Abstract
Although the genus Saccharomyces has been thoroughly studied, some species in the genus has not yet been accurately resolved; an example is S. bayanus, a taxon that includes genetically diverse lineages of pure and hybrid strains. This diversity makes the assignation and classification of strains belonging to this species unclear and controversial. They have been subdivided by some authors into two varieties (bayanus and uvarum), which have been raised to the species level by others. In this work, we evaluate the complexity of 46 different strains included in the S. bayanus taxon by means of PCR-RFLP analysis and by sequencing of 34 gene regions and one mitochondrial gene. Using the sequence data, and based on the S. bayanus var. bayanus reference strain NBRC 1948, a hypothetical pure S. bayanus was reconstructed for these genes that showed alleles with similarity values lower than 97% with the S. bayanus var. uvarum strain CBS 7001, and of 99–100% with the non S. cerevisiae portion in S. pastorianus Weihenstephan 34/70 and with the new species S. eubayanus. Among the S. bayanus strains under study, different levels of homozygosity, hybridization and introgression were found; however, no pure S. bayanus var. bayanus strain was identified. These S. bayanus hybrids can be classified into two types: homozygous (type I) and heterozygous hybrids (type II), indicating that they have been originated by different hybridization processes. Therefore, a putative evolutionary scenario involving two different hybridization events between a S. bayanus var. uvarum and unknown European S. eubayanus-like strains can be postulated to explain the genomic diversity observed in our S. bayanus var. bayanus strains.
Introduction
The genus Saccharomyces, used worldwide to produce different fermented foods and beverages, encompasses the industrially most exploited species known to man. The complex diversity of the genus Saccharomyces, including pure, hybrid and introgressed strains, makes species definition difficult and classification controversial. According to the most recent edition of ‘The Yeast, a taxonomic study’ [1], the genus Saccharomyces is composed of eight species: S. arboricolus, S. bayanus, S. cariocanus, S. cerevisiae, S. kudriavzevii, S. mikatae, S. paradoxus and S. pastorianus. Although several studies have shown that S. pastorianus comprises a group of alloploid hybrid strains originated from S. cerevisiae and a cryotolerant species similar to S. bayanus [2], [3], the last systematic revision maintained the species status for S. pastorianus [1].
In a recent study, Libkind et al. [4] isolated and characterized a new Saccharomyces species, named S. eubayanus and associated with Nothofagus spp. trees in Patagonia (Argentina). As the draft genome sequence of this species was closely related to the non S. cerevisiae portion of S. pastorianus (average divergence of 0.44%), the authors proposed S. eubayanus as the previously mentioned S. bayanus-like donor of this subgenome in S. pastorianus hybrids.
The other controversial Saccharomyces taxon is the species S. bayanus [1]. S. bayanus encompasses a group of cryotolerant strains with active fructose transport, including the former species S. abuliensis, S. bayanus, S. globosus, S. heterogenicus, S. intermedius, S. inusitatus, S. tubiformis, S. uvarum and S. willianus. Based on the quite diverse physiological [5] and genetic [6], [7] traits found among different S. bayanus strains, some authors have proposed dividing this taxon into two different species, S. bayanus and S. uvarum [8], [9]. However, the partial reproductive isolation between the strains of both groups has alternatively suggested the subdivision of the species into two varieties, bayanus and uvarum [10], which was maintained in the most recent taxonomical review of the genus Saccharomyces [1].
Rainieri et al. [11] evaluated the genetic variability of 35 yeast strains identified as S. bayanus or S. pastorianus, and observed a very complex picture. By means of PCR-RFLP and sequencing, the authors confirmed that the type strain of S. bayanus (CBS 380T) was composed of clearly differentiated ‘bayanus’ and ‘uvarum’ subgenomes. The authors identified four different genomic compositions among the studied strains: (i) a pure line named S. uvarum that included strains containing a single type of genome, with similar physiological and genetic characteristics to the type strain of the former species S. abuliensis CBS 7001; (ii) a pure line with a single type of genome named S. bayanus that included only strain NBRC 1948; (iii) a hybrid line including strains with portions of the genomes from the two pure lines, as well as alleles termed ‘Lager’ (representing a third genome present in lager brewing strains); iv) a group of S. cerevisiae/S. bayanus/Lager and S. cerevisiae/S. bayanus/S. uvarum/Lager hybrid strains (S. pastorianus). While the pure nature of strain CBS 7001 was confirmed by Libkind et al. [4], these authors together with Nguyen et al. [12] demonstrated that strain NBRC 1948 harbors a mosaic genome composed of a hybrid genetic background belonging to S. uvarum and a second unidentified species, which Nguyen et al. provisionally named S. lagerae. However, Libkind et al. identified it as belonging to the new species S. eubayanus, as well as some small introgressed regions from S. cerevisiae.
The main goal of the present study was to decipher the complexity of the S. bayanus taxon by performing PCR-RFLP analyses of 34 nuclear genes and by sequencing both nuclear and mitochondrial genes from the 46 different strains identified originally as S. bayanus or S. uvarum, including the type strains of the former species and the natural isolates from different sources (cider, wine, fruit fermentations, etc.) in the light of the discovery of the new taxon S. eubayanus. For this purpose, some S. pastorianus strains were also evaluated for comparative purposes. The putative hybridization events responsible for the genomic complexity found in the S. bayanus taxon are proposed and discussed.
Materials and Methods
Yeasts strains and media
The yeast strains used in this study, together with their sources of isolation and geographical origins, are listed in Table 1. Strains were grown on YPD (w/v: 1% of yeast extract, 2% peptone, 2% glucose) at 28°C and were maintained on YPD supplemented with 2% w/v agar.
Table 1. List of Saccharomyces strains analyzed in the present study.
| Strain reference | Original epithet | Isolation source | Geographic origin | Present characterization | |
| CECT | Other | ||||
| 1189 | CBS 6308 | Ale beer | Yorkshire (England) | S. uvarum | |
| 1369 | Unknown | Spain | S. uvarum | ||
| 1884 | S. uvarum | Wine fermentation | Mentrida (Spain) | S. uvarum | |
| 1941 | Unknown | S. eubayanus x S. uvarum | |||
| 1969T | CBS 395T | Type of S.uvarum | Juice of Ribes nigrum | Netherlands | S. uvarum |
| 1991 | DSMZ 70411 | Turbid bottled beer | S. eubayanus x S. uvarum | ||
| 10618 | Alpechin | Spain | S. uvarum | ||
| 10174 | Unknown | Spain | S. uvarum | ||
| 11035T | CBS 380T | Type of S. bayanus | Beer | S. eubayanus x S. uvarum | |
| 11036 | CBS 381 | Type of S. willianus | Spoiled beer | S. uvarum | |
| 11135 | CBS375 | Unknown | S. eubayanus x S. uvarum | ||
| 11185 | NBRC 1948 | S. bayanus | Unknown | S. eubayanus x S. uvarum | |
| 11186 | NCYC 115 | Unknown | S. eubayanus x S. uvarum | ||
| 12600 | S. ellipsoideus | Sweet wine | Alicante (Spain) | S. uvarum | |
| 12627 | S. bailli | Wine | Valladolid (Spain) | S. uvarum | |
| 12629 | S. uvarum | Must | Zaragoza (Spain) | S. uvarum | |
| 12638 | S. uvarum | Must | León (Spain) | S. uvarum | |
| 12669 | S. pastorianus | Grapes | La Rioja (Spain) | S. uvarum | |
| 12922 | S. carlsbergensis | Jerez grapes wine | Valladolid (Spain) | S. uvarum | |
| 12930 | S. bayanus | Wine | Spain | S. uvarum | |
| CBS 377 | Type of S. intermedius | Pear wine | Germany | S. uvarum | |
| CBS 378 | Unknown | Unknown | S. eubayanus x S. uvarum | ||
| CBS 424 | Type of S. globosus | Pear juice | Meggen (Switzerland) | S. eubayanus x S. uvarum | |
| CBS 425 | Type of S. heterogenicus | Fermenting apple juice | Tägerwilen (Switzerland) | S. eubayanus x S. uvarum | |
| CBS 431 | Type of S. tubiformis | Fermenting pear juice | S. uvarum | ||
| CBS 1546 | Type of S. inusitatus | Beer | Rotterdam (Netherlands) | S. eubayanus x S. uvarum | |
| CBS 2898 | Wine starter | Herrliberg (Switzerland) | S. uvarum | ||
| CBS 2946 | Unknown | Unknown | S. uvarum | ||
| CBS 2986 | Wine | Salenegg (Switzerland) | S. uvarum | ||
| CBS 3008 | Must of soft fruit | Unknown | S. eubayanus x S. uvarum | ||
| NCAIM676 | Fermented drink | Hungary | S. eubayanus x S. uvarum | ||
| NCAIM677 | Fermented drink | Hungary | S. eubayanus x S. uvarum | ||
| NCAIM789 | Carpinus betulus exudate | Babat (Hungary) | S. uvarum | ||
| NCAIM868 | Slimy material on a stump | Dorog (Hungary) | S. uvarum | ||
| S4 | Cider | Clonmel (Ireland) | S. uvarum | ||
| S10 | Cider | Clonmel (Ireland) | S. uvarum | ||
| S14 | Cider | Clonmel (Ireland) | S. uvarum | ||
| S20 | Cider | Clonmel (Ireland) | S. uvarum | ||
| ZIM 2113 | Must of Kraljevina | Dolenjska (Slovenia) | S. uvarum | ||
| ZIM 2122 | Must of Žametna črnina | Dolenjska (Slovenia) | S. uvarum | ||
| 1940NT | CBS 1538NT | Neotype of S. pastorianus | Lager beer | Denmark | S. eubayanus x S. uvarum |
| 1970 | CBS 1503 | Type of S. monacensis | Lager beer | Denmark | S. cerevisiae x S. eubayanus |
| 11037 | CBS 1513 | Type of S. carlsbergensis | Lager beer | Denmark | S. cerevisiae x S. eubayanus |
| 11000 | NCYC 2340 | Lager beer | Unknown | S. cerevisiae x S. eubayanus | |
| 1885 | S. cerevisiae | Wine | Valladolid (Spain) | S. cerevisiae x S. eubayanus | |
| S6U | Wine | Italy | S. cerevisiae x S. uvarum | ||
, neotype,
, type.
