Abstract
Objective
Determine efficacy of three non-hormonal therapies for improving menopause-related quality of life (QOL) in women with vasomotor symptoms (VMS).
Methods
12-week 3×2 randomized, controlled, factorial design trial. Peri- and postmenopausal women, ages 40-62 years, were randomized to yoga (n=107), exercise (n=106), or usual activity (n=142), and also randomized to double-blind comparison of omega-3 (n=177) or placebo (n=178) capsules. Interventions: 1) weekly 90-minute yoga classes with daily at-home practice; 2) individualized facility-based aerobic exercise training 3 times/week; and 3) 0.615 gram omega-3 supplement, 3 times/day. Outcomes: Menopausal Quality of Life Questionnaire (MENQOL) total and domain (VMS, psychosocial, physical and sexual) scores.
Results
Among 355 randomized women, average age 54.7 years, 338 (95%) completed 12-week assessments. Mean baseline VMS frequency was 7.6/day and mean baseline total MENQOL score was 3.8 (range 1-8 from better to worse) with no between-group differences. For yoga compared to usual activity, baseline to 12-week improvements were seen for MENQOL total -0.3 (95% CI -0.6 to 0.0, p=0.02), and VMS (p=0.02) and sexuality (p=0.03) domain scores. For exercise and omega-3 compared to controls, improvements in baseline to 12-week total MENQOL scores were not observed. Exercise showed benefit in the MENQOL physical domain score at 12-weeks (p=0.02).
Conclusion
All women become menopausal and many seek medical advice on ways to improve quality of life; little evidence-based information exists. We found, among healthy sedentary menopausal women, yoga appears to improve menopausal QOL - the clinical significance of our finding is uncertain due to modest effect.
Keywords: menopause quality of life, yoga, exercise, omega-3, randomized trial
INTRODUCTION
More than 38 million US women ages 45-64 years old (88%) experience daytime hot flashes or night sweats during the menopausal transition.1 Hot flashes and night sweats or vasomotor symptoms (VMS) are the cardinal symptoms of menopause. However, other menopausal symptoms - often adversely affected by VMS frequency and bother,2 such as sleep and mood disturbances, pain, difficulty concentrating and diminished energy - can affect daily functioning in work, social, leisure and sexual activities.3 Medical resources expended to alleviate these problems are substantial4 and there is a compelling need for effective treatments to relieve menopausal symptoms in midlife women.
Among symptomatic peri- and postmenopausal women with vasomotor symptoms (VMS), hormone therapy (HT) has demonstrated an improvement in QOL.5 Due to risks associated with HT among postmenopausal women6 alternative lower risk behavioral therapies have been proposed for the treatment of VMS. Studies regarding benefit of behavioral interventions for improving menopause-related QOL are less robust, but suggest that yoga7-12 and exercise,13,14 may be beneficial. Findings across studies have been inconsistent, perhaps because of different measures and outcomes of interest.14 Yoga findings, in particular, are limited by a paucity of studies, small sample sizes and lack of control groups.
Non-phytoestrogenic supplements are widely used by midlife women but have not been specifically examined for improved midlife QOL.15 Omega-3 supplements containing polyunsaturated fatty acids (PUFAs) are among the most widely consumed supplements for a variety of medical conditions.16 Studies suggest omega-3s modulate serotonergic and dopaminergic neurotransmission17-19 and may alleviate VMS.20 Two small randomized trials have examined the efficacy of omega-3s in the treatment of VMS20,21 with conflicting results.
We conducted a factorial design randomized controlled trial to evaluate the efficacy of yoga, exercise and omega-3s on VMS frequency and bother. We found no benefit from any of these interventions for VMS, but suggestive evidence that self-reported sleep quality and depressive symptoms improved slightly with exercise and yoga (findings previously reported, not adjusted for multiple comparisons).22-25 In this analysis, we report findings on the impact of yoga, exercise and omega-3s on menopause-related quality of life.
MATERIALS AND METHODS
Study design
Details about the MsFLASH Research Network, study design, and protocols are published.22-26 Briefly, we performed a multi-site, 3 by 2 factorial randomized controlled trial. Eligible women were randomized to 12 weeks of yoga, exercise, or usual activity, and simultaneously randomized to 1.8 g/day of omega-3 or placebo capsules. The study was approved by the Institutional Review Boards of all clinical sites and the Data Coordinating Center (DCC), and all participants provided written informed consent. The DCC performed centralized training, and monitored maintenance of the standardized protocol, fidelity to the intervention, and participant adherence.
Eligibility, Screening, Randomization, and Blinding
Participants were recruited February 2011 through January 2012, primarily by mass-mailing to women ages 40-62 years, using purchased lists and health-plan enrollment files, at three sites (Indianapolis, Oakland, and Seattle). Eligible women were in the menopausal transition or postmenopausal. Screening was performed centrally with standardized inclusion, exclusion, and final eligibility criteria across sites.22,24
Randomization was conducted in a secure central web-based database, utilizing a dynamic randomization algorithm to maintain comparability between study groups with respect to clinical site. An unequal allocation was employed for the behavioral interventions (3:3:4, yoga:exercise:control) and equal allocation employed for omega-3 and placebo treatments, provided in masked identical capsules and containers.
Interventions (details described elsewhere)24
Yoga:26
The yoga intervention (studio and home practice) emphasized a practice of “cooling” breathing exercises, 11-13 poses (Asanas: restorative, inverted, lateral bends or twists, forward bends, and counter-poses) previously suggested for VMS relief,7,27 and guided meditation (Yoga Nidra). Instruction was provided during 12, weekly, 90-minute classes. Daily home practice was expected for 20 minutes on days not attending class.
Exercise:23
The exercise intervention consisted of 12 weeks of three, individual cardiovascular conditioning training sessions/week at local fitness facilities, supervised by trained, certified exercise trainers. The targeted training heart rate was 50-60% of the heart rate reserve for the first month and 60-70% for the remainder of the intervention (approximately 125-145 beats/minute). Women exercised 40-60 minutes/session to achieve the energy expenditure goal of 16 kcal/kg (about 1,000-1,500 kcals/week).
Usual Activity
Women in the usual activity group were instructed to follow their usual physical activity behavior and were asked not to begin yoga or a new exercise regimen.
Omega-3 and Placebo Capsules:25
To standardize the expectation of benefit, all women in both behavioral interventions and the usual activity group received either a placebo containing olive oil or an active omega-3 capsule. The omega-3 supplement contained 425 mg ethyl eicosapentaenoic acid, 100 mg docosahexaenoic acid and 90 mg of other omega-3s. All capsules (placebo and omega-3) contained natural lemon oil rosemary extract and vitamin E.
