Abstract
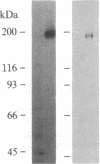
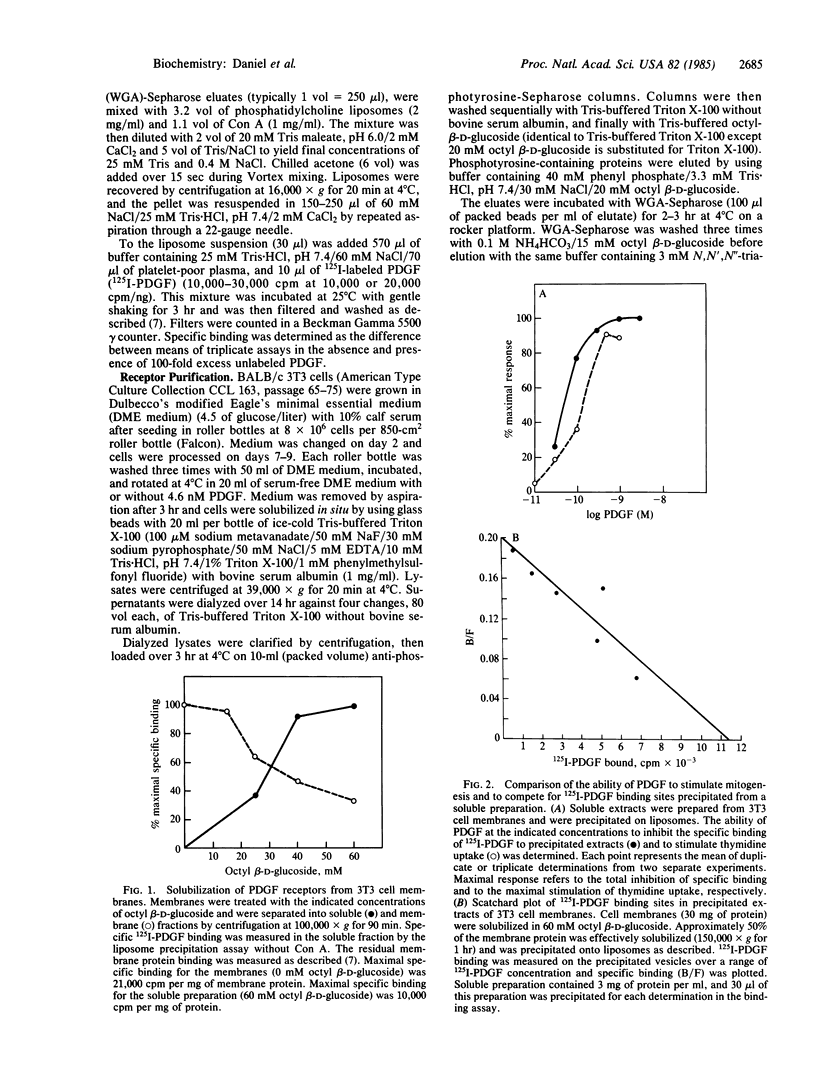
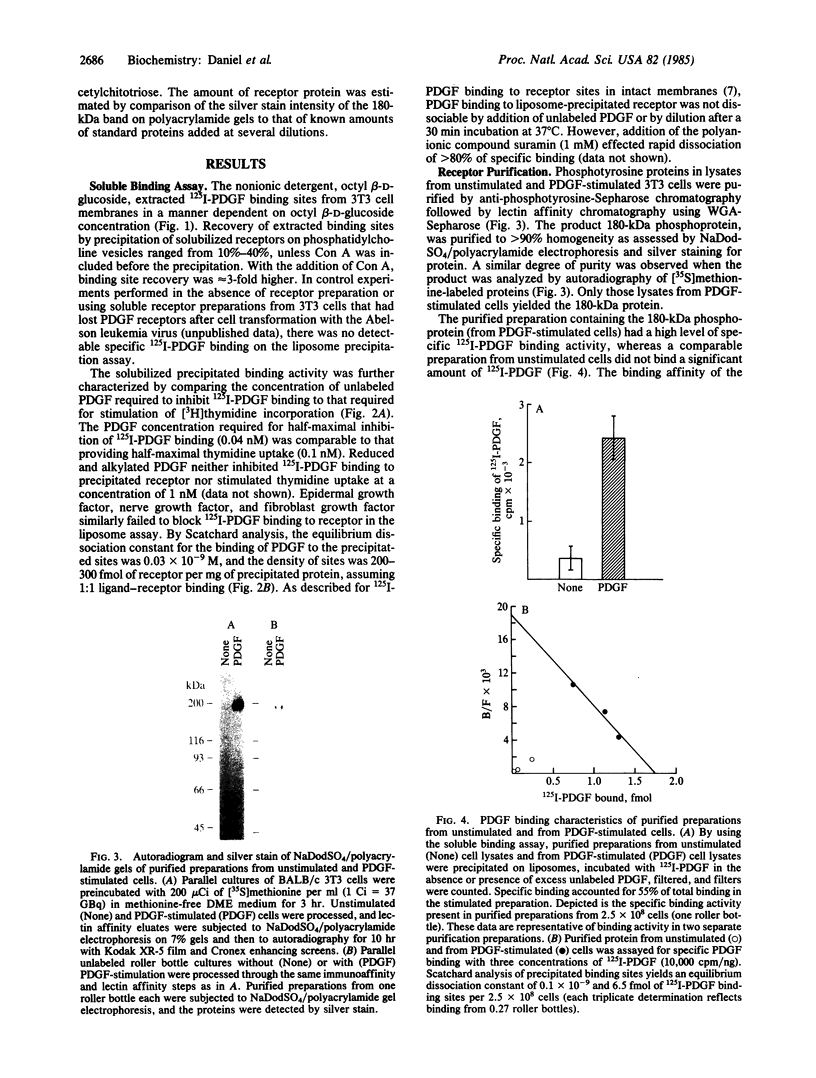
The platelet-derived growth factor (PDGF) receptor is a 180-kDa membrane glycoprotein. A protein of identical size, lectin affinity, and isoelectric point has been identified as a major substrate for PDGF-activated tyrosine kinase in stimulated 3T3 cells. We have purified this tyrosine-phosphorylated protein to homogeneity by using anti-phosphotyrosine immunoaffinity and lectin affinity steps. Demonstration that this purified tyrosine phosphoprotein is the PDGF receptor necessitated development of an assay capable of identifying specific 125I-labeled PDGF binding activity in soluble receptor preparations. PDGF receptor solubilized from 3T3 cell membranes with the detergent octyl beta-D-glucoside was precipitated on an artificial liposome matrix after receptor aggregation with concanavalin A. Precipitated binding sites display affinity and kinetic characteristics of PDGF receptors in cells and membranes. Preparations of the 180-kDa phosphoprotein that are greater than 90% homogeneous by silver stain and by [35S]methionine protein autoradiography have specific high affinity 125I-labeled PDGF binding sites (equilibrium dissociation constant, 0.1 X 10(-9) M). Binding activity enrichment in this preparation reflects an 11,000-fold purification of binding activity in intact cells. These data demonstrate that the 180-kDa substrate of the PDGF-stimulated tyrosine kinase is the PDGF receptor. Furthermore, these methods provide a means of purifying this and other tyrosine kinase substrates from growth factor-stimulated cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowen-Pope D. F., Ross R. Platelet-derived growth factor. II. Specific binding to cultured cells. J Biol Chem. 1982 May 10;257(9):5161–5171. [PubMed] [Google Scholar]