Culture collection abbreviated as follows: CECT, Colección Española de Cultivos Tipo, Valencia, Spain; CBS, Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; DSMZ, German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany; NBRC; NCAIM, National Collection of Agricultural and Industrial Microorganisms, Faculty of Food Sciences, Corvinus University of Budapest, Hungary; NCYC, National Collection of Yeast Cultures, Norwich, UK; ZIM, ZIM Culture Collection of Industrial Microorganisms, University of Ljubljana, Slovenia.
PCR amplification
The characterization of S. bayanus var. bayanus, S. bayanus var. uvarum, S. cerevisiae and S. pastorianus strains was performed by PCR amplification and the subsequent restriction analyses of 34 protein-coding genes distributed along the 16 chromosomes present in these yeasts (Figure S1 in File S1). These genes were probed to be suitable to differentiate among the species of Saccharomyces genus [13]. The oligonucleotides used as primers for the PCR amplifications are provided in Table S1 in File S1.
Although the S. bayanus var. bayanus and S. bayanus var. uvarum genomes are almost co-linear to that of S. cerevisiae, they differ in several reciprocal translocations, hence some gene regions are located in other linkage groups (Figure S1 in File S1). In this way, S. bayanus var. bayanus differs from S. cerevisiae in two reciprocal translocations among chromosomes II and IV and VIII [3], [14], while S. bayanus var. uvarum contains two other translocations between chromosomes VI and X, and between XIV and IItIV [15].
Total yeast DNA was isolated following standard procedures [16]. PCR reactions were performed in a final volume of 100 μl containing 10 μl of 10x Taq DNA polymerase buffer, 100 μM deoxynucleotides, 1 μM of each primer, 2 units of Taq DNA polymerase (BioTools, B&M Labs, Madrid, Spain) and 4 μl of DNA diluted to 1–50 ng/μl. PCR amplifications were carried out in Techgene and Touchgene thermocyclers (Techne, Cambridge, UK) as follows: initial denaturing at 95°C for 5 min, then 40 PCR cycles involving the following steps: denaturing at 95°C for 1 min, annealing at 55°C (for most genes), and extension at 72°C for 2 min, then a final extension at 72°C for 10 min. For genes ATF1, DAL1, EGT2, KIN82, MNT2, MRC1, RRI2 and UBP7, annealing was performed at 50°C.
PCR products were run on 1.4% agarose (Pronadisa, Madrid, Spain) gels in 0.5x TBE buffer. After electrophoresis, gels were stained with 0.5 μg/ml of ethidium bromide solution (AppliChem, Darmstadt, Germany) and were visualized under UV light. A 100-bp DNA ladder marker (Roche Molecular Biochemicals, Mannheim, Germany) served as a size standard.
Restriction analysis of nuclear gene regions
Simple digestions with different endonuclases were performed with 15 μl of amplified DNA to a final volume of 20 μl. Restriction endonucleases Acc I, Asp I, Asp 700I, Cfo I, Dde I, Eco RI, Hae III, Hind III, Hinf I, Msp I, Pst I, Rsa I, Sac I, Scr FI, Taq I and Xba I (Roche Molecular Biochemicals, Mannheim, Germany) were used according to the supplier's instructions. Restriction fragments were separated on 3% agarose (Pronadisa, Madrid, Spain) gel in 0.5 x TBE buffer. A mixture of 50-bp and 100-bp DNA ladder markers (Roche Molecular Biochemicals, Mannheim, Germany) served as size standards.
Amplification, cloning, sequencing and phylogenetic analysis of nuclear genes
The 34 gene regions used in this study were amplified and sequenced in NBRC 1948 strain for the genetic reconstruction of a hypothetical S. bayanus var. bayanus genome. For genes EPL1, GSY1, JIP5, KIN82, MRC1, PEX2, MAG2, NPR2 and ORC1 additional sequences were obtained (sequences obtained from strains CECT 11186 and CBS 424). Additionally, new alleles were sequenced to confirm their nature (“uvarum”, “eubayanus” or “cerevisiae” alleles). These sequences were deposited in the nucleotide databases under accession numbers KJ093508 to KJ093569.
For gene MNL1, no diagnostic restriction patterns for the differentiation of the ‘eubayanus’- and ‘uvarum’-type alleles were found. Therefore, the MNL1 PCR products were sequenced, in all the strains, for allele discrimination, and the corresponding sequences were deposited under accession numbers KJ093570 to KJ093618
The PCR products were purified using the Perfectprep Gel Cleanup Kit (Eppendorf, Hamburg, Germany) following the manufacturer's instructions, and were subsequently sequenced for allele discrimination. Sequencing was performed with the BigDye Terminator V3.1 Cycle Sequencing Kit (Applied Biosystems, Warrington, UK) according to the manufacturer's instructions. The sequencing reactions were run on a Techgene Thermal Cycler (Techne, Cambridge, UK), which was programmed as follows: an initial denaturation at 94°C for 3 min, followed by 99 cycles of denaturation at 96°C for 10 s, annealing at 50°C for 5 s, and polymerization at 60°C for 4 min. Sequences were obtained with an Applied Biosystems automatic sequencer model ABI 3730 (Applied Byosistems, Warrington, UK).
For the heterozygous strains exhibiting ambiguous nucleotide sequences, given the presence of more than one allele, the PCR amplifications were cloned and sequenced to obtain the nucleotide sequence of each allele. Cloning was carried out with the pGEM T Easy Vector System ll kit (Promega, Madison, USA) by preparing a ligation reaction with a final volume at 3.3 μL and by incubating overnight at 4°C. The transformation reaction was performed with 20 μL of competent cells JM 109 (Promega, Madison, USA) and 2 μL of the ligation reaction, and the mix was incubated by shaking at 200 rpm for 1.5 h. A volume of 120 μL was plated in LB medium (1% tryptone, 0.5% yeast extract, 1% glucose, 1.5% agar) with 100 μg/mL ampicillin, 0.5 mM IPTG, and 80 μg/mL X-Gal. Plates were incubated for 24 h at 37°C and at least 12 positive colonies were isolated for the direct PCR amplification from colony, and the subsequent sequencing was done according to the conditions described above.
Alignments were done using the Clustal W algorithm as implemented in the MEGA 4.0 software [17]. Similarities between ‘eubayanus’ and ‘uvarum’ alleles were estimated as nucleotide identities per 100 sites (%).
The jModelTest program [18] was used to estimate the evolutionary model that best represents the nucleotide divergence data provided by the MNL1 sequences by applying the Bayesian information criterion [19]. The best fitting model was the Kimura 2-paremetter model [20] with a gamma distribution (G) of substitution rates with a shape parameter of α = 0.099. A maximum likelihood (ML) tree was obtained with PHYML 3.0 [21] by applying the corresponding K2-p +G model. The statistical support for the resulting topology was assessed using a nonparametric bootstrap with 100 pseudo-replicates [22].
Amplification, sequencing and phylogenetic analysis of COX2
To establish the COX2 gene haplotypes present in the strains under study, this mitochondrial gene region was PCR-amplified and subsequently sequenced given the absence of diagnostic restriction sites. COX2 was amplified using the primers and conditions described in Belloch et al. [23]. PCR products were cleaned with the Perfectprep Gel Cleanup kit (Eppendorf, Hamburg, Germany) and both DNA strands were sequenced directly using the BigDyeTM Terminator v3.0 Cycle Sequencing kit (Applied Biosystems, Warrington, UK) following the manufacturer's instructions, in an Applied Biosystems automatic DNA sequencer Model ABI 3730.
COX2 sequences (accesion numbers AF442212, AJ938046, AJ938045, AJ966729, and JN676768 to JN676813) were aligned and analyzed with the MEGA 4 program [17]. Due to low divergences and the presence of a putative recombination, phylogenetic trees were obtained by the Neighbor-Joining method using the p-distance (uncorrected nucleotide divergence). Tree reliability was assessed using a nonparametric bootstrap with 2000 pseudo-replicates.
