176289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clifton VL, et al. The impact of iodine supplementation and bread fortification on urinary iodine concentrations in a mildly iodine deficient population of pregnant women in South Australia. Nutr J. 2013;12:32. doi: 10.1186/1475-2891-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zimmermann MB. Iodine deficiency. Endocr Rev. 2009;30:376–408. doi: 10.1210/er.2009-0011. [DOI] [PubMed] [Google Scholar]
- 34.Zava TT, Zava DT. Assessment of Japanese iodine intake based on seaweed consumption in Japan: a literature-based analysis. Thyroid Res. 2011;4:14–20. doi: 10.1186/1756-6614-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rhee SS, Braverman LE, Pino S, He X, Pearce EN. High iodine content of Korean seaweed soup: a health risk for lactating women and their infants? Thyroid. 2011;21:927–928. doi: 10.1089/thy.2011.0084. [DOI] [PubMed] [Google Scholar]
- 36.Teas J, Pino S, Critchley A, Braverman LE. Variability of iodine content in common commercially available edible seaweeds. Thyroid. 2004;14:836–841. doi: 10.1089/thy.2004.14.836. [DOI] [PubMed] [Google Scholar]
- 37.Mussig K, et al. Iodine-induced thyrotoxicosis after ingestion of kelp-containing tea. J Gen Intern Med. 2006;21:C11–C14. doi: 10.1111/j.1525-1497.2006.00416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eliason BC. Transient hyperthyroidism in a patient taking dietary supplements containing kelp. J Am Board Fam Pract. 1998;11:478–480. doi: 10.3122/jabfm.11.6.478. [DOI] [PubMed] [Google Scholar]
- 39.Teas J, et al. Seaweed and soy: companion foods in Asian cuisine and their effects on thyroid function in American women. J Med Food. 2007;10:90–100. doi: 10.1089/jmf.2005.056. [DOI] [PubMed] [Google Scholar]
- 40.Miyai K, Tokushige T, Kondo M Iodine Research Group. Suppression of thyroid function during ingestion of seaweed “Kombu” (Laminaria japonoca) in normal Japanese adults. Endocr J. 2008;55:1103–1108. doi: 10.1507/endocrj.k08e-125. [DOI] [PubMed] [Google Scholar]
- 41.Kasahara T, et al. Delayed onset congenital hypothyroidism in a patient with DUOX2 mutations and maternal iodine excess. Am J Med Genet A. 2013;161A:214–217. doi: 10.1002/ajmg.a.35693. [DOI] [PubMed] [Google Scholar]
- 42.Fuse Y, Saito N, Tsuchiya T, Shishiba Y, Irie M. Smaller thyroid gland volume with high urinary iodine excretion in Japanese schoolchildren: normative reference values in an iodine-sufficient area and comparison with the WHO/ICCIDD reference. Thyroid. 2007;17:145–155. doi: 10.1089/thy.2006.0209. [DOI] [PubMed] [Google Scholar]
- 43.Konno N, Makita H, Iizuka N, Kawasaki K. Association between dietary iodine intake and prevalence of subclinical hypothyroidism in the coastal regions of Japan. J Clin Endocrinol Metab. 1994;78:393–397. doi: 10.1210/jcem.78.2.8106628. [DOI] [PubMed] [Google Scholar]
- 44.Michikawa T, et al. Seaweed consumption and the risk of thyroid cancer in women: the Japan Public Health Center-based Prospective Study. Eur J Cancer Prev. 2012;21:254–260. doi: 10.1097/CEJ.0b013e32834a8042. [DOI] [PubMed] [Google Scholar]
- 45.Dasgupta PK, Liu Y, Dyke JV. Iodine nutrition: iodine content of iodized salt in the United States. Environ Sci Technol. 2008;42:1315–1323. doi: 10.1021/es0719071. [DOI] [PubMed] [Google Scholar]
- 46.Leung AM, Pearce EN, Braverman LE. Iodine content of prenatal multivitamins in the United States. N Engl J Med. 2009;360:939–940. doi: 10.1056/NEJMc0807851. [DOI] [PubMed] [Google Scholar]
- 47.Connelly KJ, et al. Congenital hypothyroidism caused by excess prenatal maternal iodine ingestion. J Pediatr. 2012;161:760–762. doi: 10.1016/j.jpeds.2012.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nishiyama S, et al. Transient hypothyroidism or persistent hyperthyrotropinemia in neonates born to mothers with excessive iodine intake. Thyroid. 2004;14:1077–1083. doi: 10.1089/thy.2004.14.1077. [DOI] [PubMed] [Google Scholar]