Follow-up and compensation
Participants were contacted by study staff masked to pill randomization assignment to encourage pill compliance and to evaluate tolerance at 2 and 6-weeks. Participants were compensated $50 after each clinic visit for a possible total of $150.
Data collection
Outcomes (baseline to 12-week change): Menopause Quality of Life (MENQOL), total and specific domains
The MENQOL (range 1-8) is a 29-item assessment of menopause-related QOL.28 Scoring generates a total score and 4 domain scores (vasomotor, physical, psychosocial, sexual functioning); higher scores on all scales indicate poorer quality of life. Women were asked whether an item was experienced in the past four weeks. Each item score includes non-endorsement “1” or endorsement “2” plus the bother score (0-6), for a maximum score of 8. The domain-specific score is the mean of the item scores within that domain. The total MENQOL score is the mean of the specific domain scores. Validity, internal consistency, reliability and responsiveness to change are adequate to excellent.28,29
Other Measures (Covariates)
Demographic factors were assessed by baseline questionnaire. Weight and height were measured at baseline and body mass index (BMI, kg/m2) calculated. Frequency and severity of VMS were recorded retrospectively on daily diaries completed in the morning for night sweats and in the evening for daytime hot flashes. Standardized and validated baseline questionnaires (covariates and possible effect modifiers) included: insomnia severity (7-item Insomnia Severity Index (ISI));30 subjective sleep quality (Pittsburgh Sleep Quality Index (PSQI)),31,32 depressive symptoms (8-item scale from the Patient Health Questionnaire (PHQ-8))33 and anxiety (7-item Generalized Anxiety Disorder scale (GAD-7)).34
Additional individual validated menopause QOL measures included the Hot Flash-Related Daily Interference Scale (HFRDIS),35 Perceived Stress Scale (PSS),32 Pain Intensity, Interference with Enjoyment of Life, and Interference with General Activity scale (PEG)33 and Female Sexual Function Index (FSFI),36 collected at baseline and 12-weeks.
Statistical analyses
The intent-to-treat analysis included all randomized participants with response data, which were collected regardless of intervention adherence. Baseline characteristics were compared between treatment groups using t-tests or chi-square tests.
Primary analyses consisted of treatment group contrasts from linear regression models summarizing each outcome (total MENQOL and 4 domains) at 12-weeks as a function of treatment assignment, adjusting each model for clinical center, concurrent intervention assignment, and baseline value of the outcome measure. Treatment group comparisons included yoga vs. usual activity, exercise vs. usual activity, and omega-3 vs. placebo. Analyses comparing the treatment effects of yoga and exercise were adjusted for omega-3 assignment, and the omega-3 analyses were adjusted for the behavioral intervention assignment. Sensitivity analyses were conducted to determine if the intervention effects differed among women who were adherent22-24 to the intervention.
Additional analyses assessed treatment group contrasts from linear regression models summarizing the HFRDIS (hot flash interference), PSS (stress), PEG (pain) and FSFI (sexual function) scores - at 12-weeks as a function of treatment assignment, adjusting each model for clinical center, concurrent intervention assignment, and baseline value of the outcome measure.
We hypothesized that the intervention effects on the total MENQOL might be modified by symptom thresholds measured at baseline: anxiety (GAD-7 continuous), depressive symptoms (PHQ-8 continuous), poor sleep quality (PSQI > 8) or moderate to severe insomnia (ISI > 14). Tests of interaction between treatment assignment and each of these 4 variables were performed within the linear regression models estimating mean 12-week MENQOL as a function of treatment arm, the covariate of interest and the interaction between treatment assignment and covariate; models were adjusted for clinical center, concurrent intervention assignment, and baseline outcome value. Nominal p-values were calculated for the 8 potential interactions examined. Thus, less than one p-value would be expected to be statistically significant at the 0.05 level by chance alone.
Reported p-values are based on the Wald statistic, with 2-sided p-value ≤0.05 considered statistically significant. Analyses were conducted using SAS Version 9.2 (SAS Institute, Cary, NC).
RESULTS
Sample sizes by intervention assignments, available MENQOL data for analyses, and study completion are shown in Figure 1. Overall, 78% met the yoga adherence threshold, 83% met the threshold for adherence to exercise;22-24 82% of women randomized to omega-3, and 79% of women randomized to placebo took at least 80% of dispensed pills.25
Figure 1. Participant recruitment.
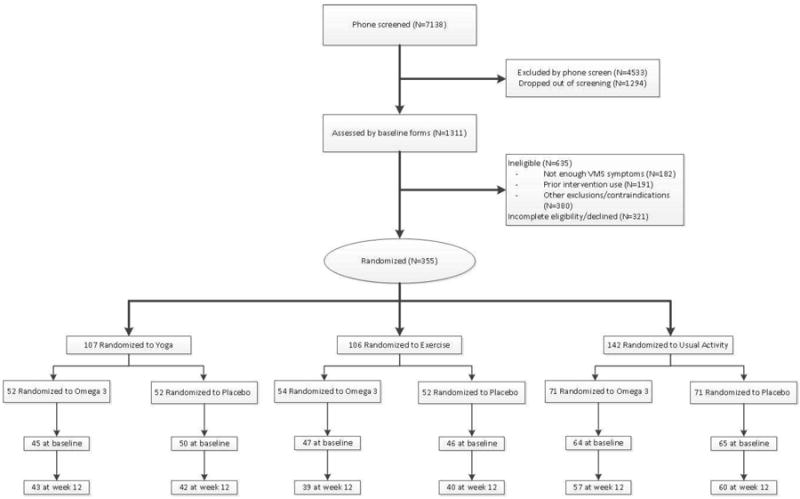
* Some women had more than one reason for exclusion
1 Participants were randomized to yoga, exercise, and usual activity in a 3: 3:4 ratio
MENQOL Total Data by MS FLASH Treatment Assignment at baseline and Week 121
1Week 12 totals include only those participants who also have data at baseline
There were no significant differences between the randomized treatment groups in baseline characteristics (Table 1) with the exception of age (exercise group older than usual activity); and ethnicity (omega-3 group more likely to be white than placebo).