Results
Genetic reconstruction of a hypothetical S. bayanus var. bayanus or ‘bayanus’ pure line
By using a set of the 34 pairs of primers (Table S1 in File S1) previously generated in our laboratory for Saccharomyces hybrids detection and characterization, the nuclear gene regions of NBRC 1948 strain were amplified and sequenced. This strain was selected as the most representative S. bayanus var. bayanus strain, because was defined by Rainieri et al. [11] as a “pure” S. bayanus var. bayanus strain. These sequences were then compared to the homologous regions of the genome sequence of strain CBS 7001(available at http://www.saccharomycessensustricto.org). This strain, also known as MCYC623, is considered as a pure S. bayanus var. uvarum strain [11].
Each pair of homologous sequences were aligned and the corresponding nucleotide similarities were estimated as shown in Table 2. From the 34 gene compared regions, identical sequence pairs for genes EPL1, GSY1, JIP5, KIN82, MRC1 and PEX2 (100% similarity), and almost identical sequences for genes MAG2, NPR2 and ORC1 (99.6 to 99.9% similarity), were observed. However, 25 homologous sequence pairs showed similarities lower than 97%, and between 86.0% for CBP2 and 96.7% for MET6 (Table 2).
Table 2. Sequence similarity for 34 protein-coding genes between the reference strain of S. bayanus var. bayanus (NBRC 1948) and the reference strains of S. bayanus var. uvarum (CBS 7001) and the ‘eubayanus’ alleles of the S. pastorianus strain Weihenstephan 34/70 (W 34/70).
| Gene | Similarity (%) between CBS 7001 and | Similarity (%) between W 34/70 ‘eubayanus’ alleles and | Similarity (%) between CBS 7001 and W 34/70 ‘eubayanus’ alleles | ||||
| NBRC 1948 | CECT 11186 | CBS 424 | NBRC 1948 | CECT 11186 | CBS 424 | ||
| APM3 | 92.7 | - | - | 100 | - | - | 92.7 |
| ATF1 | 91.2 | - | - | 99.2 | - | - | 91.8 |
| BAS1 | 91.9 | - | - | 100 | - | - | 91.9 |
| BRE5 | 86.7 | - | - | 100 | - | - | 86.7 |
| BUD14 | 92.1 | - | - | 99.9 | - | - | 92.0 |
| CAT8 | 91.9 | - | - | 99.6 | - | - | 92.1 |
| CBP2 | 86.0 | - | - | 100 | - | - | 86.0 |
| CBT1 | 91.4 | - | - | 99.4 | - | - | 91.6 |
| CYC3 | 91.5 | - | - | 100 | - | - | 91.5 |
| CYR1 | 93.2 | - | - | 100 | - | - | 93.2 |
| DAL1 | 92.0 | - | - | 99.9 | - | - | 92.2 |
| EGT2 | 88.6 | - | - | 99.7 | - | - | 88.9 |
| EPL1 | 100 | 92.6 | 92.6 | 92.7 | 99.9 | 99.9 | 92.5 |
| EUG1 | 90.3 | - | - | 99.5 | - | - | 90.4 |
| GAL4 | 91.2 | - | - | none | - | - | none |
| GSY1 | 100 | 95.4 | 95.4 | 95.4 | 99.7 | 99.7 | 95.4 |
| JIP5 | 100 | 100 | 91.9 | 91.9 | 91.9 | 96.6 | 91.9 |
| KEL2 | 87.7 | - | - | 99.9 | - | - | 87.8 |
| KIN82 | 100 | 92.3 | 99.7 | none | None | none | none |
| MAG2 | 99.9 | 99.9 | 93.9 | 94.0 | 94.0 | 100 | 93.9 |
| MET6 | 96.7 | - | - | 100 | - | - | 96.7 |
| MNL1 | 89.6 | - | - | 100 | - | - | 89.6 |
| MNT2 | 91.0 | - | - | 100 | - | - | 91.0 |
| MRC1 | 100 | 90.7 | 99.8 | 90.7 | 99.2 | 90.7 | 90.7 |
| NPR2 | 99.7 | 93.0 | 93.0 | 93.2 | 99.9 | 99.9 | 92.9 |
| OPY1 | 92.8 | - | - | 100 | - | - | 92.8 |
| ORC1 | 99.6 | 89.5 | 99.7 | 89.7 | 100 | 89.7 | 89.5 |
| PEX2 | 100 | 100 | 92.4 | 92.3 | 92.3 | 99.9 | 92.3 |
| PKC1 | 91.9 | - | - | 100 | - | - | 91.9 |
| PPR1 | 95.6 | - | - | 99.6 | - | - | 95.8 |
| RPN4 | 90.5 | - | - | 100 | - | - | 90.5 |
| RRI2 | 90.1 | - | - | 100 | - | - | 90.1 |
| UBP7 | 92.5 | - | - | 100 | - | - | 92.5 |
| UGA3 | 91.0 | - | - | 99.9 | - | - | 91.3 |
For those genes exhibited between the CBS 7001 and NBRC 1948 similarities ≥99.6% (in bold), additional sequences were obtained for S. bayanus strains CECT 11186 or CBS 424, exhibiting divergent alleles, and their similarities to CBS 7001 and W 34/70 are also provided. Those gene sequence comparisons for which S. pastorianus Weihenstephan 34/70 contains only ‘cerevisiae’ alleles are indicated by ‘none’ (i.e., no eubayanus alleles are present to compare).
To check if the nine identical or almost identical sequences found in both NBRC 1948 and CBS 7001 could be fixed characteristics of the S. bayanus species genome, sequences for those genes were obtained from two other S. bayanus var. bayanus strains, CECT 11186 (NCYC 115) and CBS 424. Three sequences from CECT 11186 (JIP5, MAG2 and PEX2) and three others from CBS 424 (KIN82, MRC1 and ORC1) were identical or almost identical to the sequences in CBS 7001 and NBRC 1948. However, all the remaining sequences analyzed in the two additional strains gave lower similarity values, around 89.5–95.4%, as compared to the reference sequences (Table 2).
By combining the sequence data from strains NBRC 1948, CECT 11186 and CBS 424, a complete set of ‘bayanus’ alleles of a hypothetical S. bayanus var. bayanus pure line (alleles with similarity values lower than 97% as compared to strain CBS 7001) was obtained.
Using the sequences obtained in this study, we performed a genome BLAST search on the non cerevisiae sub-genome of the S. pastorianus strain Weihenstephan 30/70 available in NCBI. Two homologous sequences were obtained for all genes, each corresponding to one of the two subgenomes (the S. eubayanus and S. cerevisiae subgenomes according to Libkind et al. [4]). The only exceptions were genes KIN82 and GAL4, for which only one highly similar sequence to S. cerevisiae was obtained. Sixteen gene sequences from the ‘eubayanus’ fraction of S. pastorianus were 100% identical to the sequences comprising our hypothetical S. bayanus pure line, and 15 gene sequences were almost identical (between 99.2% and 99.9% of similarity)(Table 2).
The divergent genes between the ‘uvarum’ pure line CBS 7001 and our hypothetical ‘bayanus’ pure line (the alleles from NBRC 1948, CECT 11186 or CBS 424) were also divergent between CBS 7001 and the ‘eubayanus’ Weihenstephan 34/70 gene sequences (similarities of 86.0–96.7%) (Table 2).
After considering the high similarity of the gene sequences between the ‘eubayanus’ alleles in the S. pastorianus strain and our ‘hypothetical S. bayanus var. bayanus’, we used the name ‘eubayanus’, or simply ‘E’, to designate these alleles henceforth. Within this new framework, strain CBS 7001 contained only ‘uvarum’ alleles (or simply ‘U’), but strain NBRC 1948 contained both E and an important fraction of U alleles (26.5% of the genes under study). Our results indicate that the two alleles have an average divergence of 8.4% (between 3.3% and 14%) for the analyzed gene sequences.
Characterization of the strains belonging to the S. bayanus taxon based on the presence of both alleles ‘eubayanus, E’ and ‘uvarum, U’
To characterize the complex S. bayanus taxon and to find a putative pure S. bayanus var. bayanus strain, a PCR-RFLP analysis of the 34 gene regions was performed on a panel of 46 strains deposited in culture collections under species name S. bayanus, S. uvarum or S. pastorianus (Table 1). According to the sequence differences observed between alleles E and U, only the restriction endonucleases able to differentiate both alleles for each particular gene were chosen from those proposed by González et al.[13] (Table S2 in File S1). New restriction endonucleases were used for gene sequences for which the enzymes proposed by González et al [13] did not differentiate between the two alleles (Table S2 in File S1). In order to merely avoid wrong allele type assignation due to intra-type sequence variations, we used a single restrictase to assign U or E alleles only when more than two restriction site gains/losses were observed between both alleles (because small variant of the alleles can sometimes make one fragment get cutted into 2 fragments while the rest of the pattern remains the same). Whenever this condition was not achieved with a single restrictase, additional restriction enzymes were used.