- Ek B., Heldin C. H. Characterization of a tyrosine-specific kinase activity in human fibroblast membranes stimulated by platelet-derived growth factor. J Biol Chem. 1982 Sep 10;257(17):10486–10492. [PubMed] [Google Scholar]
- Ek B., Heldin C. H. Use of an antiserum against phosphotyrosine for the identification of phosphorylated components in human fibroblasts stimulated by platelet-derived growth factor. J Biol Chem. 1984 Sep 10;259(17):11145–11152. [PubMed] [Google Scholar]
- Frackelton A. R., Jr, Tremble P. M., Williams L. T. Evidence for the platelet-derived growth factor-stimulated tyrosine phosphorylation of the platelet-derived growth factor receptor in vivo. Immunopurification using a monoclonal antibody to phosphotyrosine. J Biol Chem. 1984 Jun 25;259(12):7909–7915. [PubMed] [Google Scholar]
- Glenn K., Bowen-Pope D. F., Ross R. Platelet-derived growth factor. III. Identification of a platelet-derived growth factor receptor by affinity labeling. J Biol Chem. 1982 May 10;257(9):5172–5176. [PubMed] [Google Scholar]
- Heldin C. H., Ek B., Rönnstrand L. Characterization of the receptor for platelet-derived growth factor on human fibroblasts. Demonstration of an intimate relationship with a 185,000-Dalton substrate for the platelet-derived growth factor-stimulated kinase. J Biol Chem. 1983 Aug 25;258(16):10054–10061. [PubMed] [Google Scholar]
- Heldin C. H., Wasteson A., Westermark B. Interaction of platelet-derived growth factor with its fibroblast receptor. Demonstration of ligand degradation and receptor modulation. J Biol Chem. 1982 Apr 25;257(8):4216–4221. [PubMed] [Google Scholar]
- Huang J. S., Huang S. S., Kennedy B., Deuel T. F. Platelet-derived growth factor. Specific binding to target cells. J Biol Chem. 1982 Jul 25;257(14):8130–8136. [PubMed] [Google Scholar]