Table 3.
a. Menopause Quality of Life (MENQOL) Yoga and Exercise vs Usual Activity
| Intervention Arm | Differences – Usual Activity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Yoga | Exercise | Usual Activity | Yoga | Exercise | ||||||
|
| ||||||||||
| N | Mean (95% CI) | N | Mean (95% CI) | N | Mean (95% CI) | Mean (95% CI) | p-value2 | Mean (95% CI) | p-value | |
| MENQOL Total | 0.02 | 0.32 | ||||||||
| Baseline | 95 | 3.8 (3.6, 4.0) | 93 | 3.8 (3.5, 4.1) | 129 | 3.8 (3.6, 4.0) | 0.0 (-0.3, 0.3) | 0.0 (-0.3, 0.3) | ||
| Week 12 – baseline | 85 | -0.9 (-1.2, -0.7) | 79 | -0.8 (-1.0, -0.6) | 117 | -0.6 (-0.8, -0.5) | -0.3 (-0.6, 0.0) | -0.1 (-0.4, 0.1) | ||
|
| ||||||||||
| MENQOL -Vasomotor | 0.02 | 0.52 | ||||||||
| Baseline | 104 | 5.3 (5.0, 5.6) | 105 | 5.3 (5.0, 5.6) | 141 | 5.6 (5.4, 5.8) | -0.3 (-0.7, 0.0) | -0.3 (-0.7, 0.1) | ||
| Week 12 – baseline | 97 | -1.5 (-1.9, -1.2) | 99 | -1.2 (-1.5, -0.9) | 133 | -1.2 (-1.5, -0.9) | -0.3 (-0.8, 0.2) | 0.0 (-0.4, 0.5) | ||
|
| ||||||||||
| MENQOL - Psychosocial | 0.78 | 0.57 | ||||||||
| Baseline | 105 | 3.3 (3.0, 3.6) | 106 | 3.3 (3.0, 3.6) | 140 | 3.3 (3.1, 3.5) | 0.0 (-0.4, 0.3) | 0.0 (-0.4, 0.4) | ||
| Week 12 – baseline | 98 | -0.6 (-0.8, -0.4) | 98 | -0.6 (-0.9, -0.4) | 130 | -0.6 (-0.7, -0.4) | 0.0 (-0.3, 0.3) | -0.1 (-0.4, 0.2) | ||
|
| ||||||||||
| MENQOL - Physical | 0.13 | 0.02 | ||||||||
| Baseline | 98 | 3.2 (2.9, 3.5) | 100 | 3.2 (2.9, 3.4) | 137 | 3.3 (3.1, 3.5) | -0.1 (-0.4, 0.3) | -0.1 (-0.4, 0.2) | ||
| Week 12 – baseline | 90 | -0.6 (-0.8, -0.4) | 91 | -0.7 (-0.9, -0.5) | 129 | -0.5 (-0.6, -0.3) | -0.1 (-0.4, 0.1) | -0.2 (-0.5, 0.0) | ||
|
| ||||||||||
| MENQOL - Sexual | 0.03 | 0.41 | ||||||||
| Baseline | 102 | 3.2 (2.7, 3.6) | 100 | 3.4 (2.9, 3.9) | 137 | 3.3 (2.9, 3.7) | -0.1 (-0.7, 0.5) | 0.1 (-0.5, 0.8) | ||
| Week 12 – baseline | 95 | -0.9 (-1.3, -0.5) | 88 | -0.6 (-0.9, -0.3) | 127 | -0.4 (-0.7, -0.1) | -0.5 (-1.0, 0.0) | -0.2 (-0.6, 0.2) | ||
|
b. Menopause Quality of Life (MENQOL) Omega-3 vs Placebo Capsule
| ||||||
| Intervention Arm | ||||||
|
| ||||||
| Omega 3 | Placebo | Difference | ||||
|
| ||||||
| N | Mean (95% CI) | N | Mean (95% CI) | Mean (95% CI) | p-value | |
|
| ||||||
| MENQOL Total | 0.12 | |||||
| Baseline | 156 | 3.8 (3.6, 3.9) | 161 | 3.9 (3.7, 4.0) | -0.1 (-0.3, 0.2) | |
| Week 12 – baseline | 139 | -0.7 (-0.8, -0.5) | 142 | -0.9 (-1.0, -0.7) | 0.2 (-0.1, 0.4) | |
|
| ||||||
| MENQOL - Vasomotor | 0.06 | |||||
| Baseline | 173 | 5.5 (5.3, 5.7) | 177 | 5.3 (5.1, 5.6) | 0.2 (-0.1, 0.5) | |
| Week 12 – baseline | 164 | -1.2 (-1.5, -1.0) | 165 | -1.4 (-1.7, -1.1) | 0.2 (-0.2, 0.6) | |
|
| ||||||
| MENQOL - Psychosocial | 0.29 | |||||
| Baseline | 175 | 3.2 (3.0, 3.4) | 176 | 3.3 (3.2, 3.6) | -0.2 (-0.5, 0.1) | |
| Week 12 – baseline | 163 | -0.5 (-0.7, -0.3) | 163 | -0.7 (-0.8, -0.5) | 0.2 (-0.1, 0.4) | |
|
| ||||||
| MENQOL - Physical | 0.91 | |||||
| Baseline | 167 | 3.2 (3.0, 3.4) | 168 | 3.2 (3.0, 3.4) | 0.0 (-0.3, 0.3) | |
| Week 12 – baseline | 156 | -0.6 (-0.8, -0.4) | 154 | -0.5 (-0.7, -0.4) | 0.0 (-0.3, 0.2) | |
|
| ||||||
| MENQOL - Sexual | 0.31 | |||||
| Baseline | 167 | 3.1 (2.8, 3.5) | 172 | 3.4 (3.0, 3.8) | -0.3 (-0.8, 0.3) | |
| Week 12 – baseline | 154 | -0.5 (-0.7, -0.2) | 156 | -0.7 (-1.0, -0.4) | 0.3 (-0.1, 0.6) | |
p-values from contrasts comparing active vs. control in a linear model of outcome as a function of intervention arm and adjusted for clinical center, baseline outcome value, and concurrent interventions. MENQOL Total score range 1-8, domain scores range 1-8.
Additional factors that might affect menopause-related QOL were compared at baseline (Table 2). There were no differences between groups with the exception that women randomized to exercise had a higher mean PEG score than women in the usual activity group, and that women randomized to omega-3s had a lower mean FSFI score, than women in the placebo group. Overall, 35% percent had mild/moderate depressive symptoms, 27% had mild/moderate anxiety, and 40% had poor sleep quality. Mean hot flash interference score was 32.4 (HFRDIS range 0-100). Stress levels were relatively low with an overall mean PSS score of 13.8 (SD 7.0), similar to the standard norm of 13.7 (SD 6.6).37 Mean PEG score was low at 1.1 (SD 1.8). Sexual function was relatively poor with a mean FSFI of 18.4 (SD 10.5).