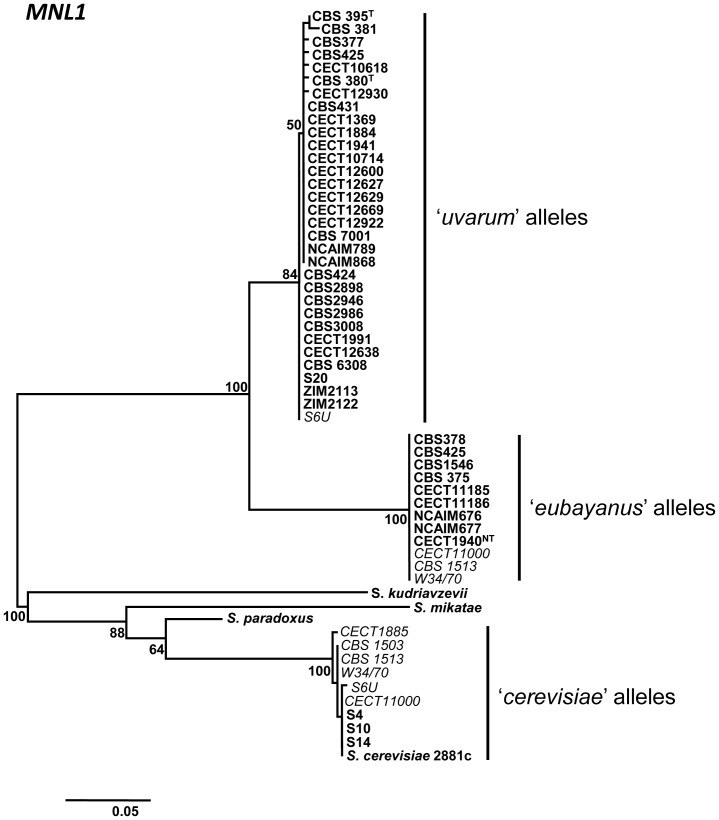
Accordingly, the restriction patterns similar to those present in reference strain S. bayanus var. uvarum CBS 7001 were named ‘U1’, while those present in the reconstructed pure S. bayanus var. bayanus and in S. pastorianus strain Weihenstephan 34/70 (from S. eubayanus) were named ‘E1’. The restriction patterns similar to those present in reference strain S. cerevisiae S288c were named ‘C1’. As we were unable to find diagnostic restriction patterns to differentiate alleles E and U for the MNL1 gene region, the analysis of the MNL1 region was done by sequencing (Figure 1).
Figure 1. Phylogenetic tree obtained with the partial sequences of the nuclear MNL1 gene.
Numbers at nodes correspond to the bootstrap values based on 1000 pseudo-replicates. The scale is in nucleotide substitutions per site. All the strains are indicated in bold, except the S. pastorianus ones.
Following the procedures described before, we obtained a complete characterization of all the strains listed in Table 1. These results are summarized in Figure 2 and Tables S4 and S5 in File S1. Some strains exhibited alternative restriction patterns, which differed by one restriction site gains/losses from the C1, E1 or U1 patterns present in the reference strains. These new alleles were sequenced and their similarities with the reference C, U and E alleles were tested. These new alleles were named C, E or U (depending on the closest allele), followed by an ordinal number from 2 onward, as shown in Table S3 in File S1.
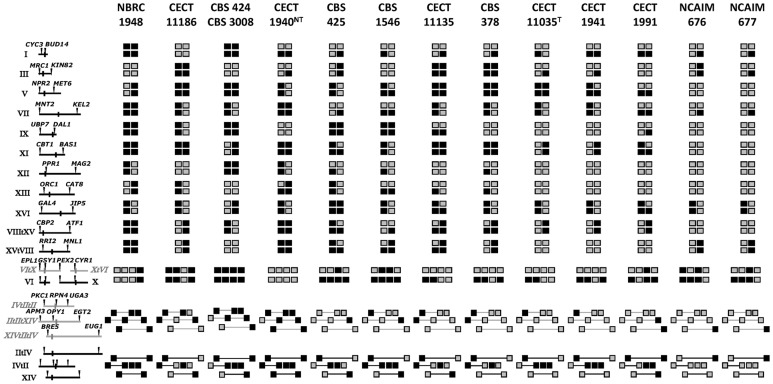
Figure 2. RFLPs of 34 nuclear genes from the S. bayanus strains analyzed in this work.
Each square corresponds to a copy of each gene region according to its chromosome location, indicated in the map on the left. ‘eubayanus’ alleles are indicated as black squares and ‘uvarum’ alleles as gray squares. The genes order differs between S. bayanus var. bayanus and S. bayanus var. uvarum, as depicted, due to the presence of two translocations. The first involves chromosomes VI and X in S. bayanus var. bayanus and chromosomes VItX and XtVI in S. bayanus var. uvarum, while the second involves chromosomes IItIV, IVtII and XIV in S. bayanus var. bayanus and chromosomes IItIItIV, IVtIItII and XIVtIItIV in S. bayanus var. uvarum.
Twenty-seven of the 46 strains showed only U alleles for 33 of the 34 analyzed nuclear gene sequences. Seven of them showed U alleles for the 34 analyzed genes and twenty showed a C2 allele for PEX2 gene region, being the most frequent alternative allele detected among the analyzed strains (20 of the 27 strains bearing only U alleles showed this C2 allele). Among them, 13 different nuclear genotypes were observed due to the presence of alternative U2 alleles for different gene regions (Table S4 in File S1). These new allele variants were observed only for genes MNT2, UBP7, BAS1, RRI2 and BRE5. Most of these strains exhibited only one allele for the 34 analyzed genes, except for strains ZIM 2122 and NCAIM 868, which were heterozygous U1/U2 for genes RRI2 and BAS1, respectively. Finally, Irish cider strains S4, S10, and S14 contained a similar combination of alleles U1 and U2 to that found in strains CBS 2946 and NCAIM 789 for all genes analyzed, except for gene MNL1 (Table S4 in File S1), for which they showed a ‘cerevisiae’ (C) allele, as observed after the sequence analysis (Figure 1).
Fourteen strains contained different combinations of alleles U and E (Table S5 in File S1), indicating their ‘uvarum’ x ‘eubayanus’ hybrid nature. These included strains NBRC 1948, the type strain of S. bayanus CBS 380T and the putative neotype strain of S. pastorianus CECT 1940NT. It was possible to clearly differentiate these U x E hybrids into two groups (types I and II) according to their genetic constitution. To obtain a more illustrative picture of this situation, we represent the genetic constitution of these strains containing alleles U and E in Figure 2. The strains included in Type I (strains NBRC 1948, CECT 11186, CBS 424 and CBS 3008) appeared to be homozygous for all 34 genes under study (Figure 2); while the alloploid strains that presented some genes in heterozygosis (U/E alleles) were included in Type II (Figure 2 and Table S5 in File S1). The total number of heterozygous E/U loci varied from 9% in strain NCAIM 676 to 44% in strain CBS 1546. Alternative E2 alleles were observed for genes DAL1 (strains CBS 424 and CBS 3008) and BAS1 (strains CBS 424, CBS 3008, CBS 425 and CECT 1991), while alternative U2 alleles were observed for genes MNT2, UBP7, BAS1, RRI1 and BRE5 in different strains (Table S5 in File S1).
Another group of strains included those identified as S. pastorianus and were, therefore, characterized by the additional presence of ‘cerevisiae’ (C) alleles (Table S6 in File S1). Among them, wine commercial strain S6U exhibited alleles U and C for 33 genes and alleles C1 and C2 for PEX2 gene. Three strains, including the former type strains of S. carlsbergensis (CBS 1513 = CECT 11037) and S. monacensis (CBS 1503 = CECT 1970) and one wine strain (CECT 1885), contained different combinations of alleles E and C. Finally, lager brewing strain CECT 11000 contained the three types of alleles (E, C and U), although alleles U were found for only five gene regions, including MRC1, NPR2, KEL2, GSY1 and EGT2 (Table S6 in File S1). Interestingly, all the previously mentioned yeasts (except S6U) exhibited alleles E2 for two genes: BAS1 and BRE5.
According to our data, no pure strains bearing 100% E alleles were found among our S. bayanus strains. Based on the presence of alleles E and U, it was possible to divide the S. bayanus strains analyzed in this work into three groups: (i) a ‘S. bayanus var. uvarum’ pure-line group that includes those strains containing only U, in which some limited S. cerevisiae introgressions may have occurred, as with strains S04, S10 and S14, showing a C allele in the subtelomeric gene MNL1 or the 20 strains showing a C2 allele in the subtelomeric gene PEX2; (ii) a homozygous ‘S. bayanus var. bayanus’ group including strains with both alleles E and U in homozygosis (Type I); (iii) an alloploid ‘S. bayanus var. bayanus’ group containing strains with both alleles E and U in heterozygosis (Type II).
It was also possible to divide the S. pastorianus strains into three groups: (i) hybrids with alleles C, E and U (ii) hybrids with alleles C and E and (iii) hybrids with alleles C and U.