- 49.Emder PJ, Jack MM. Iodine-induced neonatal hypothyroidism secondary to maternal seaweed consumption: a common practice in some Asian cultures to promote breast milk supply. J Paediatr Child Health. 2011;47:750–752. doi: 10.1111/j.1440-1754.2010.01972.x. [DOI] [PubMed] [Google Scholar]
- 50.Shumer DE, Mehringer JE, Braverman LE, Dauber A. Acquired Hypothyroidism in an Infant Related to Excessive Maternal Iodine Intake: Food for Thought. Endocr Pract. 2013;9:729–731. doi: 10.4158/EP13017.CO. [DOI] [PubMed] [Google Scholar]
- 51.American Thyroid Association. ATA Statement on the Potential Risks of Excess Iodine Ingestion and Exposure. 2013 doi: 10.1089/thy.2014.0331. [online], http://www.thyroid.org/ata-statement-on-the-potential-risks-of-excess-iodine-ingestion-and-exposure. [DOI] [PMC free article] [PubMed]
- 52.Minelli R, Gardini E, Bianconi L, Salvi M, Roti E. Subclinical hypothyroidism, overt thyrotoxicosis and subclinical hypothyroidism: the subsequent phases of thyroid function in a patient chronically treated with amiodarone. J Endocrinol Invest. 1992;15:853–855. doi: 10.1007/BF03348819. [DOI] [PubMed] [Google Scholar]
- 53.Danzi S, Klein I. Amiodarone-induced thyroid dysfunction. J Intensive Care Med. doi: 10.1177/0885066613503278. http://dx.doi.org/10.1177/0885066613503278. [DOI] [PubMed]
- 54.Bogazzi F, Tomisti L, Bartalena L, Aghini-Lombardi F, Martino E. Amiodarone and the thyroid: a 2012 update. J Endocrinol Invest. 2012;35:340–348. doi: 10.3275/8298. [DOI] [PubMed] [Google Scholar]
- 55.Eskes SA, et al. Treatment of amiodarone-induced thyrotoxicosis type 2: a randomized clinical trial. J Clin Endocrinol Metab. 2012;97:499–506. doi: 10.1210/jc.2011-2390. [DOI] [PubMed] [Google Scholar]
- 56.Tomisti L, et al. Total thyroidectomy in patients with amiodarone-induced thyrotoxicosis and severe left ventricular systolic dysfunction. J Clin Endocrinol Metab. 2012;97:3515–3521. doi: 10.1210/jc.2012-1797. [DOI] [PubMed] [Google Scholar]
- 57.Bogazzi F, et al. Preparation with iopanoic acid rapidly controls thyrotoxicosis in patients with amiodarone-induced thyrotoxicosis before thyroidectomy. Surgery. 2002;132:1114–1117. doi: 10.1067/msy.2002.128561. [DOI] [PubMed] [Google Scholar]
- 58.Bogazzi F, et al. Color flow Dopploer sonography rapidly differentiates type I and type II amiodarone-induced thyrotoxicosis. Thyroid. 1997;7:541–545. doi: 10.1089/thy.1997.7.541. [DOI] [PubMed] [Google Scholar]
- 59.Tomisti L, et al. Effects of amiodarone, thyroid hormones and CYP2C9 and VKORC1 polymorphisms on warfarin metabolism: a review of the literature. Endocr Pract. 2013;19:1043–1049. doi: 10.4158/EP13093.RA. [DOI] [PubMed] [Google Scholar]
- 60.Rhee CM, Bhan I, Alexander EK, Brunelli SM. Association between iodinated contrast media exposure and incident hyperthyroidism and hypothyroidism. Arch Intern Med. 2012;172:153–159. doi: 10.1001/archinternmed.2011.677. [DOI] [PubMed] [Google Scholar]
- 61.Nimmons GL, Funk GF, Graham MM, Pagedar NA. Urinary iodine excretion after contrast computed tomography scan: implications for radioactive iodine use. JAMA Otolaryngol Head Neck Surg. 2013;139:479–482. doi: 10.1001/jamaoto.2013.2552. [DOI] [PubMed] [Google Scholar]
- 62.Padovani RP, et al. One month is sufficient for urinary iodine to return to its baseline value after the use of water-soluble iodinated contrast agents in post-thyroidectomy patients requiring radioiodine therapy. Thyroid. 2012;22:926–930. doi: 10.1089/thy.2012.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alkhuja S, Pyram R, Odeyemi O. In the eye of the storm: Iodinated contrast medium induced thyroid storm presenting as cardiopulmonary arrest. Heart Lung. 2013;42:267–269. doi: 10.1016/j.hrtlng.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 64.Gartner W, Weissel M. Do iodine-containing contrast media induce clinically relevant changes in thyroid function parameters of euthyroid patients within the first week? Thyroid. 2004;14:521–524. doi: 10.1089/1050725041517075. [DOI] [PubMed] [Google Scholar]