Table 2.
Baseline Factors Related to Menopausal Quality of Life
| Baseline Characteristic | Total No. of Participants | Behavioral Intervention | Omega-3 | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Yoga (N=107) | Exercise (N=106) | Usual Activity (N=142) | Active (N=177) | Placebo (N=178) | ||
|
| ||||||
| Depression score (PHQ-8), mean (SD) | 4.0 (3.6) | 4.0 (4.2) | 4.1 (3.6) | 3.6 (3.5) | 4.4 (4.0) | |
| % Mild+ depression (≥ 5) | 126 | 38.3 | 34.0 | 34.5 | 33.3 | 37.6 |
|
| ||||||
| Anxiety score (GAD-7), mean (SD) | 3.2 (3.8) | 3.4 (4.1) | 3.0 (3.0) | 2.9 (3.4) | 3.5 (3.8) | |
| % Mild+ anxiety (≥ 5) % | 95 | 27.1 | 27.4 | 26.1 | 24.3 | 29.2 |
|
| ||||||
| ISI score, mean (SD) | 11.8 (5.4) | 11.5 (5.9) | 12.2 (5.2) | 11.8 (5.2) | 12.0 (5.7) | |
| % Moderate+ insomnia (> 14) | 264 | 32.7 | 31.1 | 33.8 | 32.2 | 33.1 |
|
| ||||||
| PSQI score, mean (SD) | 7.7 (3.4) | 7.8 (3.4) | 8.4 (3.3) | 7.9 (3.3) | 8.2 (3.4) | |
| % Poor sleep quality (> 8) | 294 | 34.6 | 40.6 | 43.7 | 35.6 | 44.4 |
|
| ||||||
| HFRDIS, mean (SD) | 336 | 31.7 (21.3) | 31.8 (22.5) | 33.6 (21.4) | 32.4 (20.0) | 32.5 (23.2) |
|
| ||||||
| PSS score, mean (SD) | 347 | 13.5 (7.0) | 14.1 (7.3) | 13.6 (6.9) | 13.5 (7.1) | 14.0 (6.9) |
|
| ||||||
| PEG score, mean (SD) | 353 | 1.2 (2.0) | 0.8 (1.1) | 1.3 (2.0) | 1.0 (1.7) | 1.3 (1.8) |
|
| ||||||
| FSFI score, mean (SD) | 290 | 18.5 (10.5) | 16.8 (10.6) | 19.6 (10.4) | 20.0 (10.3) | 16.8 (10.6) |
|
| ||||||
| Treadmill test duration (min), mean (SD) | 355 | 10.4 (3.1) | 9.6 (2.9) | 10.3 (2.9) | 10,3 (2.9) | 9.9 (3.1) |
PHQ-8 = Patient Health Questionaire-8 (Range 0 – 20); GAD-7 = Generalized Anxiety Disorder-7 (range 0-17); ISI = Insomnia Severity Index (range 0-27); PSQI = Pittsburgh Sleep Quality Index (range 1-17); HFRDIS =Hot Flash-Related Daily Interference Scale (range 0-100); PSS = Perceived Stress Score (range 0-32); PEG = Pain Intensity, Interference with Enjoyment of Life, and Interference with General Activity scale (range 0-10); FSFI = Female Sexual Function Index (range 2-36); SD = standard deviation.
Mean total MENQOL score was 3.8 (range 1-8) at baseline, with no between-group differences at baseline. The yoga intervention resulted in significantly greater improvement in MENQOL scores at 12-weeks as compared to the usual activity group in adjusted linear regression models (p=0.02), but there were no group differences between exercise and usual activity or omega-3 and placebo (Table 3). The mean difference in change from baseline to 12-weeks in total MENQOL score for the yoga intervention, as compared to usual activity, was -0.3 (95% confidence interval (CI) -0.6, 0.0). Statistically significant differences in MENQOL domain scores favoring the yoga intervention were observed for the Vasomotor (-0.3; 95% CI -0.8, 0.2; p=0.02) and Sexual domains (-0.5; 95% CI -1.0, 0.0; p=0.03). For exercise and omega-3, evaluation of the four MENQOL domains showed only a statistically significant treatment group difference favoring the exercise group for the Physical domain (-0.2; 95% CI -0.5, 0.0; p=0.02), and no domain scores varied between the omega-3 and placebo groups.
There was no significant difference between yoga and usual activity when sexual function was evaluated by the validated and more detailed FSFI (p=0.58) (Table 4). HFRDIS scores declined (i.e. improved) in the yoga group relative to usual activity by 12 weeks (group difference -3.4; 95% CI -9.0, 2.3; p=0.03), but changes in stress and pain did not differ between the yoga and usual activity groups. Hot flash interference, stress, pain and sexual function showed no improvement with exercise or omega-3 interventions over usual care or placebo, respectively.
Table 4.
Outcome Differences at Week 12 by Intervention Arm: Hot Flash Interference (HFRDIS), Stress (PSS), Pain (PEG) and Sexual Function (FSFI)
| Intervention | Yoga | Usual Activity | Difference1 | Exercise | Usual Activity | Difference1 | Omega-3 | Placebo | Difference1 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| N | N | Mean (95% CI) | p-value1 | N | N | Mean (95% CI) | p-value1 | N | N | Mean (95% CI) | p-value1 | |
| HFRDIS | 0.03 | 0.14 | 0.59 | |||||||||
| Baseline | 103 | 133 | -1.9 (-7.4, 3.6) | 100 | 133 | -1.8 (-7.5, 3.9) | 167 | 169 | -0.1 (-4.8, 4.5) | |||
| Week 12 – baseline | 95 | 122 | -3.4 (-9.0, 2.3) | 95 | 122 | -2.0 (-7.7, 3.7) | 158 | 154 | -1.6 (-6.2, 3.0) | |||
|
| ||||||||||||
| PSS | 0.19 | 0.97 | 0.08 | |||||||||
| Baseline | 102 | 139 | -0.1 (-1.9, 1.7) | 106 | 139 | 0.5 (-1.3, 2.3) | 172 | 175 | -0.6 (-2.0, 0.9) | |||
| Week 12 – baseline | 95 | 132 | 0.9 (-0.6, 2.4) | 101 | 132 | -0.2 (-1.8, 1.4) | 164 | 164 | 1.1 (-0.2, 2.5) | |||
|
| ||||||||||||
| PEG | 0.75 | >0.99 | 0.64 | |||||||||
| Baseline | 105 | 142 | -0.1 (-0.6, 0.4) | 106 | 142 | -0.5 (-0.9, -0.1) | 175 | 178 | -0.3 (-0.6, 0.1) | |||
| Week 12 – baseline | 99 | 135 | 0.0 (-0.5, 0.5) | 100 | 135 | 0.3 (-0.2, 0.7) | 166 | 168 | 0.0 (-0.4, 0.5) | |||
|
| ||||||||||||
| FSFI | 0.58 | 0.31 | 0.61 | |||||||||
| Baseline | 86 | 119 | -1.1 (-4.1, 1.8) | 85 | 119 | -2.9 (-5.8, 0.1) | 147 | 143 | 3.2 (0.8, 5.6) | |||
| Week 12 – baseline | 76 | 104 | 0.8 (-1.1, 2.6) | 75 | 104 | -0.5 (-2.7, 1.6) | 129 | 126 | -1.0 (-2.7, 0.6) | |||
Active – control differences for each intervention.