About the origin of mitochondrial DNA in S. bayanus
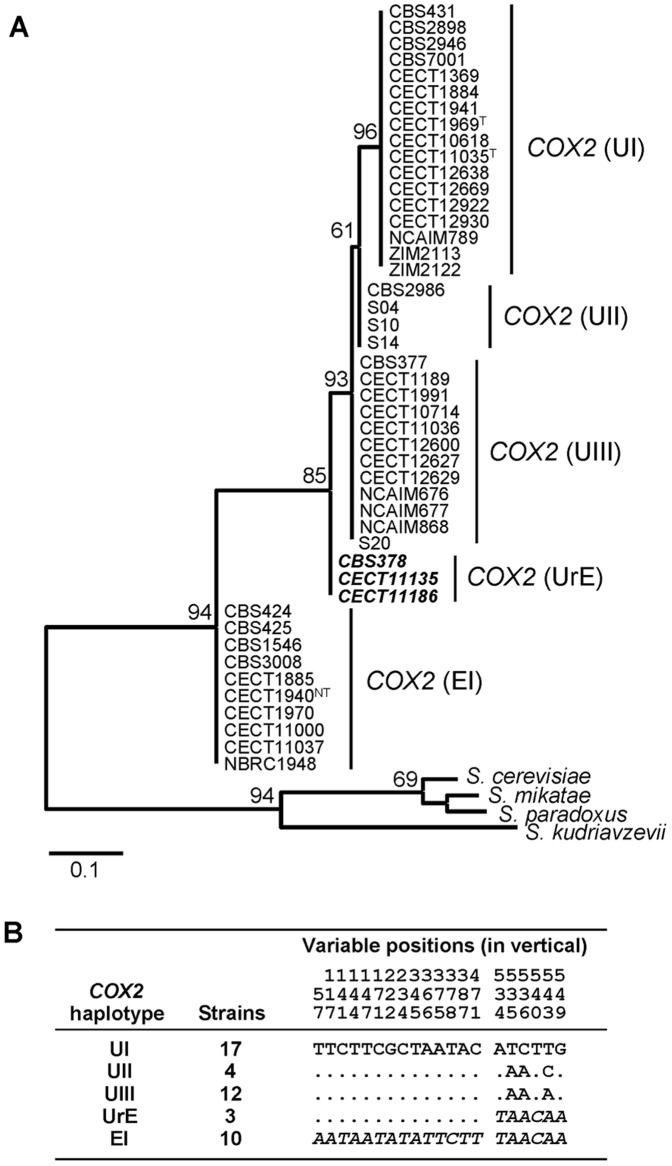
In order to obtain a more complete picture of the identity of the S. bayanus strains studied, we also analyzed the nature of their mtDNA. For this purpose, we evaluated mitochondrial gene COX2 from all 46 strains. Due to the difficulties in unveiling COX2 variability in Saccharomyces by restriction analyses, we performed direct sequencing. Five groups of strains were separated according to the COX2 phylogenetic analysis (Figure 3A). The strains possessing only U haplotypes for the 34 analyzed genes (the ‘uvarum’ pure line strains) were separated into three COX2 haplotypes: U-I, U-II and U-III. Haplotype U-I, found in reference strain CBS 7001, was the most frequent among our strains. Haplotype U-II was shared by cider strains and wine strain CBS2986, and haplotype U-III was observed in nine strains of diverse origins (Figure 3A).
Figure 3. Phylogenetic analysis of the partial sequences of the mitochondrial COX2 gene.
A- NJ tree. Numbers at nodes correspond to the bootstrap values based on 1000 pseudo-replicates. The scale is in nucleotide substitutions per site. Strains with a controversial adscription are indicated in bold. B- Variable positions on the different COX2 haplotypes. A dot indicates the presence of the same nucleotide at this position. Haplotype U-I is used as a reference.
All the S. pastorianus strains CBS 1503, CBS 1513, CECT 1885 and CECT 11000 showed the same haplotype E–I, postulated as being received from the S. eubayanus progenitor according to the phylogenetic analysis of the sequences. The only exception was strain S6U, which exhibited an S. cerevisiae COX2 haplotype (Figure 3A).
S. bayanus hybrids strains, with alleles U and E in their nuclear genes, exhibited four different COX2 haplotypes which did not cluster together in the gene phylogeny. Some of their COX2 sequences clustered with ‘uvarum’ haplotypes U–I, U-II and U-III, and others did so with the ‘eubayanus’ haplotype E-I present in S. pastorianus strains (Figure 3A). Interestingly, three S. bayanus hybrid strains, CECT 11186, CBS 375 and CBS 378, exhibited a COX2 haplotype located in the phylogenetic tree at an intermediate position between the uvarum and eubayanus allele (E–I). A detailed analysis of the variable positions of the COX2 sequences (Figure 3B) showed that the 5′region of this haplotype was identical to the ‘uvarum’ haplotype sequences, but differed from the ‘eubayanus’ sequence, while the 3′region was identical to the ‘eubayanus’ sequence and differed from the ‘uvarum’. This result are indicative that these three hybrid strains may exhibit a putative recombinant COX2 haplotype (called UrE), which could result from the recombination between the uvarum and eubayanus COX2 genes. To check this putative recombination, we performed separate phylogenetic analyses of the 5′ and 3′ COX2 regions (Figure S2 in File S1) corresponding to nucleotide positions 1 to 525 and nucleotides 526 to 582, respectively. Accordingly in the 5′region phylogeny, the UrE haplotype clustered with the uvarum haplogroup and with the eubayanus E-I allele in the 3′ region phylogeny.
Discussion
Most studies about complex species “S. bayanus” coincide on the existence of two well-differentiated groups of strains: the molecularly and physiologically heterogeneous group of strains belonging to S. bayanus var. bayanus, and the homogenous group of strains pertaining to S. bayanus var. uvarum [1]. These two varieties have even been considered to be two different species (S. bayanus and S. uvarum, respectively) by other authors because of their partial reproductive isolation [8], [9]. However, the genetically heterogeneous nature of the ‘bayanus’ variety, as several works have demonstrated [4], [11], [12], makes it difficult to obtain reliable information about hybridization data to evaluate the reproductive isolation between these two varieties. Together with the discovery of the pure species S. eubayanus and the association of this new taxon with the ‘bayanus-like’ subgenome of S. pastorianus, Libkind et al. [4] proposed the use of S. eubayanus and S. uvarum as descriptors of species, but restricted the name S. bayanus to the hybrid lineages between pure species. S. eubayanus has not been detected in Europe; however, in order to explain its necessary contact with a S. cerevisiae ale strain to generate the hybrid S. pastorianus, it is feasible that this species inhabits a specific niche environment still to be sampled in this continent, as suggested by Gibson et al. [24].
For the purpose of finding a European strain of S. eubayanus, a set of 46 European strains obtained from different sources and annotated as S. bayanus in different culture collections have been genetically characterized. It is interesting to note that most analyzed strains (∼85%) were diploid (preliminary results not shown). As expected, most of the gene alleles found in the S. bayanus var. bayanus reference strain NBRC 1948 were divergent (6–8% of nucleotide divergence) as compared to the same ones in the S. bayanus var. uvarum reference strain CBS 7001. These divergence values were similar to those found between the pure lines of S. eubayanus and S. bayanus var. uvarum [4]. Contrarily, a significant fraction of identical or almost identical alleles was found between NBRC 1948 and CBS 7001 (27% of the genes under study). In a similar study, but with 35 S. bayanus and S. pastorianus strains (only nine strains coincide with our study), Rainieri et al., [11] have also identified alleles with high similarity between strains NBRC 1948 and CBS 7001. In their study, the authors considered that those alleles correspond to cases in which both the ‘bayanus’ and ‘uvarum’ varieties show same or similar allelic variants. In our work, these kinds of identical or almost identical alleles between the two varieties are considered ‘uvarum’ (U) due to the divergence found between the genes common to NBRC 1948 and CBS 7001 and the non S. cerevisiae portion in S. pastorianus Weihenstephan 34/70. Accordingly, these genes also evidence the non-pure nature of strain NBRC 1948. According to our results, the alleles named ‘bayanus’ by Rainieri et al.[11], which differed from ‘uvarum’ alleles in only a few nucleotidic positions, must be reconsidered to be ‘uvarum’ variants. Following the same argument, the ‘lager’ alleles in Rainieri et al. [11] must correspond to the real ‘bayanus’ alleles because they demonstrate a homology percentage of around 89–94% between these lager and ‘uvarum’ alleles, which are similar results to those observed in the present work between ‘uvarum’ and ‘eubayanus’ alleles.
The reconstructed S. bayanus var. bayanus pure line, which contains a combination of alleles present in different hybrid S. bayanus strains, shows a similarity of 99–100% with the non S. cerevisiae subgenome of the fully sequenced S. pastorianus lager strain Weihenstephan 34/70. After considering the genetic similarity demonstrated between S. eubayanus and the non S. cerevisiae portion of S. pastorianus [4], and as no complete database containing the whole S. eubayanus genome exists, we assigned the name ‘eubayanus’ instead of ‘bayanus’ to the non uvarum alleles in the S. bayanus var. bayanus strains (S. eubayanus x S. uvarum hybrids) analyzed in our work. Following the idea proposed by Gibson et al. [24], this hypothetical genotype may represent the genotype exhibited by a European pure line of S. eubayanus.
Of the 46 strains analyzed, 7 only exhibited U alleles for the 34 analyzed gene regions, 17 exhibited U alleles for 33 gene regions and a C allele for PEX2, and 3 exhibited U alleles for 32 gene regions and C alleles for PEX2 and MNL1, and hence, they can be considered pure S. bayanus var. uvarum or S. uvarum strains. These strains were isolated mainly from grapes, grape must or wine, but also from pear or apple ciders, while a few were isolated from other sources; i.e., spoiled and ale beers, alpechin (olive mill waste), or tree exudates. Low variation in allele composition was observed among these strains in the S. uvarum group. This intraspecific homogeneity has also been evidenced in recent studies using microsatellite loci analyses [25], [26]. Nevertheless, the presence of heterozygous strains in this group can be considered evidence for a certain degree of interbreeding among the strains of this variety. The sequence analysis of mitochondrial gene COX2 is also in accordance with this homogeneity, which was detected in the nuclear DNA for all the S. uvarum strains. COX2 is a highly variable gene that has proved most informative in determining the interspecies phylogenetic relationships in the Saccharomyces-Kluyveromyces complex [23], [27] and different interspecific hybrids of the genus Saccharomyces [13], [28], [29].