- Macara I. G., Marinetti G. V., Balduzzi P. C. Transforming protein of avian sarcoma virus UR2 is associated with phosphatidylinositol kinase activity: possible role in tumorigenesis. Proc Natl Acad Sci U S A. 1984 May;81(9):2728–2732. doi: 10.1073/pnas.81.9.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura J., Huang J. S., Deuel T. F. Platelet-derived growth factor stimulates tyrosine-specific protein kinase activity in Swiss mouse 3T3 cell membranes. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4303–4307. doi: 10.1073/pnas.79.14.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike L. J., Bowen-Pope D. F., Ross R., Krebs E. G. Characterization of platelet-derived growth factor-stimulated phosphorylation in cell membranes. J Biol Chem. 1983 Aug 10;258(15):9383–9390. [PubMed] [Google Scholar]
- Puma P., Buxser S. E., Watson L., Kelleher D. J., Johnson G. L. Purification of the receptor for nerve growth factor from A875 melanoma cells by affinity chromatography. J Biol Chem. 1983 Mar 10;258(5):3370–3375. [PubMed] [Google Scholar]
- Schneider W. J., Beisiegel U., Goldstein J. L., Brown M. S. Purification of the low density lipoprotein receptor, an acidic glycoprotein of 164,000 molecular weight. J Biol Chem. 1982 Mar 10;257(5):2664–2673. [PubMed] [Google Scholar]
- Sugimoto Y., Whitman M., Cantley L. C., Erikson R. L. Evidence that the Rous sarcoma virus transforming gene product phosphorylates phosphatidylinositol and diacylglycerol. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2117–2121. doi: 10.1073/pnas.81.7.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Y., Williams L. T. A v-sis oncogene protein produced in bacteria competes for platelet-derived growth factor binding to its receptor. J Biol Chem. 1984 Sep 10;259(17):10645–10648. [PubMed] [Google Scholar]
- Williams L. T., Tremble P. M., Lavin M. F., Sunday M. E. Platelet-derived growth factor receptors form a high affinity state in membrane preparations. Kinetics and affinity cross-linking studies. J Biol Chem. 1984 Apr 25;259(8):5287–5294. [PubMed] [Google Scholar]