p-values from contrasts comparing active vs. control for each protocol in a linear model of outcome as a function of intervention arm and adjusted for clinical center, baseline outcome value, and concurrent interventions.
HFRDIS =Hot Flash-Related Daily Interference Scale (range 0-100); PSS = Perceived Stress Score (range 0-32); PEG = Pain Intensity, Interference with Enjoyment of Life, and Interference with General Activity scale (range 0-10); FSFI = Female Sexual Function Index (range 2-36).
COMMENT
Little evidenced-based information is available for women with menopausal symptoms considering behavioral changes to improve quality of life. We found that, relative to usual activity, a 12-week program of yoga slightly improved menopause-related QOL and reduced the extent to which hot flashes interfered with a woman’s daily function among women with VMS, but that exercise and omega-3 supplements had no effect on these measures. Among the individual MENQOL domains, we found slight benefit for VMS and sexual function domains (but not for the physical and psychosocial domains) from the yoga intervention and benefit for only the physical domain for the exercise intervention.
All of the significant menopause-related QOL differences and the difference observed in the hot flash daily interference measure with the yoga intervention were small, and therefore clinical relevance of our findings may be modest at best. For example, mean total MENQOL score at baseline was 3.8 (range 1-8) with a mean diminution of 0.9 in the yoga intervention and a decrease of 0.6 in the usual activity group at 12-weeks. Significant MENQOL domain-specific differences observed (VMS, physical, sexual) were of similarly small magnitudes and were not supported by other individual validated measures of factors potentially related to QOL: pain (PEG), sexual function (FSFI), and VMS daily diaries.22 While similar questionnaires may purport to measure the same outcomes, differences in the way they are delivered (e.g. diary vs. global scale) may lead women to respond differently (counts vs. global impression). Aside from these differences, the statistically significant differences found could be due to chance alone.
Few studies have evaluated menopause-related QOL in menopausal women practicing yoga. Most were conducted in special populations – women with insomnia,11 breast cancer,12 and those specifically taking aromatase inhibitors.38 All found benefit, but studies were limited by an extremely high withdrawal rate (23% overall, 63% yoga group),11 and wait-list12 or no controls;38 results should be interpreted with caution. A 3-arm trial (n=162) found a “positive affect” on QOL in both walking and yoga intervention groups compared to wait-list controls.39 We previously reported that among healthy women, 12 weeks of yoga class plus home practice did not improve VMS frequency or bother compared with usual activity, but yoga was associated with minor improvements in sleep quality, insomnia symptoms, and depressive symptoms.22 In the analyses reported here, we found modest diminished hot flash interference with daily activities, comparing yoga to usual activity, findings consistent with two smaller studies that lacked control groups.7,9 The inclusion of control groups in our study with opportunity for expectation of benefit (all women received a pill - placebo or omega 3 fatty acid tablets) is critical for interpreting these mixed findings.
Consistent with other studies on exercise in midlife, we found that the exercise intervention improved the MENQOL physical function domain as compared with usual activity, but exercise was not associated with improvement in overall menopause-related QOL.40,41 Two studies reporting on health-related QOL among postmenopausal women found benefit with increased physical activity, but specifics as to whether women were experiencing significant VMS, as in our study population, were lacking in one,13 and the other evaluated breast cancer survivors with a home-based exercise program.42 In addition, both studies used a different QOL measure that is not specific to menopause-related symptoms. Consistent with the lack of menopause-related QOL benefit from exercise, we did not observe improvement in hot flash interference, stress, pain or sexual function.
In our study, omega-3 supplements were not associated with improvements in menopausal QOL, hot flash interference, stress, pain or sexual function.
The rationale for evaluating whether yoga and or exercise improves QOL is based on the hypothesis that sympathetic nervous system (SNS) and parasympathetic nervous (PNS) system imbalances occur at midlife. Behavioral interventions impact both SNS and PNS function and stress reactivity, supporting the hypothesis that yoga and/or exercise might shift the balance toward sympathetic dominance and improve perceived QOL. Yoga or exercise could also decrease autonomic arousal through changes in circulating neurotransmitters and hormone concentrations,43 leading to improved perceived QOL. In addition, improved QOL with exercise is hypothesized to occur through increasing circulation of beta endorphins, or potentially as a relief or distraction from worries or stress.44,45 Analyses of physiologic measures of the SNS and PNS balance (heart rate variability and salivary cortisol) among our participants are ongoing to guide biologic interpretation of our findings.
Limitations of our study deserve mention. Although women were recruited from community-based samples, participants were primarily sedentary, had at least two hot flashes/day and were compensated for their participation. Generalizability to other populations must be thoughtfully considered. The impact of reimbursement is uncertain. Women who self-selected, and paid for, similar classes in the community might be more (due to their financial investment) or less (due to lack of reimbursement) motivated to adhere to the program. They may have been a select group motivated to seek treatment and had treatment expectations. Mean sum baseline MENQOL scores for all groups were 3.8 (yoga, exercise and usual activity), comparable to another MsFLASH study evaluating an SSRI vs placebo (3.7-3.9) 46, but lower than a study evaluating hormonal therapies (4.4-4.5) 47. The 12-week treatment interval was brief, but most likely sufficient for determining long-term non-hormonal treatment efficacy.42 We examined multiple potential moderating factors of treatment response, but other factors likely exist. We cannot rule out the possibility that other more intensive yoga or exercise regimens or a different omega-3 formulation might have a more salutary effect on menopause-related QOL. This study analyzed multiple outcomes, thus significant findings may be due to chance.