Twenty strains from the S. uvarum group, isolated from Irish cider, wine, beer, as well as different unfermented musts and natural environments, exhibited a S. cerevisiae introgression in gene PEX2, located in a subtelomeric region of the translocated S. uvarum chromosome VItX; 3 of them, isolated from Irish cider, presented a second introgression in gene MNL1, also located in a subtelomeric region of the translocated S. uvarum chromosome XVtVIII. The presence of S. cerevisiae subtelomeric sequences has been previously reported for the S. bayanus var. bayanus [9], [30] and S. bayanus var. uvarum [25] strains. According to the above-cited authors, the S. cerevisiae sequences in the S. bayanus genomes are the result of introgression following unstable interspecies hybridization [31], [32]. Introgression may be particularly effective for regaining lost traits, which were functional in a common ancestor; in other words, introgression often serves as a repair or replacement strategy [33]. The two introgressed strains identified by Naumova et al. [25] as S. bayanus var. uvarum are also included in the present study where we demonstrate that they contain ‘eubayanus’ alleles in nine different gene regions; hence they must be reclassified as hybrid S. bayanus var. bayanus strains. Introgressed S. cerevisiae telomeric Y′ sequences have also been described in three cider S. bayanus var. uvarum strains from Brittany and Normandy, France [34]. As Naumova et al. proposed [25], introgressed S. bayanus var. uvarum strains could be isolated if both, S. cerevisiae and S. bayanus var. uvarum, strains co-exist in the same environment, allowing hybridization [35], and, according our results, introgressions in subtelomeric regions seem to be quite frequent.
Origin of the S. bayanus var. bayanus genome complexity
The situation of the strains classified as S. bayanus var. bayanus is more complex due to the presence of different combinations of ‘uvarum’ and ‘eubayanus’ alleles in their nuclear genomes, as well as mtDNA of different origins, as indicated by the presence of the ‘uvarum’ or ‘eubayanus’ COX2 haplotypes, as well as a rare possible recombinant haplotype.
The recombination between mitochondrial DNAs from different parental strains has already been described for S. cerevisiae in early studies into yeast mitochondrial genetics [36]. In S. cerevisiae, mitochondria from the two parental spores can fuse in the zygote after mating. In these fused mitochondria, parental mtDNAs mix and recombine to generate a recombinant lineage that can be established as homoplasmic during mitochondrial vegetative segregation [36]. COX2 recombination was also postulated to occur in natural S. cerevisiae x S. kudriavzevii hybrids [37], and in this study, we showed that it could also have occurred in S. bayanus hybrids.
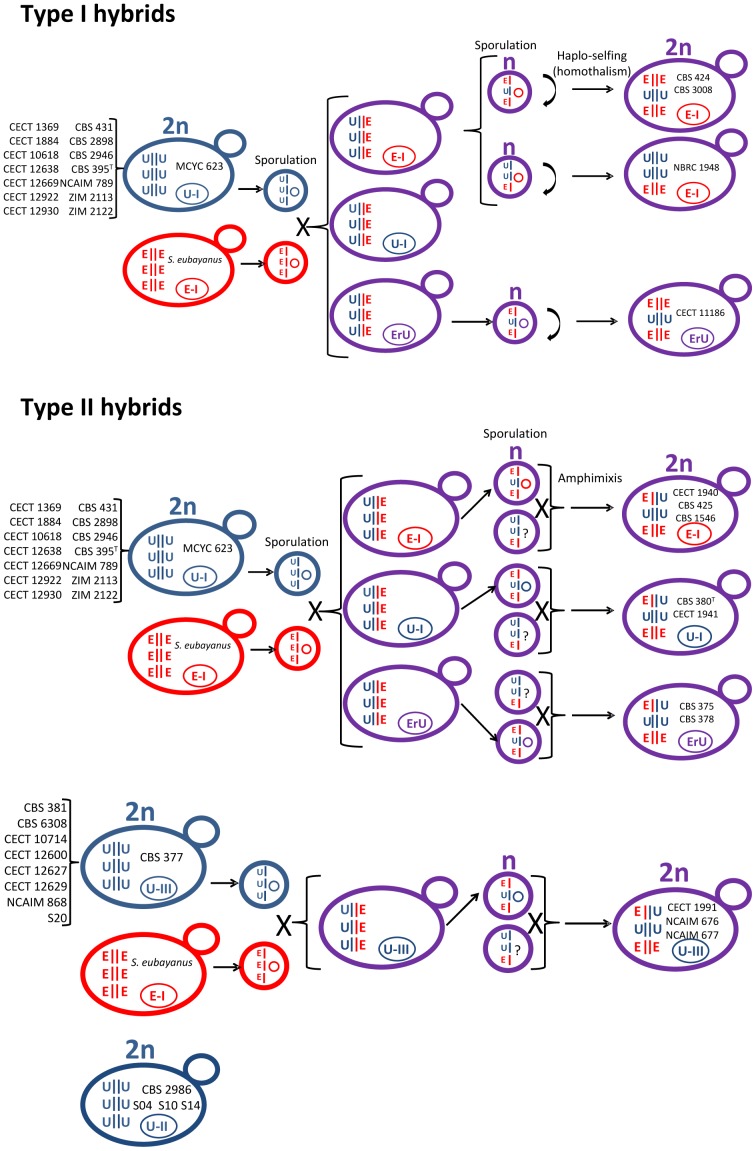
Based on the complexity observed in nuclear and mtDNA genes, we propose a scheme (Figure 4) to summarize the generation of all the different S. bayanus var. bayanus strains resulting from several hybridization events between pure strains from the ‘uvarum’ group and a strain possessing only ‘eubayanus’ alleles, which is related to the recently described Patagonian S. eubayanus, and is also similar to a European S. eubayanus strain.
Figure 4. Possible origins of the S. bayanus (S. uvarum x S. eubayanus) hybrid strains.
Based on the fact that two different ‘uvarum’ COX2 alleles were detected among the strains in the ‘bayanus’ hybrid group, we hypothesized that at least two ‘uvarum’ pure strains were involved in the origin of the complete set of S. bayanus strains studied here. Regardless of the parental ‘uvarum’ involved in hybridization, the first generation of hybrids would possess a complete set of chromosomes from each parental species, and should show reduced fertility according to the low spore viability (7%) exhibited by the artificial hybrids between S. eubayanus and S. uvarum generated by Libkind et al. [4]. In addition, Liti et al. [38] had shown that a sequence divergence of over 5% between strains considerably reduces spore viability, and the average genome divergence between S. eubayanus and S. uvarum is ∼6.9% [4].
After hybridization, and perhaps in a fermenting environment context where these hybrids are found and can reach extremely large yeast populations, some of these hybrids can sporulate to generate some viable spores (<7%) that can give rise to the two hybrid types I and II (Figure 4) described in this work. Type I hybrids can arise through sporulation and haplo-selfing (homothallism), while Type II hybrids do so by sporulation and amphimixis, or by mating those spores carrying different a genetic background (heterothallism) (Figure 4). In this ancestral hybrid, recombination between “uvarum” and “eubayanus” chromosomes was also possible during sporulation.
Hybrid speciation has been performed under laboratory conditions from artificial S. cerevisiae x S. paradoxus hybrids [39] and, despite being uncommon, it has been described in plants [40]. This is partly due to the ability to self-fertilize, which produces identical homolog pairs for every chromosome (except at the mating-type locus on chromosome III), thus avoiding any incompatibility that might arise by fusion with other gametes, even from the same parent [39].
Although the initial hybrids obtained from S. eubayanus and S. uvarum mating show low spore viability [4], derived type I hybrids would probably recover a higher fertility due to their homozygosity. Contrarily, type II hybrids bearing an important proportion of genes in heterozygosis (E–U) should show lower fertility than the type I hybrids. According to this hypothesis, the larger number of heterozygous genes in hybrids, the lower their fertility. In this sense, spore viability for some of these strains was evaluated by Naumov [10]. In that study, Naumov observed 48% and 7% of spore viabilities for strains CBS 380 and CBS 425, respectively, corresponding to heterozygous type II strains in the present study, and 77% of spore viability for CBS 424, a homozygous type I strain.
It is important to note that, with the exception of the work by Libkind et al. [4], all previous studies evaluated the viability of the hybrids generated by mating a S. bayanus var. uvarum pure strain (i.e., CBS 7001) and different S. bayanus var. bayanus strains, such as NBRC 1948 or CBS 380, which correspond according to this study to S. bayanus hybrid lines. In this context, the analysis of hybrid fertility is confusing and extremely variable because they really correspond to backcrosses between hybrids and representative strains of the parental species, while the fertility of the progeny depends on the genome constitution of the hybrid.