- 65.Koroscil TM, Pelletier PR, Slauson JW, Hennessey J. Short-term effects of coronary angiographic contrast agents on thyroid function. Endocr Pract. 1997;3:219–221. doi: 10.4158/EP.3.4.219. [DOI] [PubMed] [Google Scholar]
- 66.Ozkan S, et al. Thyroid functions after contrast agent administration for coronary angiography: a prospective observational study in euthyroid patients. Anadolu Kardiyol Derg. 2013;13:363–369. doi: 10.5152/akd.2013.134. [DOI] [PubMed] [Google Scholar]
- 67.Kochi MH, Kaloudis EV, Ahmed W, Moore WH. Effect of in utero exposure of iodinated intravenous contrast on neonatal thyroid function. J Comput Assist Tomogr. 2012;36:165–169. doi: 10.1097/RCT.0b013e31824cc048. [DOI] [PubMed] [Google Scholar]
- 68.Thaker V, Levine B-S, Leung AM, Braverman LE. Neonatal iodine-induced hypothyroidism after cardiac arteriography; Presented at ENDO; 2013. [Google Scholar]
- 69.Conn JJ, Sebastian MJ, Deam D, Tam M, Martin FI. A prospective study of the effect of nonionic contrast media on thyroid function. Thyroid. 1996;6:107–110. doi: 10.1089/thy.1996.6.107. [DOI] [PubMed] [Google Scholar]
- 70.Martin FI, Tress BW, Colman PG, Deam DR. Iodine-induced hyperthyroidism due to nonionic contrast radiography in the elderly. Am J Med. 1993;95:78–82. doi: 10.1016/0002-9343(93)90235-h. [DOI] [PubMed] [Google Scholar]
- 71.van der Molen AJ, Thomsen HS, Morcos SK Contrast Media Safety Committee, European Society of Urogenital Radiology (ESUR) Effect of iodinated contrast media on thyroid function in adults. Eur Radiol. 2004;14:902–907. doi: 10.1007/s00330-004-2238-z. [DOI] [PubMed] [Google Scholar]
- 72.Gordon CM, Rowitch DH, Mitchell ML, Kohane IS. Topical iodine and neonatal hypothyroidism. Arch Pediatr Adolesc Med. 1995;149:1336–1339. doi: 10.1001/archpedi.1995.02170250042006. [DOI] [PubMed] [Google Scholar]
- 73.Linder N, et al. Topical iodine-containing antiseptics and subclinical hypothyroidism in preterm infants. J Pediatr. 1997;131:434–439. doi: 10.1016/s0022-3476(97)80071-6. [DOI] [PubMed] [Google Scholar]
- 74.Vermeulen H, et al. Benefit and harm of iodine in wound care: a systematic review. J Hosp Infect. 2010;76:191–199. doi: 10.1016/j.jhin.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 75.Ader AW, et al. Effect of mouth rinsing with two polyvinylpyrrolidone-iodine mixtures on iodine absorption and thyroid function. J Clin Endocrinol Metab. 1988;66:632–635. doi: 10.1210/jcem-66-3-632. [DOI] [PubMed] [Google Scholar]
- 76.McMonigal KA, et al. Thyroid function changes related to use of iodinated water in the U.S. Space Program. Aviat Space Environ Med. 2000;71:1120–1125. [PubMed] [Google Scholar]
- 77.Georgitis WJ, McDermott MT, Kidd GS. An iodine load from water-purification tablets alters thyroid function in humans. Mil Med. 1993;158:794–797. [PubMed] [Google Scholar]
- 78.Pearce EN, et al. Effects of chronic iodine excess in a cohort of long-term American workers in West Africa. J Clin Endocrinol Metab. 2002;87:5499–5502. doi: 10.1210/jc.2002-020692. [DOI] [PubMed] [Google Scholar]
- 79.Nauman J, Wolff J. Iodide prophylaxis in Poland after the Chernobyl reactor accident: benefits and risks. Am J Med. 1993;94:524–532. doi: 10.1016/0002-9343(93)90089-8. [DOI] [PubMed] [Google Scholar]
- 80.Pearce EN, Pino S, Bazrafshan HR, Lee SL, Braverman LE. Sources of dietary iodine: bread, cows’ milk, and infant formula in the Boston area. J Clin Endocrinol Metab. 2004;89:3421–3424. doi: 10.1210/jc.2003-032002. [DOI] [PubMed] [Google Scholar]
- 81.Allegrini M, Pennington JAT, Tanner JT. Total diet study: determination of iodine intake by neutron activation analysis. J Am Diet Assoc. 1983;83:18–24. [PubMed] [Google Scholar]