Study strengths are that the interventions were designed specifically for midlife women, both peri- and postmenopausal women, the omega-3 formulation had excellent quality control data, the sample size was large, with a low dropout rate, and participants were adherent to therapy. All participants took either omega-3 or placebo capsules, thus providing the expectancy of benefit in all women. Validated measures for VMS, sleep, stress, pain, sexual function, and mood assisted in the interpretation of MENQOL findings. The majority of VMS intervention trials have not evaluated treatment effects on menopause-related QOL. For those that have, the available QOL measures are numerous and of variable quality.48 Our rationale for using the MENQOL for the sFLASH trials is based on the breadth of its domains, salutary psychometric properties, brevity and sensitivity to change over time.
All women become menopausal and many seek medical advice on ways to improve quality of life; little evidence-based information exists. Future studies are needed to better understand the physiologic basis for and maximization of benefit from behavioral therapies for menopausal symptoms. Providers may advise women that yoga slightly improves menopause quality of life; exercise and omega-3 supplements do not.
Table 1.
Baseline Demographic and Clinical Characteristics by Intervention Arm
| Baseline Characteristic | Total No. of Participants | Behavioral Intervention | Omega-3 | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Yoga (N=107) | Exercise (N=106) | Usual Activity (N=142) | Active (N=177) | Placebo (N=178) | ||
|
| ||||||
| % | % | % | % | % | ||
|
| ||||||
| Age at screening (years), mean (SD) | 355 | 54.3 (3.9) | 55.8 (3.6) | 54.2 (3.5) | 54.4 (3.6) | 55.0 (3.8) |
| < 50 | 19 | 6.5 | 1.9 | 7.0 | 5.6 | 5.1 |
| 50 - 54 | 162 | 46.7 | 40.6 | 48.6 | 49.2 | 42.1 |
| 55 - 59 | 130 | 36.4 | 37.7 | 35.9 | 35.6 | 37.6 |
| ≥ 60 | 44 | 10.3 | 19.8 | 8.5 | 9.6 | 15.2 |
|
| ||||||
| Race | ||||||
| White | 228 | 63.6 | 66.0 | 63.4 | 70.6 | 57.9 |
| African American | 93 | 23.4 | 25.5 | 28.9 | 25.4 | 27.0 |
| Other* | 34 | 13.1 | 8.5 | 7.7 | 4.0 | 15.2 |
|
| ||||||
| College graduate | 221 | 64.5 | 54.7 | 66.2 | 64.4 | 60.1 |
|
| ||||||
| Employment status | ||||||
| Retired or no employment | 49 | 13.1 | 16.0 | 12.7 | 15.3 | 12.4 |
| Full / Part -time | 267 | 72.0 | 77.4 | 76.1 | 76.3 | 74.2 |
| Other | 38 | 14.0 | 6.6 | 11.3 | 8.5 | 12.9 |
|
| ||||||
| Married/living with partner | 236 | 68.2 | 62.3 | 68.3 | 70.1 | 62.9 |
|
| ||||||
| Current smoker | 32 | 7.5 | 7.5 | 11.3 | 9.0 | 9.0 |
|
| ||||||
| ≥ 7 alcohol drinks / week | 60 | 13.1 | 17.9 | 19.0 | 21.5 | 12.4 |
|
| ||||||
| Body Mass Index (m/kg2), mean (SD) | 27.1 (4.6) | 26.8 (3.9) | 26.9 (4.6) | 26.8 (4.4) | 27.1 (4.3) | |
| ≥ 30 | 88 | 27.1 | 23.6 | 23.9 | 23.7 | 25.8 |
|
| ||||||
| Menopause status | ||||||
| Postmenopausal | 286 | 74.8 | 84.9 | 81.7 | 82.5 | 78.7 |
| Perimenopausal | 69 | 25.2 | 15.1 | 18.3 | 17.5 | 21.3 |
|
| ||||||
| Hot flashes / day at Screening, mean (SD) | 7.4 (3.8) | 7.3 (3.3) | 8.0 (4.1) | 7.7 (3.9) | 7.6 (3.8) | |
| ≥ 9 | 114 | 29.9 | 30.2 | 35.2 | 33.9 | 30.3 |
|
| ||||||
| Hysterectomy | 64 | 15.9 | 23.6 | 15.5 | 19.8 | 16.3 |
|
| ||||||
| Bilateral Oophorectomy | 32 | 7.5 | 10.4 | 9.2 | 10.2 | 7.9 |
|
| ||||||
| Self-reported health | ||||||
| Excellent / Very Good | 220 | 58.9 | 59.4 | 66.2 | 61.6 | 62.3 |
| Good | 119 | 36.4 | 37.7 | 28.2 | 33.9 | 33.1 |
| Fair | 15 | 4.7 | 1.9 | 5.6 | 4.5 | 3.9 |
Other = 18% Hispanic, 23% American Indian, 35% Asian/ Pacific Islander and 24% other groups with <5 individuals.
SD = standard deviation
Acknowledgments
We thank Dr. D. Lee Alekel, PhD, Program Director of Women’s Health, Division of Extramural Research, NCCAM, for review of the yoga protocol. We thank Ms. Lisa Temposky for her contributions to the implementation of the yoga intervention.
Support: This study was funded by the National Institutes of Health as a cooperative agreement of National Institute of Aging (NIA), Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Center for Complementary and Alternative Medicine (NCCAM), Office of Research on Women’s Health (ORWH) and grants U01 AG032656, U01AG032659, U01AG032669, U01AG032682, U01AG032699, U01AG032700 from NIA. There was partial support from UL1RR02571 (Indiana University). The omega-3 study supplement (ω-3, n-3, or polyunsaturated fatty acids) was manufactured as EPA and donated, with matching placebo, by Nordic Naturals, Watsonville, CA.
Role of the Sponsor: The National Institutes of Health had no role in the collection, analysis, and interpretation of the data. NIH staff critically reviewed the study protocol and drafts of the manuscript prior to journal submission. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health. Nordic Naturals, Inc. had no role in the design and conduct of the study, the collection, management, analysis, and interpretation of the data, or in the preparation of the manuscript.