Saccharomyces pastorianus
As previously mentioned, the most popular examples of hybrid yeasts are those involved in lager beer production included in the taxa S. pastorianus (syn. S. carlsbergensis and S. monacensis), which originated from natural hybridization between S. cerevisiae and S. eubayanus [4]. Although the different S. pastorianus strains possess chromosomes from both species [41], [42], mitochondrial DNA was always acquired from the non cerevisiae parental [43]. Recent comparative genome hybridization studies have shown that S. pastorianus strains came about as a result of at least two independent hybridization events [12], [44]. These hybridizations generated the two main groups of lager brewing strains, including Frohberg-type lager strains (i.e., Weihenstephan 34/70) and Saaz-type lager strains (i.e., S. monacensis CBS 1503 = CECT 1970).
Our study also agrees partly with these data as Saaz-type lager strains CECT 1885, CECT 11000, CBS 1503 (the type strain of S. monacensis) and CBS 1513 (the type strain of S. carlsbergensis) exhibit an E2 fixed allele for genes BRE5 and BAS1, whilst strain Weihenstephan 34/70 (a Frohberg-type lager strain) possesses an E1 allele for the same gene regions.
No ‘cerevisiae’ alleles were detected in strain CECT 1940, considered the type strain of S. pastorianus. This fact indicates that two different strains can be found under the same name, CECT 1940. The literature reports this confusion as to the use of the neotype of S. pastorianus several times, and it has been attributed to the misuse of two of its neotype strains: CBS1538NT (from the CBS) and NRRLY-1551 (from the ARS collection in the past). A recent study by Nguyen et al. [12], together with the proteomic data reported by Joubert et al. [45], demonstrate that NRRLY-1551 has been misidentified and should be reclassified as S. bayanus. Our results coincide with the data reported by Nguyen et al [12], but we also observed that the strain CECT 1940 used in this work (probably originating from strain NRRLY-1551, and not from CBS 1538) is a S. bayanus hybrid strain that bears both the ‘uvarum’ and ‘eubayanus’ alleles.
Finally, while strain S6U seems to have been originated from the hybridization of a S. bayanus var. uvarum pure strain (such as CBS 381, CBS 431 and CECT 1884) and a S. cerevisiae, the remaining S. pastorianus strains evaluated in this work were obtained from the hybridization between a S. eubayanus strain (strains Weihenstephan 34/70, CECT 1885, CBS 1503 and CBS 1513) or S. bayanus (bearing both ‘eubayanus’ and ‘uvarum’ alleles; i.e., CECT 11000) and a S. cerevisiae strain. The fact that wine strain S. pastorianus CECT 1885 shows ‘eubayanus’ alleles does not fall in line with the hypothesis of Naumova et al. [30], which suggests that the non ‘cerevisiae’ parental of S. pastorianus wine strains is always a S. bayanus var. uvarum, unless this strain is a contaminant strain from brewing environments.
In this work, the complexity of “S. bayanus” species group was deciphered using the restriction analysis of 34 genes used to differentiate ‘uvarum’ (U) and ‘eubayanus’ (E) alleles. From the 48 analyzed strains none was a pure S. bayanus var. bayanus/S. eubayanus strain. The ‘uvarum’ group showed a high intraspecific homogeneity, although a certain degree of interbreeding among the strains of this variety was shown. The situation of the ‘bayanus’ group is more complex, all these stains are hybrids between S. uvarum and S. eubayanus and can be divided in two subgroups: type I or homozygous strains and type II or heterozygous strains. A scheme summarizing the generation of all the different S. bayanus var. bayanus is proposed.
Supporting Information
Contains the files: Figure S1 Chromosome composition and gene order in different Saccharomyces species. A- S. cerevisiae. B- S. eubayanus. C- S. uvarum. Figure S2 Phylogenetic analysis of the 5′ and 3′ regions of the mitochondrial COX2 gene. A- 5′ region. B- 3′ region. Table S1 Gene regions under restriction analysis and primers used for PCR amplification. Chromosome (Chr) positions of the genes correspond to S. cerevisiae, for other arrangements present in the other strains see Figure S1. Table S2 Composite restriction patterns deduced from the gene region sequences of the eubayanus -type alleles, present in the reference strains S. bayanus NBRC 1948, CECT 11186, CBS 424 or S. pastorianus Weihenstephan 34/70, the uvarum alleles exhibited by S. uvarum CBS 7001, and the cerevisiae -type alleles present in S. cerevisiae S288c. These composite patterns for each gene region have been named after the initial of the allele-type name followed by the order numeral 1. Chromosome (Chr) positions of the genes correspond to S. cerevisiae, for other arrangements present in the other strains see Figure S1. Table S3 Alternative restriction patterns exhibited by S. bayanus or S. uvarum strains differing by one or two restriction site gains/losses (indicated in bold) from those found in the reference strains. Table S4 Conformation of the S. uvarum strains for each gene region according to the composite restriction patterns exhibited. For a description of the composite restriction patterns, see Tables S2 and S3. Mitochondrial COX2 sequence haplotypes are described in Figure 2. Table S5 Conformation of the S. bayanus strains with eubayanus - and uvarum -type alleles according to the composite restriction patterns exhibited. For a description of the composite restriction patterns, see Tables S2 and S3. Mitochondrial COX2 sequence haplotypes are described in Figure 2. Table S6 Conformation of the S. pastorianus strains with eubayanus - cerevisiae - or uvarum -type alleles according to the composite restriction patterns exhibited. For a description of the composite restriction patterns, see Tables S2 and S3. Mitochondrial COX2 sequence haplotypes are described in Figure 2, except S6U COX2, which is similar to S. cerevisiae (C) COX2.
(ZIP)
Funding Statement
This work was supported by grants AGL2009-12673-CO2 (01 and 02) and AGL2012-39937-CO2 (01 and 02) from the Spanish Government to AQ and EB, respectively. LP-T and CAL acknowledge to CSIC and Fundación Carolina for the I3P and Formación Permanente fellowships respectively. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Vaughan-Martini A, Martini A (2011) Chapter 61 - Saccharomyces Meyen ex Reess (1870). In: The Yeasts (Fifth Edition). London: Elsevier. pp. 733–746.
- 2. Casaregola S, Nguyen HV, Lapathitis G, Kotyk A, Gaillardin C (2001) Analysis of the constitution of the beer yeast genome by PCR, sequencing and subtelomeric sequence hybridization. Int J Syst Evol Microbiol 51: 1607–1618. [DOI] [PubMed] [Google Scholar]
- 3. Nakao Y, Kanamori T, Itoh T, Kodama Y, Rainieri S, et al. (2009) Genome sequencing of the lager brewing yeast, an interspecies hybrid. DNA Research 16: 115–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Libkind D, Hittinger CT, Valério E, Gonçalves C, Dover J, et al. (2011) Microbe domestication and the identification of the wild genetic stock of lager-brewing yeast. Proc Natl Acad Sci USA 108: 14539–14544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rainieri S, Zambonelli C, Hallsworth JE, Pulvirenti A, Giudici P (1999) Saccharomyces uvarum, a distinct group within Saccharomyces sensu stricto. FEMS Microbiol Lett 177: 177–185. [DOI] [PubMed] [Google Scholar]
- 6. Nguyen HV, Gaillardin C (1997) Two subgroups within the Saccharomyces bayanus species evidenced by PCR amplification and restriction polymorphism of the non-transcribed spacer 2 in the ribosomal DNA unit. Syst Appl Microbiol 20: 286–294. [Google Scholar]
- 7. Nguyen HV, Lepingle A, Gaillardin C (2000) Molecular typing demonstrates homogeneity of Saccharomyces uvarum strains and reveals the existence of hybrids between S. uvarum and S. cerevisiae including the S. bayanus type strain CBS 380. Syst Appl Microbiol 23: 71–85. [DOI] [PubMed] [Google Scholar]
- 8. Pulvirenti A, Nguyen HV, Caggia C, Giudici P, Rainieri S, et al. (2000) Saccharomyces uvarum, a proper species within Saccharomyces sensu stricto. FEMS Microbiol Lett 192: 191–196. [DOI] [PubMed] [Google Scholar]
- 9. Nguyen HV, Gaillardin C (2005) Evolutionary relationships between the former species Saccharomyces uvarum and the hybrids Saccharomyces bayanus and Saccharomyces pastorianus; reinstatement of Saccharomyces uvarum (Beijerinck) as a distinct species. FEMS Yeast Res 5: 471–483. [DOI] [PubMed] [Google Scholar]
- 10. Naumov GI (2000) Saccharomyces bayanus var. uvarum comb. nov., a new variety established by genetic analysis. Microbiology 69: 338–342. [PubMed] [Google Scholar]