Footnotes
Sites and Author Contributions:
The network sites that participated in this study were: Seattle, WA (Group Health Research Institute: Principal Investigators Katherine M. Newton, PhD and Susan D. Reed, MD, MPH; co-Investigators: Cathryn Booth-LaForce, PhD, Karen J. Sherman, PhD; yoga consultant: Robin Rothenberg, BS, CYTh); Indianapolis, IN (Indiana University; Principal Investigator: Janet S Carpenter, PhD, RN, FAAN, co-Investigator: Lee Learman, MD, PhD); and Oakland, CA (Kaiser Permanente Division of Research; Principal Investigators: Barbara Sternfeld, PhD and Bette Caan, PhD). The Network Data Coordinating Center is based in Seattle, WA (Fred Hutchinson Cancer Research Center: Principal Investigators: Andrea Z. LaCroix, PhD and Garnet Anderson, PhD; co-Investigators: Rebecca A. Seguin PhD and Julie R Hunt, PhD). The chairperson is Kristine E. Ensrud, MD, University of Minnesota. Other Principal investigators of the MsFlash Network that contributed to this study include Lee Cohen, MD and Hadine Joffe, MD, MSc, Massachusetts General Hospital; Ellen W. Freeman, PhD, University of Pennsylvania. Dr. Katherine Guthrie and Joseph Larson had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the analyses. All authors made substantial contributions to the study and this manuscript. None were compensated for the manuscript preparation.
Financial Disclosures:
Dr. Freeman has research support from Forest Laboratories, Inc. and Bionovo; Dr. Cohen has research support from Astra-Zeneca Pharmaceuticals, Bristol-Myers Squibb, Cephalon, Inc., Ortho-McNeil Janssen, Sunovion Pharmaceuticals, Inc. and is a consultant for Noven Pharmaceuticals; Dr. Joffe has research support from Cephalon/Teva, is on the advisory board for Noven Pharmaceuticals and is an unpaid consultant for Sunovion. Dr. Ensrud serves as a DSMB consultant for Merck Sharpe & Dohme. Dr. Dunn received honorarium/royalties from the Japanese Sport Psychiatry Association and Human Kinetics, Inc. All other authors have no financial disclosures to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Williams RE, Kalilani L, DiBenedetti DB, et al. Frequency and severity of vasomotor symptoms among peri- and postmenopausal women in the United States. Climacteric. 2008;11:32–43. doi: 10.1080/13697130701744696. [DOI] [PubMed] [Google Scholar]
- 2.Avis NE, Colvin A, Bromberger JT, et al. Change in health-related quality of life over the menopausal transition in a multiethnic cohort of middle-aged women: Study of Women’s Health Across the Nation. Menopause. 2009;16:860–869. doi: 10.1097/gme.0b013e3181a3cdaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams RE, Levine KB, Kalilani L, Lewis J, Clark RV. Menopause-specific questionnaire assessment in US population-based study shows negative impact on health-related quality of life. Maturitas. 2009;62:153–159. doi: 10.1016/j.maturitas.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Williams RE, Kalilani L, DiBenedetti DB, et al. Healthcare seeking and treatment for menopausal symptoms in the United States. Maturitas. 2007;58:348–358. doi: 10.1016/j.maturitas.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Barnabei VM, Cochrane BB, Aragaki AK, et al. Menopausal symptoms and treatment-related effects of estrogen and progestin in the Women’s Health Initiative. Obstet Gynecol. 2005;105:1063–1073. doi: 10.1097/01.AOG.0000158120.47542.18. [DOI] [PubMed] [Google Scholar]
- 6.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 7.Booth-LaForce C, Thurston RC, Taylor MR. A pilot study of a Hatha yoga treatment for menopausal symptoms. Maturitas. 2007;57:286–295. doi: 10.1016/j.maturitas.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Elavsky S, McAuley E. Physical activity and mental health outcomes during menopause: a randomized controlled trial. Ann Behav Med. 2007;33:132–142. doi: 10.1007/BF02879894. [DOI] [PubMed] [Google Scholar]
- 9.Cohen BE, Kanaya AM, Macer JL, et al. Feasibility and acceptability of restorative yoga for treatment of hot flushes: a pilot trial. Maturitas. 2007;56:198–204. doi: 10.1016/j.maturitas.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Chattha R, Raghuram N, Venkatram P, Hongasandra NR. Treating the climacteric symptoms in Indian women with an integrated approach to yoga therapy: a randomized control study. Menopause. 2008;15:862–870. doi: 10.1097/gme.0b013e318167b902. [DOI] [PubMed] [Google Scholar]
- 11.Afonso RF, Hachul H, Kozasa EH, et al. Yoga decreases insomnia in postmenopausal women: a randomized clinical trial. Menopause. 2012;19:186–193. doi: 10.1097/gme.0b013e318228225f. [DOI] [PubMed] [Google Scholar]
- 12.Carson JW, Carson KM, Porter LS, Keefe FJ, Seewaldt VL. Yoga of Awareness program for menopausal symptoms in breast cancer survivors: results from a randomized trial. Support Care Cancer. 2009;17:1301–1309. doi: 10.1007/s00520-009-0587-5. [DOI] [PubMed] [Google Scholar]
- 13.Vallance JK, Murray TC, Johnson ST, Elavsky S. Quality of life and psychosocial health in postmenopausal women achieving public health guidelines for physical activity. Menopause. 2010;17:64–71. doi: 10.1097/gme.0b013e3181b6690c. [DOI] [PubMed] [Google Scholar]
- 14.Sternfeld B, Dugan S. Physical activity and health during the menopausal transition. Obstet Gynecol Clin North Am. 2011;38:537–566. doi: 10.1016/j.ogc.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey RL, Gahche JJ, Lentino CV, et al. Dietary supplement use in the United States, 2003-2006. J Nutr. 2011;141:261–266. doi: 10.3945/jn.110.133025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hooper L, Thompson RL, Harrison RA, et al. Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: systematic review. BMJ. 2006;332:752–760. doi: 10.1136/bmj.38755.366331.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hibbeln JR, Linnoila M, Umhau JC, et al. Essential fatty acids predict metabolites of serotonin and dopamine in cerebrospinal fluid among healthy control subjects, and early- and late-onset alcoholics. Biol Psychiatry. 1998;44:235–242. doi: 10.1016/s0006-3223(98)00141-3. [DOI] [PubMed] [Google Scholar]