- 11. Rainieri S, Kodama Y, Kaneko Y, Mikata K, Nakao Y, et al. (2006) Pure and mixed genetic lines of Saccharomyces bayanus and Saccharomyces pastorianus and their contribution to the lager brewing strain genome. Appl Environ Microbiol 72: 3968–3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen HV, Legras JL, Neuveglise C, Gaillardin C (2011) Deciphering the Hybridisation History Leading to the Lager Lineage Based on the Mosaic Genomes of Saccharomyces bayanus Strains NBRC1948 and CBS380T. PLOS One 6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. González SS, Barrio E, Querol A (2008) Molecular characterization of new natural hybrids between Saccharomyces cerevisiae and Saccharomyces kudriavzevii from brewing. Appl Environ Microbiol 74: 2314–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ryu SL, Murooka Y, Kaneko Y (1996) Genomic reorganization between two sibling yeast species, Saccharomyces bayanus and Saccharomyces cerevisiae . Yeast 12: 757–764. [DOI] [PubMed] [Google Scholar]
- 15. Kellis M, Patterson N, Endrizzi M, Birren BW, Lander ES (2003) Sequencing and comparison of yeast species to identify genes and regulatory elements. Nature 423: 241–254. [DOI] [PubMed] [Google Scholar]
- 16. Querol A, Barrio E, Huerta T, Ramón D (1992) Molecular monitoring of wine fermentations conducted by active dry yeast strains. Appl Environ Microbiol 58: 2948–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24: 1596–1599. [DOI] [PubMed] [Google Scholar]
- 18. Posada D (2008) jModelTest: phylogenetic model averaging. Mol Biol Evol 25: 1253–1256. [DOI] [PubMed] [Google Scholar]
- 19. Posada D, Buckley TR (2004) Model selection and model averaging in phylogenetics: Advantages of akaike information criterion and Bayesian approaches over likelihood ratio tests. Syst Biol 53: 793–808. [DOI] [PubMed] [Google Scholar]
- 20. Kimura M (1980) A simple method for estimating evolutionary rate of base substitution through comparative studies of nucleotide sequences. J Mol Evol 16: 111–120. [DOI] [PubMed] [Google Scholar]
- 21. Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, et al. (2010) New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst Biol 59: 307–321. [DOI] [PubMed] [Google Scholar]
- 22. Felsenstein J (1985) Confidence limits on phylogenies with a molecular clock. Syst Zool 34: 152–161. [Google Scholar]
- 23. Belloch C, Querol A, García MD, Barrio E (2000) Phylogeny of the genus Kluyveromyces inferred from mitochondrial cytochrome-c oxidase II gene. Int J Syst Evol Microbiol 50: 405–416. [DOI] [PubMed] [Google Scholar]
- 24. Gibson BR, Storgards E, Krogerus K, Vidgren V (2013) Comparative physiology and fermentation performance of Saaz and Frohberg lager yeast strains and the parental species Saccharomyces eubayanus . Yeast 30: 255–266. [DOI] [PubMed] [Google Scholar]
- 25. Naumova ES, Naumov GI, Michailova YV, Martynenko NN, Masneuf-Pomarede I (2011) Genetic diversity study of the yeast Saccharomyces bayanus var. uvarum reveals introgressed subtelomeric Saccharomyces cerevisiae genes. Res Microbiol 162: 204–213. [DOI] [PubMed] [Google Scholar]
- 26. Masneuf-Pomarède I, Le Jeune C, Durrens P, Lollier M, Aigle M, et al. (2007) Molecular typing of wine yeast strains Saccharomyces bayanus var. uvarum using microsatellite markers. Syst Appl Microbiol 30: 75–82. [DOI] [PubMed] [Google Scholar]
- 27. CP, Robnett CJ (2003) Phylogenetic relationships among yeasts of the “Saccharomyces complex” determined from multigene sequence analyses. FEMS Yeast Res 1554: 1–16. [DOI] [PubMed] [Google Scholar]
- 28. González SS, Barrio E, Gafner J, Querol A (2006) Natural hybrids from Saccharomyces cerevisiae, Saccharomyces bayanus and Saccharomyces kudriavzevii in wine fermentations. FEMS Yeast Res 6: 1221–1234. [DOI] [PubMed] [Google Scholar]
- 29. Peris D, Lopes C, Belloch C, Querol A, Barrio E (2012) Comparative genomics among Saccharomyces cerevisiae x Saccharomyces kudriavzevii natural hybrid strains isolated from wine and beer reveals different origins. BMC Genomics 13: 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Naumova ES, Naumov GI, Masneuf-Pomarède I, Aigle M, Dubourdieu D (2005) Molecular genetic study of introgression between Saccharomyces bayanus and S. cerevisiae . Yeast 22: 1099–1115. [DOI] [PubMed] [Google Scholar]
- 31. Dujon B (2006) Yeasts illustrate the molecular mechanisms of eukaryotic genome evolution. Trends Genet 22: 375–387. [DOI] [PubMed] [Google Scholar]
- 32. Hall C, Brachat S, Dietrich FS (2005) Contribution of Horizontal Gene Transfer to the Evolution of Saccharomyces cerevisiae . Eukaryot Cell 4: 1102–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rieseberg LH (2009) Evolution: Replacing Genes and Traits through Hybridization. Current biology: CB 19: R119–R122. [DOI] [PubMed] [Google Scholar]
- 34. Naumov GI, Nguyen HV, Naumova ES, Michel A, Aigle M, et al. (2001) Genetic identification of Saccharomyces bayanus var. uvarum, a cider-fermenting yeast. Int J Food Microbiol 65: 163–171. [DOI] [PubMed] [Google Scholar]
- 35. Suárez-Valles B, Pando-Bedriñana R, Fernández-Tascón N, Querol A, Rodríguez-Madrera R (2007) Yeast species associated with the spontaneous fermentation of cider. Food Microbiol 24: 25–31. [DOI] [PubMed] [Google Scholar]
- 36. Berger KH, Yaffe MP (2000) Mitochondrial DNA inheritance in Saccharomyces cerevisiae . Trends Microbiol 8: 508–513. [DOI] [PubMed] [Google Scholar]
- 37. Peris D, Belloch C, Lopandic K, Álvarez-Pérez JM, Querol A, et al. (2012) The molecular characterization of new types of Saccharomyces cerevisiae x S. kudriavzevii hybrid yeasts unveils a high genetic diversity. Yeast 29: 81–91. [DOI] [PubMed] [Google Scholar]
- 38. Liti G, Barton DBH, Louis EJ (2006) Sequence diversity, reproductive isolation and species concepts in Saccharomyces . Genetics 174: 839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Greig D, Louis EJ, Borts RH, Travisano M (2002) Hybrid speciation in experimental populations of yeast. Science 298: 1773–1775. [DOI] [PubMed] [Google Scholar]
- 40. Rieseberg LH (1997) Hybrid origins of plant species. Annu Rev Ecol Syst 28: 359–389. [Google Scholar]
- 41.Kodama Y, Kielland-Brandt MC, Hansen J (2005) Lager brewing yeast. In: Sunnerhagen P, Piškur J, editors. Comparative Genomics: using fungi as models. Berlin, Germany: Springer-Verlag. pp. 145–164.
- 42. Yamagishi H, Ogata T (1999) Chromosomal structures of bottom fermenting yeasts. Syst Appl Microbiol 22: 341–353. [DOI] [PubMed] [Google Scholar]
- 43. Piškur J, Smole S, Groth C, Petersen RF, Pedersen MB (1998) Structure and genetic stability of mitochondrial genomes vary among yeasts of the genus Saccharomyces . Int J Syst Bacteriol 48: 1015–1024. [DOI] [PubMed] [Google Scholar]
- 44. Dunn B, Sherlock G (2008) Reconstruction of the genome origins and evolution of the hybrid lager yeast Saccharomyces pastorianus . Genome Res 18: 1610–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Joubert R, Brignon P, Lehmann C, Monribot C, Gendre F, et al. (2000) Two-dimensional gel analysis of the proteome of lager brewing yeasts. Yeast 16: 511–522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Contains the files: Figure S1 Chromosome composition and gene order in different Saccharomyces species. A- S. cerevisiae. B- S. eubayanus. C- S. uvarum. Figure S2 Phylogenetic analysis of the 5′ and 3′ regions of the mitochondrial COX2 gene. A- 5′ region. B- 3′ region. Table S1 Gene regions under restriction analysis and primers used for PCR amplification. Chromosome (Chr) positions of the genes correspond to S. cerevisiae, for other arrangements present in the other strains see Figure S1. Table S2 Composite restriction patterns deduced from the gene region sequences of the eubayanus -type alleles, present in the reference strains S. bayanus NBRC 1948, CECT 11186, CBS 424 or S. pastorianus Weihenstephan 34/70, the uvarum alleles exhibited by S. uvarum CBS 7001, and the cerevisiae -type alleles present in S. cerevisiae S288c. These composite patterns for each gene region have been named after the initial of the allele-type name followed by the order numeral 1. Chromosome (Chr) positions of the genes correspond to S. cerevisiae, for other arrangements present in the other strains see Figure S1. Table S3 Alternative restriction patterns exhibited by S. bayanus or S. uvarum strains differing by one or two restriction site gains/losses (indicated in bold) from those found in the reference strains. Table S4 Conformation of the S. uvarum strains for each gene region according to the composite restriction patterns exhibited. For a description of the composite restriction patterns, see Tables S2 and S3. Mitochondrial COX2 sequence haplotypes are described in Figure 2. Table S5 Conformation of the S. bayanus strains with eubayanus - and uvarum -type alleles according to the composite restriction patterns exhibited. For a description of the composite restriction patterns, see Tables S2 and S3. Mitochondrial COX2 sequence haplotypes are described in Figure 2. Table S6 Conformation of the S. pastorianus strains with eubayanus - cerevisiae - or uvarum -type alleles according to the composite restriction patterns exhibited. For a description of the composite restriction patterns, see Tables S2 and S3. Mitochondrial COX2 sequence haplotypes are described in Figure 2, except S6U COX2, which is similar to S. cerevisiae (C) COX2.
(ZIP)