- 18.Chalon S. Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins Leukot Essent Fatty Acids. 2006;75:259–269. doi: 10.1016/j.plefa.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Carlezon WA, Jr, Mague SD, Parow AM, et al. Antidepressant-like effects of uridine and omega-3 fatty acids are potentiated by combined treatment in rats. Biol Psychiatry. 2005;57:343–350. doi: 10.1016/j.biopsych.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 20.Lucas M, Asselin G, Merette C, Poulin MJ, Dodin S. Effects of ethyl-eicosapentaenoic acid omega-3 fatty acid supplementation on hot flashes and quality of life among middle-aged women: a double-blind, placebo-controlled, randomized clinical trial. Menopause. 2009;16:357–366. doi: 10.1097/gme.0b013e3181865386. [DOI] [PubMed] [Google Scholar]
- 21.Campagnoli C, Abba C, Ambroggio S, et al. Polyunsaturated fatty acids (PUFAs) might reduce hot flushes: an indication from two controlled trials on soy isoflavones alone and with a PUFA supplement. Maturitas. 2005;51:127–134. doi: 10.1016/j.maturitas.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Newton KM, Carpenter JS, Guthrie KA, et al. Methods for the design of vasomotor symptom trials: the Menopausal Strategies: Finding Lasting Answers to Symptoms and Health network. Menopause. 2013 doi: 10.1097/GME.0b013e31829337a4. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sternfeld B, Guthrie KA, Ensrud KE, et al. Efficacy of Exercise for Menopausal Symptoms: A Randomized Controlled Trial. Menopause. 2013 doi: 10.1097/GME.0b013e31829e4089. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sternfeld B, LaCroix A, Caan BJ, et al. Design and methods of a multi-site, multi-behavioral treatment trial for menopausal symptoms: The MsFLASH experience. Contemp Clin Trials. 2012;35:25–34. doi: 10.1016/j.cct.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen LS, Joffe H, Guthrie KA, et al. Efficacy of Omega 3 Treatment for Vasomotor Symptoms: A Randomized Controlled Trial. Menopause. 2013 doi: 10.1097/GME.0b013e31829e40b8. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newton KM, Reed SD, Guthrie KA, et al. Efficacy of Yoga for Vasomotor Symptoms: A Randomized Controlled Trial. Menopause. 2013 doi: 10.1097/GME.0b013e31829e4baa. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lasater JH. Relax and Renew: Restful Yoga for Stressful Times. Berkeley, CA: Rodmell Press; 1995. [Google Scholar]
- 28.Hilditch JR, Lewis J, Peter A, et al. A menopause-specific quality of life questionnaire: development and psychometric properties. Maturitas. 1996;24:161–175. doi: 10.1016/s0378-5122(96)82006-8. [DOI] [PubMed] [Google Scholar]
- 29.Lewis JE, Hilditch JR, Wong CJ. Further psychometric property development of the Menopause-Specific Quality of Life questionnaire and development of a modified version, MENQOL-Intervention questionnaire. Maturitas. 2005;50:209–221. doi: 10.1016/j.maturitas.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 30.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 31.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 32.Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res. 1998;45:5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- 33.Kroenke K, Strine TW, Spitzer RL, et al. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114:163–173. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 34.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 35.Carpenter JS. The Hot Flash Related Daily Interference Scale: a tool for assessing the impact of hot flashes on quality of life following breast cancer. J Pain Symptom Manage. 2001;22:979–989. doi: 10.1016/s0885-3924(01)00353-0. [DOI] [PubMed] [Google Scholar]
- 36.Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 37.Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The Social Psychology of Health: Claremont Symposium on Applied Social Psychology. Newbury Park, CA: Sage; 1988. pp. 31–67. [Google Scholar]
- 38.Galantino ML, Greene L, Archetto B, et al. A qualitative exploration of the impact of yoga on breast cancer survivors with aromatase inhibitor-associated arthralgias. Explore (NY) 2012;8:40–47. doi: 10.1016/j.explore.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Elavsky S, McAuley E. Physical activity, symptoms, esteem, and life satisfaction during menopause. Maturitas. 2005;52:374–385. doi: 10.1016/j.maturitas.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 40.Sternfeld B, Quesenberry CP, Jr, Husson G. Habitual physical activity and menopausal symptoms: a case-control study. J Womens Health. 1999;8:115–123. doi: 10.1089/jwh.1999.8.115. [DOI] [PubMed] [Google Scholar]
- 41.Mirzaiinjmabadi K, Anderson D, Barnes M. The relationship between exercise, Body Mass Index and menopausal symptoms in midlife Australian women. Int J Nurs Pract. 2006;12:28–34. doi: 10.1111/j.1440-172X.2006.00547.x. [DOI] [PubMed] [Google Scholar]
- 42.Duijts SF, van BM, Oldenburg HS, et al. Efficacy of cognitive behavioral therapy and physical exercise in alleviating treatment-induced menopausal symptoms in patients with breast cancer: results of a randomized, controlled, multicenter trial. J Clin Oncol. 2012;30:4124–4133. doi: 10.1200/JCO.2012.41.8525. [DOI] [PubMed] [Google Scholar]
- 43.Devi SK, Chansouria JPN, Malhotra OP, Udupa KN. Certain neuroendocrine responses following the practice of Kundalini yoga. Alternative Medicine. 1986;1:247–255. [Google Scholar]
- 44.Fox KR. The influence of physical activity on mental well-being. Public Health Nutr. 1999;2:411–418. doi: 10.1017/s1368980099000567. [DOI] [PubMed] [Google Scholar]
- 45.Taylor AH, Fox KR. Effectiveness of a primary care exercise referral intervention for changing physical self-perceptions over 9 months. Health Psychol. 2005;24:11–21. doi: 10.1037/0278-6133.24.1.11. [DOI] [PubMed] [Google Scholar]
- 46.LaCroix AZ, Freeman EW, Larson J, et al. Effects of escitalopram on menopause-specific quality of life and pain in healthy menopausal women with hot flashes: A randomized controlled trial. Maturitas. 2012;73:361–368. doi: 10.1016/j.maturitas.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Utian W, Yu H, Bobula J, et al. Bazedoxifene/conjugated estrogens and quality of life in postmenopausal women. Maturitas. 2009;63:329–335. doi: 10.1016/j.maturitas.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Zollner YF, Acquadro C, Schaefer M. Literature review of instruments to assess health-related quality of life during and after menopause. Qual Life Res. 2005;14:309–327. doi: 10.1007/s11136-004-0688-z. [DOI] [PubMed] [Google Scholar]


